Abstract
Background: Previous studies of the goat heart subjected to prolonged atrial pacing induced sustained atrial fibrillation (AF). Structural changes included marked accumulation of glycogen in atrial myocytes. Aims: In the present study, we hypothesized that glycogen deposition in canine atrial myocytes promotes paroxysmal forms of AF and is involved in fibrosis development in the later stages of AF. Material & methods: In dogs under pentobarbital anesthesia, tissues were obtained from the right and left atrial appendages (LAA/RAA). Periodic acid Schiff (PAS) and Masson’s trichrome staining of the LAA/RAA from normal dogs, and those subjected to atrial pacing induced AF for 48 h or 8 weeks determined glycogen and collagen concentrations, respectively, using morphometric analysis. Results: At baseline, there was a significant greater concentration of glycogen in the LAA than the RAA (P ≤ 0.05). Compared to the RAA, the LAA glycogen, was dense and locked against the intercalated discs. After pacing induced AF for 48 hours and 8 weeks there was a marked increase in glycogen deposition, significantly greater than in the baseline state (P ≤ 0.05). There was a similar and progressive increase in collagen concentrations in each group (P ≤ 0.05). Conclusions: The differential in glycogen concentration, in conjunction with other factors, neural and electrophysiological, provide a basis for the greater propensity of the left atrium for paroxysmal AF, at baseline and 48 hours of pacing induced AF. The marked increase in collagen at 8 weeks of pacing provides a substrate for sustained AF. Evidence is presented linking glycogen accumulation and fibrosis as factors in the persistent forms of AF.
Keywords: Atrial fibrillation, glycogen, fibrosis
Introduction
A recently completed study by Embi et al. [1] suggested that differences in the distribution and concentration of glycogen in the right and left atrial appendages provided presumptive evidence for the well-known greater propensity of the left atrium for atrial fibrillation (AF) than the right. The seminal studies by Allessie and his associates more than 3 decades ago [2-5] strongly supported the multiple wavelet hypothesis developed by Moe and Abildscov provided strong evidentiary confirmation for the multiple wavelet hypothesis [6,7] as a mechanism underlying AF. Using the goat heart as their model the Netherland’s investigators showed that sustained AF could be achieved by long-term rapid atrial pacing [4]. Moreover, Ausma et al. [8] demonstrated that both electrophysiological and structural remodeling occurred as AF was maintained by pacing and then became spontaneously sustained. One striking characteristic of the structural remodeling was the marked accumulation of glycogen within cells that had lost their contractile elements and had increased in size. The mechanism for glycogen accumulation and its consequences were not directly addressed other than there being an abnormality of glucose metabolism in the context of pacing induced AF [9]. In the present study we have extended the study by Embi et al. by determining the differential amounts of glycogen and collagen in the right and left atrial appendages in dogs subjected to pacing induced AF for 48 hours and after AF was sustained by pacing for 8 weeks.
Materials and methods
This study protocol was reviewed and approved by the institutional Animal Care and Use Committee of the First Affiliated Hospital of Xinjiang Medical University. The investigation conforms to the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Eighteen healthy mongrel dogs of either sex weighing 18 ± 2.12 kg, age 2-3 years, were anaesthetized with sodium pentobarbital (initial bolus 30 mg/kg IV, 50-100 mg as needed for maintenance, every 1-2 hours). After intubation, ventilation was initiated with room air delivered by a positive pressure respirator. Core body temperature was maintained at 36.5 ± 1.5°C. Standard limb ECG leads were continuously recorded to determine the heart rate and rhythm. Blood pressure (BP) was continuously monitored via a pressure transducer attached to a plastic sheath inserted into the right femoral artery.
The dogs were divided into three groups: Group 1, 6 dogs were not paced. Group 2, dogs (n = 6) were studied after 48 hours during which sustained AF was induced by rapid LA pacing [2,3]. Group 3, dogs (n = 6) were studied after 8 weeks of pacing induced AF. The dogs were examined periodically for the presence of AF that persisted by turning off the pacemaker.
Group1: Control group implanted pacemaker without pacing (n = 6).
Group 2: After a left lateral thoracotomy, the heart was exposed and placed in a pericardial cradle. A bipolar lead was sewn on the left atrial appendage. The pericardium was closed. The lead was then connected to a generator (made by Fudan University), which was placed in the pocket between the left chest muscle layers. The chest was closed. After recovery for approximately 1 week, the pacemakers were turned on to deliver rapid right atrium (LA) pacing at 600 beats/min to induce and maintain AF for 48 hours.
Group 3: The procedure was the same with Group 2, but pacing time was 8 weeks.
Tissue collection and processing
To analyze the morphologic and histological changes, hearts were obtained after the animals were euthanized by administration of a high dose of sodium pentobarbital. Tissues obtained from the RAA, LAA were fixed in 4% paraformaldehyde for 48 h at 4°C, the specimens were then processed for paraffin impregnation. Five μm thick sections were cut and mounted on clean glass slides. These sections were processed for Periodic acid-Schiff (PAS)and Masson trichrome’s staining in accordance with standard procedures for morphologic analysis. PAS and Masson’s trichrome staining was used to measure the amount of glycogen and collagen separately in the tissues taken from the animals after 48 hours and 8 weeks of pacing induced AF.
Morphometric analysis of histological images
Images of the stained sections were captured with Axio Vision software (Axio Vision 4.1 Zeiss. German) using a CCD camera attached to an inverted light microscope (Carl Zeiss, German). Images of all tissues were acquired at the same time with a constant set of imaging parameters on the microscope and imaging software.
Histomorphometry of PAS-positive cells and interstitial collagen were determined using 20 × and 40 × magnification separately by an experienced and independent cell scientist in a blinded manner. Quantitative measurements of the PAS positive area and collagen were assessed using Image Pro Plus 6.0 image analysis software (Media Cybernetics, Inc.). Glycogen and fibrosis were reported as area covered by staining which were then compared to the total area of the tissue in the image.
An area of interest (PAS+, collagen fibers) was selected by performing the segmentation command. Artifacts and objects of non-interest were manually deleted from the selected group of objects. Then the overall glycogen area (μm2) and total fiber area (μm2) were measured from the total area of a captured image using the ‘area’ tools. Six or more randomly captured images were analyzed for each group.
Statistical analysis
Statistical analyses were performed using SPSS (version 17.0). All values are presented as mean ± standard deviation. Data were summarized for analysis using Excel 2010. A paired t test was used for comparisons of LAA and RAA in the same group. Statistical comparisons of multiple group means were obtained by variance analysis (ANOVA). A p value of < 0.05 was considered significant.
Results
Histological studies
Glycogen in the RAAs highlighted the intercalated discs (arrow, Figure 1A) associated with varying amounts of glycogen granules extending from the intercalated discs (arrows) into the myocytes. In the LAAs, the glycogen often showed dense accumulation which coalesced against the intercalated disc (arrows, Figure 1B). Furthermore, the dense glycogen extended into and occupied the sarcoplasm suggesting it may affect longitudinal as well as the side-to-side myocyte conduction (Figure 1B, arrowhead). Unobstructed intercalated discs were sparse compared to the RAAs in control group.
Figure 1.
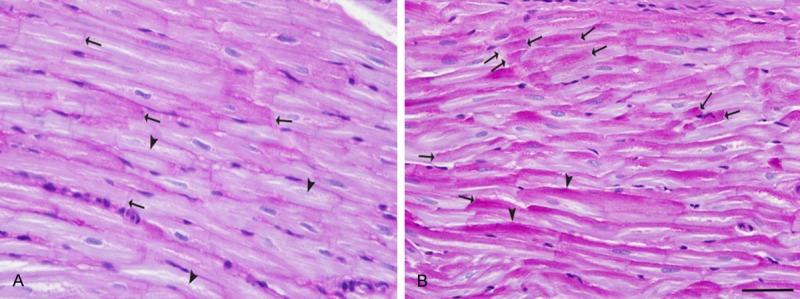
Differential glycogen concentration and distribution in the RAA and LAA as shown by PAS staining. A. In the RAAs glycogen deposition delicately highlighted the intercalated discs (arrows). Also glycogen deposition was seen along and within several myocytes (arrowheads). B. In contrast to the RAAs, in the LAAs glycogen often coalesced against intercalated discs (arrows) with dense tails of glycogen extending into the cells along the lateral wall at the myocyte-myocyte junctions (arrowheads). Periodic-acid Schiff (PAS) stain. Bar = 50 μm.
Figure 2 shows a graphic comparison of the concentrations of glycogen (determined morphometrically) in the LAA and RAA in the control and after pacing induced AF for 48 hours and 8 weeks. As determined by morphometric analysis, the concentration of glycogen was significantly greater in the LAA than in the RAA in the control state as well as after 48 hours of pacing induced AF (P ≤ 0.05). However, after 8 weeks of pacing induced AF, higher concentrations of glycogen were seen in both the LAA and RAA compared to the baseline state.
Figure 2.
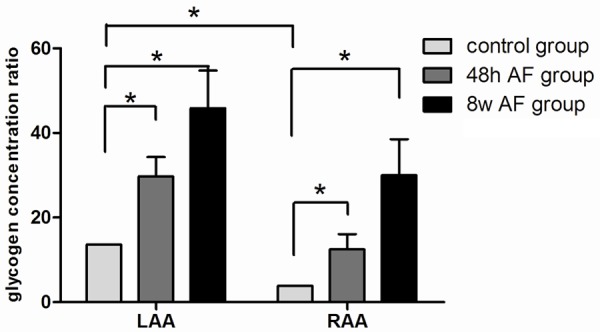
Graphic representation of the glycogen concentration in RAA and LAA in the baseline state and after pacing induced AF for 48 hours and 8 weeks. Atrial fibrillation promoted glycogen deposition. The level of glycogen deposition in 48 hour AF group was significantly higher than that in the control group and was the highest in the 8 week group. Values are represented as mean ± standard deviation, asterisk (*) indicates a P < 0.05 compared with control group. See text for further discussion.
Figure 3 Collagen distribution was assessed histologically with Masson’s trichrome stain. In RAA, LAA sections, the collagen was distributed throughout the tissue (Figure 3A, 3D, arrows) along with supporting blood vessels and myocytes. In the RAA, the collagen was distributed with multiple foci of more pronounced separation between myocytes and surrounding blood vessels with incipient connective tissue within the spaces between myofibrils (Figure 3B, 3C, 3E, 3F, arrows).
Figure 3.
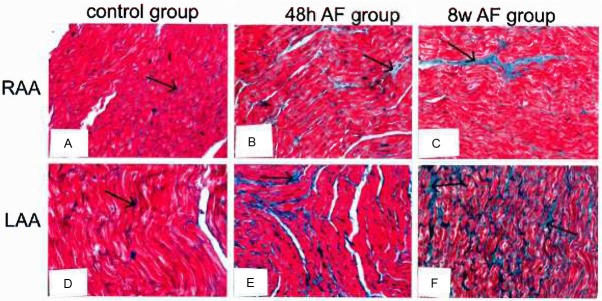
Differential concentrations of collagen in the LAA and RAA in the baseline state and after pacing induced AF for 48 hours and 8 weeks by Masson’s trichrome staining. Collagen deposition was clearly seen as patches of fibrosis (arrow) and other sites particularly between adjacent groups of myocytes. In the RAA, similar fibrotic patches were observed but connective tissue development (light blue staining) was present showing marked separation of myofibrils.
Based upon morphometric analysis, there was a progressive and significant increase in the concentration of collagen in both the LAA and RAA when 48 hours and 8 weeks of pacing induced AF were compared with the baseline state (Figure 4).
Figure 4.
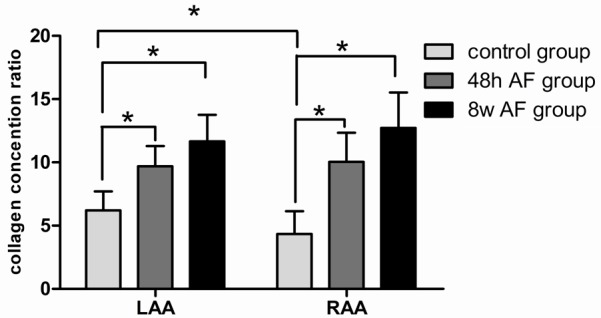
Graphic presentation of collagen deposition in the three study groups. The 8 week AF group showed dense collagen deposits compared with the 48 hour AF group which showed moderate collagen, whereas, the control group indicated slight collagen deposition. There was a progressive increase in collagen so that the 48 hour and 8 week groups were significantly greater than that detected in the baseline state. Values are represented as mean ± standard deviation, asterisk (*) indicates a P < 0.05 compared with control group.
Discussion
We found a significantly greater concentration of glycogen in the LAA compared to the RAA starting in the baseline state and progressing as pacing-induced AF was maintained for 48 hours. At 8 weeks, the glycogen concentration was significantly greater in the LAA than in the RAA. Notably, the distribution of the LAA glycogen was seen in the dense accumulation of glycogen at the intercalated discs and extending into the myocytes. These findings suggest that the dense glycogen accumulation at these sites could impede electrical conduction both longitudinally and laterally between adjacent myocytes. The implications of these findings are discussed below.
Since 1998, AF has received considerable attention mainly due to the advent of catheter ablation as an effective treatment of drug resistant AF. Invariably the introduction to many of these reports points out that AF is the most common cardiac arrhythmia encountered clinically. The mechanistic basis for AF can be a combination of a single or multiple firing sites, such as the pulmonary veins [10,11], the myocardium within the ligament of Marshall [12], the atrial sleeve of the superior vena cava [13]; and/or multiple reentrant circuits engaging one or both atria [4-7].
The question arises, are there intrinsic properties of the atria, both in the normal state and in response to the progressive stages of AF (paroxysmal, persistent, long-standing persistent) that predisposes the atria, particularly the left atrium for AF, above and beyond the well-known electrophysiological remodeling [4]? Previous experimental [14] and clinical [15] studies have demonstrated that a hyperactive state of the intrinsic cardiac autonomic nervous system can be a critical component for the initiation and maintenance of AF. It is interesting to note that these intrinsic neural structures, ganglionated plexi, are located on the left atrium at the PV-atrial junctions. Do structural differences in glycogen concentration and distribution between the LAA and RAA also contribute to the propensity for AF, even in the baseline state and during the progressive stages of AF?
In the present study, in the baseline state, we found a significantly greater concentration of glycogen in the LAA compared to the RAA. Notably, the distribution of the LAA glycogen was seen in dense accumulations at the intercalated discs and extending into the myocytes. We hypothesized the large glycogen molecules provide an impediment to electrical conduction both longitudinally and laterally. These heterogeneously distributed islands of impaired conduction create a non-uniform wavefront of activation with areas of slow conduction predisposing for reentry circuit development more likely to occur in the LAA than the right RAA.
In support of this hypothesis, several experimental [16,17] and clinical [18] reports have noted the greater propensity for AF residing in the left rather than the right atrium. Specifically, Dhillon et al. [19] studied gap junction resistivity and measured conduction velocity in guinea pig atria and found significant differences between the left and right atria. They found that the left atrial gap junction resistivity was significantly higher and conduction velocity significantly lower compared to the right atrium. Moreover, addition of a gap junction uncoupling agent slowed atrial conduction more in the LAA than in the RAA.
Tai et al. [20] compared activation of the left and right atrial in control and heart failure dogs. They designated 3 different forms of activation: Type 1, a single broad wavefront; Type 2, a nonuniform wavefront associated with conduction block and/or slow conduction or the presence of two wavelets; Type 3, the presence of 3 or more wavelets associated with areas of slow conduction and multiple arcs of conduction block. In the right atrium only 2 of the 5 control dogs had Type 2 activation whereas in the left atrium, all 5 control dogs had Type 2 activation. Furthermore, the left atrium had a greater dispersion of refractoriness (a biomarker for AF susceptibility) than the right atrium.
The findings of the present study confirmed the previous findings of Ausma et al. [8,21] who showed, in the goat model, several structural changes that occurred with progressive pacing-induced AF until the arrhythmias was sustained without pacing. They noted that marked glycogen accumulation was associated with enlarged myocytes and loss of myofibrils (myolysis). They also stated that, “The degree of glycogen accumulation and myolysis increased till 8 weeks and did not increase further…” [21]. In the present study we found that the greatest increase of collagen (fibrosis) occurred at 8 weeks of pacing induced AF. In this regard, Park et al. [22] looked for myofibroblasts in left atrial appendage tissues taken from patients with AF (n = 17) and those in sinus rhythm (n = 15) during cardiac surgery. Of interest, they found a significant difference in atrial remodeling between the AF and non-AF groups. There was a direct relationship between the presence of myofibroblasts and fibrosis. More relevant to the present study, the sites of myofibroblasts accumulation co-localized with PAS positive cells. It would appear that the sites of glycogen accumulation not only leads to disparities of conduction but also sets the stage for myolysis and myocytes replacement with fibrotic areas. The importance of fibrosis as a substrate for AF has been emphasized since the early studies of Spach et al. [23,24] who viewed fibrosis as an electrophysiological impediment to cell to cell connectivity both longitudinally and laterally leading to a fractionated wavefront favoring reentry formation.
A specific finding of our study was the reversal of the trend for increased concentration of glycogen in between the RAA and LAA in the 8 week group when AF became sustained. This was associated with the marked increase in fibrosis in both the RAA and LAA. In clinical studies of successful catheter ablation in patients with chronic [25] or long-lasting persistent AF, “Termination of AF was achieved in both atria suggesting a biatrial substrate for chronic AF” [25]. Also, these patients had a larger right atrial dimension with evidence of a right atrial driving site in a number of these patients with long-standing AF.
The hypothesis we put forth for the intrinsic propensity for AF in the baseline state was also proposed by Ausma et al. [20] for the goats with the chronic and sustained form of AF induced by long term pacing: “…cellular structural remodeling, including gap junctional remodeling, might result in…heterogeneity of wave front propagation…thereby be responsible for conduction block and reentry that stabilizes AF. The increased stability (sustainability) of AF occurred in the same time domain as the structural remodeling…which is suggestive of a relationship.”
In conclusion, the differential in glycogen concentration, in conjunction with other factors, neural and electrophysiological, provide a basis for the greater propensity of the left atrium for paroxysmal AF at baseline and with 48 hour pacing induced AF. Whereas, the marked increase in collagen at 8 weeks of pacing induced AF provides a substrate for sustained AF without pacing. Evidence has been presented linking glycogen accumulation and fibrosis as factors in the long-standing persistent forms of AF.
Acknowledgements
Grants from the International Science & Technology Cooperation Program of China to Dr. Hou; The Xinjiang Key Laboratory for Medical Animal Model Research of China to Ling Zhang.
Disclosure of conflict of interest
None.
References
- 1.Embi AA, Scherlag BJ, Ritchey JW. Glycogen and the propensity for atrial fibrillation: intrinsic anatomic differences in glycogen in the left and right atria in the goat heart. North Amer J Med Sci. 2014;6:510–5. doi: 10.4103/1947-2714.143282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without involvement of an anatomical obstacle. Circ Res. 1977;41:9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- 3.Allessie MA, Wijffels MC, Kirchhof CJ. Experimental models of arrhythmias: toys or truth? Eur Heart J. 1994;15(Suppl A):2–8. doi: 10.1093/eurheartj/15.suppl_a.2. [DOI] [PubMed] [Google Scholar]
- 4.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 5.Allessie MA, Lammers WJEP, Bonke FIM, Hollen J. Experimental evaluation of Moe’1(1):s multiple wavelet hypothesis of atrial fibrillation. Cardiac Electrophysiology and Arrhythmias. 1985:265–276. [Google Scholar]
- 6.GK M. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn Ther. 1962;140:183–188. [Google Scholar]
- 7.Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J. 1964;67:200–220. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 8.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 9.Knaapen MW, Vrolijk BC, Wenink AC. Ultrastructural changes of the myocardium in the embryonic rat heart. Anat Rec. 1997;248:233–241. doi: 10.1002/(SICI)1097-0185(199706)248:2<233::AID-AR10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Jais P, Haissaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, Clémenty J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95:572–576. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 11.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 12.Hwang C, Wu TJ, Doshi RN, Peter CT, Chen PS. Vein of marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–1505. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 13.Tsai CF, Tai CT, Hsieh MH, Lin WS, Yu WC, Ueng KC, Ding YA, Chang MS, Chen SA. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102:67–74. doi: 10.1161/01.cir.102.1.67. [DOI] [PubMed] [Google Scholar]
- 14.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Malcolme-Lawes LC, Lim PB, Wright I, Kojodjojo P, Koa-Wing M, Jamil-Copley S, Dehbi HM, Francis DP, Davies DW, Peters NS, Kanagaratnam P. Characterization of the left atrial neural network and its impact on autonomic modification procedures. Circ-Arrhythmia Elec. 2013;6:632–640. doi: 10.1161/CIRCEP.113.000193. [DOI] [PubMed] [Google Scholar]
- 16.Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation. 2001;103:2631–2636. doi: 10.1161/01.cir.103.21.2631. [DOI] [PubMed] [Google Scholar]
- 17.Niwano S, Kojima J, Fukaya H, Sato D, Moriguchi M, Niwano H, Masaki Y, Izumi T. Arrhythmogenic difference between the left and right atria during rapid atrial activation in a canine model of atrial fibrillation. Circ J. 2007;71:1629–1635. doi: 10.1253/circj.71.1629. [DOI] [PubMed] [Google Scholar]
- 18.Mary-Rabine L, Albert A, Pham TD, Hordof A, Fenoglio JJ, Malm JR, Rosen MR. The relationship of human atrial cellular electrophysiology to clinical function and ultrastructure. Circ Res. 1983;52:188–199. doi: 10.1161/01.res.52.2.188. [DOI] [PubMed] [Google Scholar]
- 19.Dhillon PS, Gray R, Kojodjojo P, Jabr R, Chowdhury R, Fry CH, Peters NS. Relationship between gap-junctional conductance and conduction velocity in mammalian myocardium. Circ-Arrhythmia Elec. 2013;6:1208–1214. doi: 10.1161/CIRCEP.113.000848. [DOI] [PubMed] [Google Scholar]
- 20.Tai CT, Lo LW, Lin YJ, Chen SA. Arrhythmogenic difference between the left and right atria in a canine ventricular pacing-induced heart failure model of atrial fibrillation. Pace. 2012;35:188–195. doi: 10.1111/j.1540-8159.2011.03250.x. [DOI] [PubMed] [Google Scholar]
- 21.Ausma J, Litjens N, Lenders MH, Duimel H, Mast F, Wouters L, Ramaekers F, Allessie M, Borgers M. Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat. J Mol Cell Cardiol. 2001;33:2083–2094. doi: 10.1006/jmcc.2001.1472. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Pak HN, Lee S, Park HK, Seo JW, Chang BC. The clinical significance of the atrial subendocardial smooth muscle layer and cardiac myofibroblasts in human atrial tissue with valvular atrial fibrillation. Cardiovasc Pathol. 2013;22:58–64. doi: 10.1016/j.carpath.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res. 1988;62:811–832. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]
- 24.Rostock T, Steven D, Hoffmann B, Servatius H, Drewitz I, Sydow K, Müllerleile K, Ventura R, Wegscheider K, Meinertz T, Willems S. Chronic atrial fibrillation is a biatrial arrhythmia data from catheter ablation of chronic atrial fibrillation aiming arrhythmia termination using a sequential ablation approach. Circ-Arrhythmia Elec. 2008;1:344–353. doi: 10.1161/CIRCEP.108.772392. [DOI] [PubMed] [Google Scholar]
- 25.Hocini M, Nault I, Wright M, Veenhuyzen G, Narayan SM, Jaïs P, Lim KT, Knecht S, Matsuo S, Forclaz A, Miyazaki S, Jadidi A, O’Neill MD, Sacher F, Clémenty J, Haïssaguerre M. Disparate Evolution of right and left atrial rate during ablation of long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2010;55:1007–1016. doi: 10.1016/j.jacc.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]


