Abstract
Previous studies on the prognostic value of osteopontin (OPN) and β-catenin in colorectal carcinoma (CRC) revealed conflicting results. To date, only two immunohistochemical studies investigated their association in CRC with discrepant results. Moreover, the relevance of their co-expression to clinicopathological parameters was not previously reported. This study was designed to investigate the relationship between these markers and prognostic parameters in CRC and study further the relationship between them. Immunohistochemical expression of OPN and β-catenin was evaluated in 72 CRCs. Cytoplasmic OPN was detected in 45.83% of CRCs while normal mucosa was immunonegative. Strong continuous membranous β-catenin was present in normal mucosa. However, abnormal membranous, nuclear and cytoplasmic expressions were observed in 36.11%, 31.94% and 52.78% of CRCs, respectively. A highly significant relationship was detected between each of OPN and nuclear β-catenin expression and lymph node metastasis (P = 0.0001 and 0.004 respectively), depth of invasion (P = 0.001 and 0.004 respectively), TNM stages (P = 0.0001 and 0.001 respectively) and Dukes’ stages (P = 0.0001 and 0.004 respectively). A significant association was found between OPN and distant metastases. A strong agreement was observed between OPN and nuclear β-catenin (kappa = 0.656). A highly significant relationship was found between their co-expression and poor prognostic parameters. OPN overexpression and nuclear β-catenin expression appeared to be associated with unfavorable prognostic factors in CRC. A direct relationship was observed between them. Further understanding their role in colorectal carcinogenesis as well as targeting the interaction between them might be effective in the future development of therapeutic agents for CRC patients.
Keywords: Colorectal carcinoma, osteopontin, β-catenin, immunohistochemistry
Introduction
Colorectal cancer (CRC) is the third most common human malignancy and the second highest cause of cancer-related death worldwide [1]. Despite the tremendous progress in treatment, the overall mortality of CRC is still approximately 40% [2]. The prognosis of CRC remains poor, particularly for patients with metastasis [3]. Unfortunately, the molecular mechanisms underlying CRC metastasis are not completely understood [4].
Osteopontin (OPN) is a matrix glycoprotein secreted by a variety of cell types including osteoclasts, endothelial cells, epithelial cells, and activated immune cells such as macrophages and T cells [5]. It is also known as bone sialoprotein I, early T lymphocyte activation 1 and secreted phosphoprotein 1 [6]. Human OPN gene is located on chromosome 4q21-q25 and consists of seven exons encoding the OPN protein with 314 amino acid residues [7]. OPN has a role in cell adhesion, chemotaxis, prevention of apoptosis, invasion, migration and anchorage-independent growth of tumor cells. Extensive research has demonstrated the pivotal participation of OPN in the regulation of cell signaling which controls neoplastic and malignant transformation [8]. OPN is a new marker for colorectal tumorigenesis [9]; however, its association with prognostic parameters in CRC revealed conflicting results [3,9,10].
The molecular mechanisms that regulate the expression of OPN are incompletely understood. Previous studies indicated that up-regulation of OPN is dependent on aberrant activation of the Wnt pathway by mutation of the adenomatous polyposis coli (APC) tumor suppressor gene [9]. β-catenin is a central component of the Wnt signal pathway [11].
β-catenin is a 92-kDa multifunctional protein that, in its membrane location, links the intracellular part of the E-cadherin complex to actin cytoskeleton, which is a critical step in morphogenesis and maintenance of tissue integrity [12]. Alternatively, through Wnt signaling mediated stabilization, β-catenin may act as a down-stream transcriptional trans-activator of several target genes [13]. Alterations in β-catenin protein expression levels have been shown to contribute to the malignant character of various carcinomas and are likely to affect both intercellular adhesion and signal transduction, which are believed to be two independent functions of β-catenin protein [14].
Despite its crucial role in colorectal carcinogenesis, previous studies on altered β-catenin expression as a prognostic marker in CRC have been conflicting, with some studies reporting a favorable or no prognostic value while others reporting an association with poor clinical outcome [15-19].
To the best of our knowledge, only two immunohistochemical studies investigated the association between OPN and β-catenin in CRC with discrepant results [9,22]. Moreover, the relevance of their coexpression to clinicopathological parameters was not previously reported. This study was designed to evaluate osteopontin and β-catenin immunohistochemical expression in CRC relating the results with clinicopathological prognostic parameters and to study further the relationship between these two proteins.
Material and methods
Tissue and patient data
The current study was conducted on 72 cases of primary colorectal carcinoma (CRC). Cases were obtained from the Archives of the Pathology Lab. of Ain-Shams University Specialized Hospital. Such cases were diagnosed during the period from January 2010 to January 2012. All patients underwent radical resection of colorectal cancer with adequate surgical resection margins (~5 cm or more away from the tumors). In addition, normal colorectal tissues for each case were obtained from the surgical resection margins as control group.
The surgical and histopathology reports were reviewed to determine age and sex of patients, tumor size (greatest dimension), and site of colonic primary tumor as well as the presence of distant metastases. Hematoxylin and Eosin stained slides were examined to re-evaluate and verify the histopathologic diagnosis, histologic grade according to World Health Organization tumor differentiation [20] and lymph node metastasis. TNM staging according to the American Joint Committee on Cancer (AJCC) [21] and Dukes’ stage were performed. Normal colorectal tissues were also confirmed by histology.
Patients who had received chemotherapy and/or radiotherapy prior to surgery were excluded from the study as they could lead to an incorrect evaluation since these treatments could influence the interpretation of immunohistochemistry.
Ethics statement
All patients who participated in this study signed a written, informed consent before surgery. The study was approved by the Research Ethics Committee at Ain Shams Medical University.
Immunohistochemical staining
Four micrometer sections of formalin-fixed and paraffin-embedded samples of all CRC cases in addition to the normal colonic tissues were prepared. Immunohistochemical staining was performed using primary antibodies; Rabbit polyclonal antibody against human OPN (ready to use, clone: E247; Thermo Fisher Scientific Inc., Fremont, CA) and mouse monoclonal antibody against β-catenin (dilution 1:100, clone β-Catenin-1, DAKO). Avidin-Biotin immunoperoxidase complex technique was used according to Hsu et al. [23] by applying the super sensitive detection kit (Biogenex, CA, USA). The prepared tissue sections were fixed on poly-L- lysine coated slides overnight at 37°C. They were deparaffinized and rehydrated through graded alcohol series. Then the sections were heated in a microwave oven in 10 mM citrate buffer (pH 6.0) for 10 min. After the blocking of endogenous peroxidase and incubation in Protein Block Serum-Free Solution (Dako Cytomation) for 20 min, the sections were incubated overnight at 4°C with OPN antibody and 30 min at room temperature with β-catenin. Biotinylated antimouse immunoglobulin and streptavidin conjugated to horseradish peroxidase were then added. Finally, 3,3’-diaminobenzidine as the substrate or chromogen was used to form an insoluble brown product. Finally, the sections were counterstained with hematoxylin and mounted. Sections of gastric carcinoma and breast carcinoma were used as positive control for OPN and β-catenin respectively. Negative control sections were incubated with normal mouse or rabbit serum instead of the primary antibody.
Interpretation of immunohistochemical staining
Immunohistochemical analysis of osteopontin and β-catenin was blindly performed by two independent pathologists (the authors) without any prior knowledge of the clinicopathological data. Analysis was performed using computerized Image Analyzing Software (Special SIS starter. version 3.2, Olympus, Germany) connected to an Olympus microscope (model BX51, Olympus Japan).
The cytoplasmic expression of OPN was evaluated according to the percentage of positive cells in 10 randomly selected areas at a magnification of 400 ×. No staining or staining in fewer than 10% of cells was considered negative, and staining of 10% or more of cells was considered positive [24].
Concerning β-catenin expression, all membranous, cytoplasmic and nuclear staining were evaluated independently. No membranous staining or positive membranous staining in less than 10% of cells was considered abnormal membranous expression. Strong nuclear staining of more than 10% of cells was considered positive/abnormal nuclear expression. Cytoplasmic staining of more than 10% of cells was considered positive/abnormal cytoplasmic expression [25]. The percentage of positively stained cells was estimated in 10 randomly selected areas at a magnification of 400 ×.
Statistical analysis
Continuous variables are expressed as mean and Standard Deviation. Categorical variables are expressed as frequencies and percents. Student t test was used to assess the statistical significance of the difference between two study group mean. Chi square and Fisher’s exact test was used to examine the relationship between categorical variables. Kappa statistics was used to compute the measure of agreement between two investigational methods Kappa’s value < 0.20 was defined as poor agreement; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, large; and 0.81 to 1.00, very good or almost perfect. A significance level of P < 0.05 was used in all tests. Differences were considered highly significant when P ≤ 0.01. All statistical procedures were carried out using SPSS version 15 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Clinical and pathological data for the studied colorectal carcinoma cases are represented in Table 1. The mean age of the patients was 61.7 years (Standard deviation, 14.4; range, 25-80 years). The mean size of the tumor was 6.2 cm (Standard deviation, 2.5; range, 3-13 cm).
Table 1.
Relationship between osteopontin expression and clinicopathological parameters of the studied CRC cases (n = 72)
| Variable | n (%) | Osteopontin expression | ||
|---|---|---|---|---|
|
| ||||
| Positive | Negative | P-value | ||
|
| ||||
| n (%) | n (%) | |||
|
| ||||
| 33 (45.83) | 39 (54.17) | |||
| Gender | ||||
| Male | 35 (48.6) | 16 (45.71) | 19 (54.29) | 0.984 |
| Female | 37 (51.4) | 17 (45.95) | 20 (54.05) | NS |
| Site | ||||
| Right & transverse colon | 50 (69.44) | 22 (44.00) | 28 (56.00) | 0.638 |
| Left colon & rectum | 22 (30.56) | 11 (50.00) | 11 (50.00) | NS |
| Degree of differentiation | ||||
| Well | 10 (13.89) | 5 (50.00) | 5 (50.00) | 0.798 |
| Moderate | 38 (52.78) | 16 (42.11) | 22 (57.89) | NS |
| Poor | 24 (33.33) | 12 (50.00) | 12 (50.00) | |
| Lymph nodal status | ||||
| Positive | 40 (55.56) | 31 (77.50) | 9 (22.50) | 0.0001 |
| Negative | 32 (44.44) | 2 (6.25) | 30 (93.75) | HS |
| Distant metastases | ||||
| Present | 14 (19.44) | 11 (78.57) | 3 (21.43) | 0.006 |
| Absent | 58 (80.56) | 22 (37.93) | 36 (62.07) | HS |
| Depth of invasion | ||||
| T2 | 15 (20.83) | 2 (13.33) | 13 (86.67) | 0.001 |
| T3 | 45 (62.50) | 21 (46.67) | 24 (53.33) | HS |
| T4 | 12 (16.67) | 10 (83.33) | 2 (16.67) | |
| TNM Stage | ||||
| I | 13 (18.06) | 0 (0.00) | 13 (100.00) | 0.0001 |
| II | 19 (26.39) | 2 (10.53) | 17 (89.47) | HS |
| III | 26 (36.11) | 20 (76.92) | 6 (23.08) | |
| IV | 14 (19.44) | 11 (78.57) | 3 (21.43) | |
| Dukes’ stage | ||||
| A | 13 (18.06) | 0 (0.00) | 13 (100.00) | 0.0001 |
| B | 19 (26.39) | 2 (10.53) | 17 (89.47) | HS |
| C | 40 (55.55) | 31 (77.50) | 9 (22.50) | |
Chi square test.
Expression of OPN and its relationship with clinicopathological parameters
In normal colorectal gland epithelia, OPN only displayed weak staining in < 10% of the cells, therefore all the cases were determined as negative (Figure 1A). However, 33 (45.83%) out of 72 CRC cases revealed positive cytoplasmic OPN expression (Figure 1B-D). OPN was expressed in macrophages, in normal and carcinoma tissue, which were thus used as inner positive control.
Figure 1.
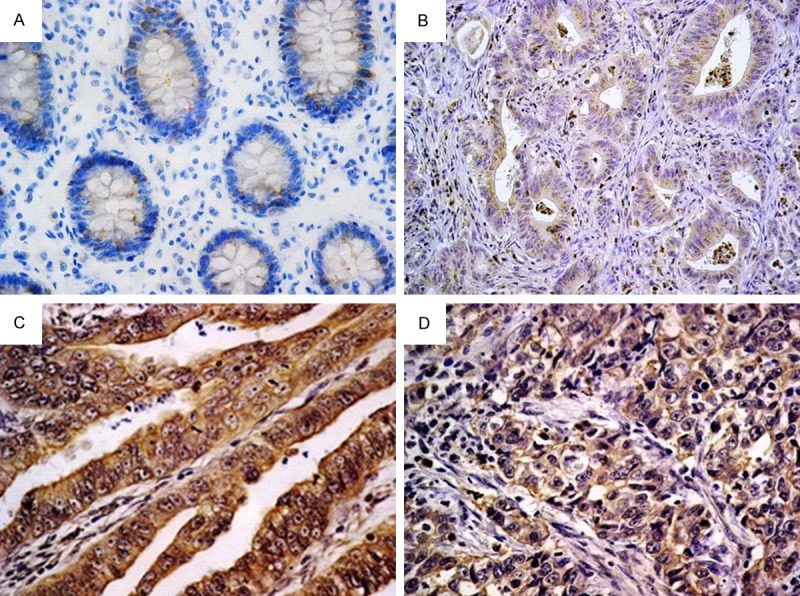
OPN expression in normal colonic epithelium and colorectal carcinoma: (A) Weak cytoplasmic expression in a small number of normal gland epithelial cells (negative expression). (B) Well differentiated adenocarcinoma showing weak cytoplasmic OPN expression. Surrounding macrophages exhibited strong positivity. (C) Moderately differentiated adenocarcinoma showing intense cytoplasmic OPN expression. (D) Poorly differentiated adenocarcinoma showing moderate cytoplasmic OPN expression [Immunohistochemistry, original magnification, (A-D) × 400].
A highly significant relationship was detected between OPN expression and the presence of lymph node metastasis (P = 0.0001), presence of distant metastases (P = 0.006), increased depth of invasion (P = 0.001), advanced TNM stages (P = 0.0001) and advanced Dukes’ stages (P = 0.0001). However, OPN expression was not significantly associated with gender (P = 0.984), age (P = 0.940; student t test), tumor size (P = 0.401; student t test) and site (P = 0.638), and histological differentiation (P = 0.798). The relationship between OPN expression and clinicopathological parameters is summarized in Table 1.
Expression of β-catenin and its relationship with clinicopathological parameters
In normal colonic epithelium, strong continuous β-catenin expression was present in the cell membrane with no cytoplasmic or nuclear staining (Figure 2A). In colorectal carcinoma, decreased expression and changes of localization of β-catenin were observed. Abnormal membranous expression was observed in 36.11% (26/72) of patients. In comparison to the normal epithelium, aberrant expression of nuclear and cytoplasmic β-catenin was detected in 31.94% (23/72) and 52.78% (38/72) of patients, respectively (Figure 2B-D). Combined cytoplasmic and nuclear β-catenin expression was observed in 16 out of 72 CRCs (22.22%).
Figure 2.
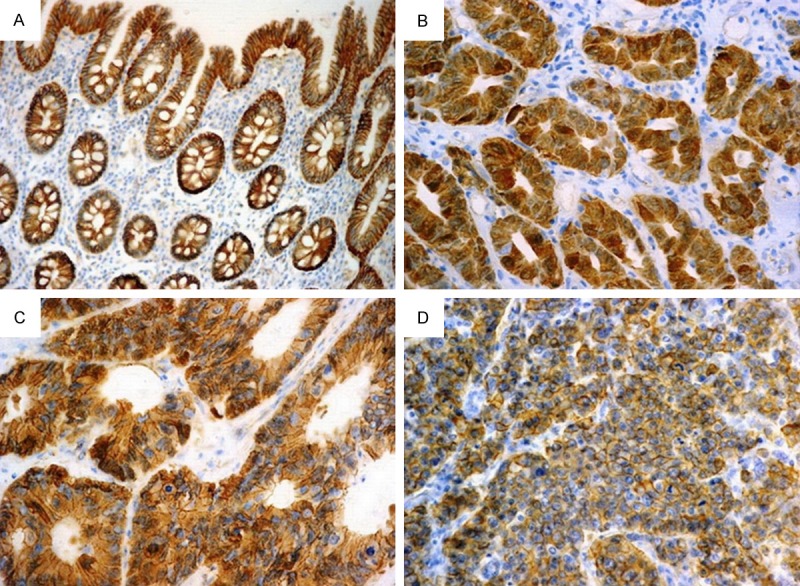
Different staining patterns for β-catenin in normal colonic epithelium and colorectal carcinoma: (A) Normal colonic epithelium showing strong continuous membranous β-catenin expression. (B) Well differentiated adenocarcinoma showing strong nuclear and cytoplasmic β-catenin expression. (C) Moderately differentiated adenocarcinoma showing strong nuclear and moderate cytoplasmic and membranous β-catenin expression. (D) Poorly differentiated adenocarcinoma showing strong membranous and moderate cytoplasmic β-catenin expression [Immunohistochemistry, original magnification, (A) × 200; (B-D) × 400].
There was a highly significant association between increased nuclear β-catenin expression and presence of lymph node metastases (P = 0.004), increased depth of invasion (P = 0.004), advanced stage according to TNM classification (P = 0.001), and advanced Dukes’ stage (P = 0.004). However, no significant relationship was detected between nuclear β-catenin expression and gender (P = 0.927), age (P = 0.386; student t test), tumor size (P = 0.386; student t test), and site (P = 0.988), degree of tumour differentiation (P = 0.362) and distant metastases (P =1.000) (Table 2).
Table 2.
Relationship between patterns of β-catenin expression and clinicopathological parameters of the studied CRC cases (n = 72)
| Variable | n | β-catenin expression | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Nuclear | Cytoplasmic | ||||||
|
| |||||||
| Positive | Negative | P-value | Positive | Negative | P-value | ||
|
|
|
||||||
| n (%) | n (%) | n (%) | n (%) | ||||
|
|
|
||||||
| 23 (31.94) | 49 (68.06) | 38 (52.78) | 34 (47.22) | ||||
| Gender | |||||||
| Male | 35 | 11 (31.43) | 24 (68.57) | 0.927* | 20 (57.14) | 15 (42.86) | 0.471* |
| Female | 37 | 12 (32.43) | 25 (67.57) | NS | 18 (48.65) | 19 (51.35) | NS |
| Site | |||||||
| Right & transverse colon | 50 | 16 (32.00) | 34 (68.00) | 0.988* | 28 (56.00) | 22 (44.00) | 0.409* |
| Left colon & rectum | 22 | 7 (31.82) | 15 (68.18) | NS | 10 (45.45) | 12 (54. 55) | NS |
| Degree of differentiation | |||||||
| Well | 10 | 5 (50.00) | 5 (50.00) | 0.362* | 5 (50.00) | 5 (50.00) | 0.975* |
| Moderate | 38 | 12 (31.58) | 26 (68.42) | NS | 20 (52.63) | 18 (47.37) | NS |
| Poor | 24 | 6 (25.00) | 18 (75.00) | 13 (54.17) | 11 (45.83) | ||
| Lymph nodal status | |||||||
| Positive | 40 | 20 (50.00) | 20 (50.00) | 0.004* | 25 (62.50) | 15 (37.50) | 0.065* |
| Negative | 32 | 3 (9.37) | 29 (90.63) | HS | 13 (40.63) | 19 (59.37) | NS |
| Distant metastases | |||||||
| Present | 14 | 4 (28.57) | 10 (71.43) | 1.00** | 8 (57.14) | 6 (42.86) | 0.715* |
| Absent | 58 | 19 (32.76) | 39 (67.24) | NS | 30 (51.72) | 28 (48.28) | NS |
| Depth of invasion | |||||||
| T2 | 15 | 0 (0.00) | 15 (100.00) | 0.004** | 8 (53.33) | 7 (46.67) | 0.548* |
| T3 | 45 | 19 (42.22) | 26 (57.78) | HS | 22 (48.89) | 23(51.11) | NS |
| T4 | 12 | 4 (33.33) | 8 (66.67) | 8 (66.67) | 4 (33.33) | ||
| TNM Stage | |||||||
| I | 13 | 0 (0.00) | 13(100.00) | 0.001** | 7 (53.85) | 6 (46.15) | 0.158* |
| II | 19 | 3 (15.79) | 16 (84.21) | HS | 6 (31.58) | 13 (68.42) | NS |
| III | 26 | 16 | 10 | 17 (65.38) | 9 (34.62) | ||
| IV | 14 | 4 (28.57) | 10 (71.43) | 8 (57.14) | 6 (42.86) | ||
| Dukes’ stage | |||||||
| A | 13 | 0 (0.00) | 13 (100.00) | 0.004* | 7 (53.85) | 6 (46.15) | 0.084* |
| B | 19 | 3 (15.79) | 16 (84.21) | HS | 6 (31.58) | 13 (68.42) | NS |
| C | 40 | 20 (50.00) | 20 (50.00) | 25 (62.50) | 15 (37.50) | ||
Chi square test;
Fisher exact test.
No significant relationship was observed between cytoplasmic β-catenin expression and different clinicopathological parameters (Table 2).
Agreement between OPN and β-catenin immunostaining in colorectal carcinoma
Positive OPN expression was detected in 69.53% of the positive cytoplasmic β-catenin cases while in only 29.41% of the negative cases. Moreover, positive OPN expression was observed in 95.65% of the positive nuclear β-catenin cases while in only 22.45% of the negative cases. All the cases that showed combined cytoplasmic and nuclear β-catenin (16 cases) were positive for OPN expression. There was a fair agreement between OPN and cytoplasmic β-catenin (kappa = 0.309), a strong agreement between OPN and nuclear β-catenin (kappa = 0.656), and a moderate agreement (kappa = 0.505) between OPN and combined cytoplasmic and nuclear β-catenin (Figure 3; Table 3).
Figure 3.
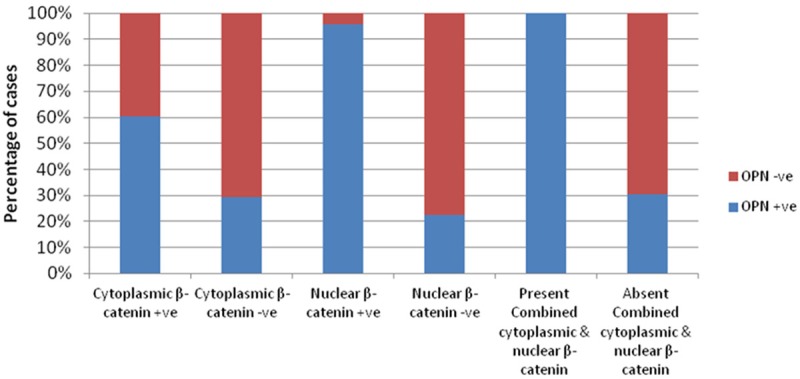
Agreement between OPN expression and each of cytoplasmic β-catenin, nuclear β-catenin and combined cytoplasmic and nuclear β-catenin expression in CRC cases.
Table 3.
Agreement between OPN expression and each of cytoplasmic β-catenin, nuclear β-catenin and combined cytoplasmic and nuclear β-catenin expression in CRC cases (n = 72)
| OPN | Kappa | P | Sig | ||
|---|---|---|---|---|---|
|
|
|||||
| Positive | Negative | ||||
|
| |||||
| n (%) | n (%) | ||||
|
| |||||
| 33 (45.83) | 39 (54.17) | ||||
| Cytoplasmic β-catenin | |||||
| Positive (n = 38) | 23 (60.53) | 15 (39.47) | 0.309 | 0.01 | HS |
| Negative (n = 34) | 10 (29.41) | 24 (70.59) | |||
| Nuclear β-catenin | |||||
| Positive (n = 23) | 22 (95.65) | 1 (4.35) | 0.656 | 0.0001 | HS |
| Negative (n = 49) | 11 (22.45) | 38 (77.55) | |||
| Combined cytoplasmic and nuclear β-catenin | |||||
| Present (n =16) | 16 (100.00) | 0 (0.00) | 0.505 | 0.0001 | HS |
| Absent (n = 56) | 17 (30.36) | 39 (69.64) | |||
Co-expression of OPN and nuclear β-catenin and its relationship with clinicopathological parameters
Co-expression of OPN and nuclear β-catenin was detected in 22 of the 72 CRCs (30.56%). A highly significant relationship was observed between co-expression of OPN and nuclear β-catenin and presence of lymph node metastases (P = 0.0001), increased depth of tumor invasion (P = 0.005), advanced TNM stage (P = 0.0001) and Dukes’ stage (P = 0.0001) (data are not tabulated) (Figure 4).
Figure 4.
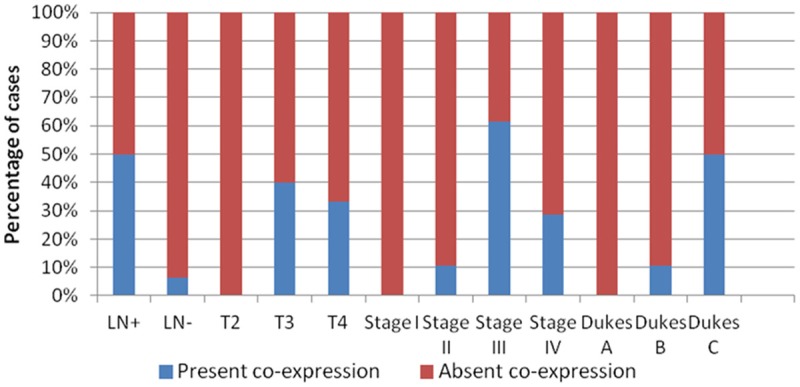
Relationship between OPN & nuclear β-catenin co-expression and prognostic parameters.
Discussion
Colorectal cancer is one of the most frequent malignancies in developed countries, and one of the leading causes of worldwide cancer-related morbidity and mortality [26]. Approximately 50% of CRC patients have metastases in the liver, but only 10%-20% of these patients can undergo surgical resection. The 5-year survival rate following surgical resection of metastatic tumor is only 30%-40% [27,28]. The molecular mechanisms underlying CRC metastasis are not completely understood [4].
OPN is a secretory calcium-binding phosphorylated glycoprotein that plays an important role in bone metabolism. At the molecular level, OPN plays important roles in cellular adhesion and migration, tissue repair, and signal transduction as well as in the invasion and metastasis of several cancers [29]. OPN is significantly associated with survival rate in several cancers and has value as a marker of clinical tumor progression [30]. As far as CRC is concerned, OPN was identified as a leading marker among the screening of 12,000 genes, and has been correlated with tumorigenesis, invasion and metastasis [9,30].
In the present study, OPN expression was detected in 45.83% of the studied CRC cases, while normal colonic epithelium was immunonegative. These results are consistent with previous studies showing up-regulation of OPN in certain neoplastic epithelia [31,32], while normal epithelia exhibited low levels or even negative expression. Previously, OPN expression was observed in 49.4% of CRC cases while normal colorectal gland epithelia revealed negative expression [3]. Moreover, Rhode et al. [9] found slightly higher rates of OPN expression (56%) in CRC cases. Consequently, these findings support previous studies implicating OPN in malignant transformation of colonic epithelium.
Previous studies on the prognostic value of OPN in CRC revealed conflicting results. Boudjadi et al. [10] did not find any correlation between OPN immunostaining index and clinicopathological parameters. However, in agreement with Li et al. [3], our study revealed a highly significant relationship between OPN expression and poor prognostic parameters as lymph node metastases and advanced Dukes’ stage that relates OPN to tumor progression and metastasis. Moreover, a significant association between OPN expression and depth of tumor invasion as well as advanced TNM stages was observed in the current work. However, Li et al. [3] didn’t find any relationship between them.
Concomitant with a previous study [9], our research indicated a positive relationship between OPN expression in CRC cases and distant metastasis. Rhode et al. [9] additionally reported that OPN expression was maintained in their studied liver metastasis that showed even higher expression than the primary colonic tumors. Invasion and metastasis are important characteristics of malignant tumors. The whole process of metastasis includes angiogenesis, adhesion, degradation, movement, and reattachment [33]. Adhesion factors are essentially involved in the process. OPN is probably one of numerous metastasis- related prognostic factors of CRC. It decreases the homotypic adhesion among CRC cells and enhances the heterotypic adhesion ability between CRC cells and endothelium cells [34].
Parallel to Li et al. [3], the present study revealed no significant association between OPN expression and gender or histological differentiation.
The mechanisms involved in the up-regulation of OPN in cancers remain unclear, and it is presumed that activation of OPN is controlled by complex regulation pathways due to diverse regulatory sequences in the promoter regions [3]. Previous studies indicated that up-regulation of OPN is dependent on aberrant activation of the Wnt pathway by mutation of the APC tumor suppressor gene. The main tumor suppressing function of APC resides in its capacity to regulate β-catenin, a central component of the Wnt signal pathway [11]. As a consequence of tumorigenic mutations in APC, β-catenin becomes stabilized and shuttles into the nucleus, where it binds to proteins of the T-cell factor family and serves as an essential co-activator of transcription contributing to tumor progression [35]. The level of β-catenin in the nucleus is an indicator of an active Wnt signaling pathway [36]. The most strongly up-regulated presumed Wnt-targets was OPN gene (16-fold up-regulated) [9].
To the best of our knowledge, there are limited data in the literature [9,22] concerning the relationship between OPN and β-catenin in CRC. In agreement with Rhode et al. [9], the present study indicated that increased OPN expression was significantly associated with elevated nuclear and cytoplasmic β-catenin staining. In contrast, Mole et al. [22] reported no correlation between these two proteins. In the current research, 30% of positive OPN cases revealed negative cytoplasmic β-catenin expression and 33% showed negative nuclear β-catenin expression. This could be due to additional genetic defects not related to the Wnt/β-catenin pathway.
β-catenin was originally identified as a membrane component of the cadherin-mediated cell-cell adhesion system and it is now widely recognized as a critical element of the Wnt signal pathway [37]. In the absence of Wnt ligands, β-catenin is located at the plasma membrane in adherent complexes, while its level in the cytoplasm is very low. Free cytosolic β-catenin is phosphorylated in a complex formed by APC, Axin, glycogen synthase kinase 3β (GSK3β) and casein kinase I (CKI). Phosphorylated β-catenin is ubiquitinated by ubiquitin ligase protein (βTrCP) and then degraded in the proteasome. Binding one of the Wnt ligands to the receptors FzD/LRP5/6 triggers signal inactivating the destruction complex [38]. Consequently, β-catenin accumulates in the cell and undergoes translocation to the nucleus where it activates specific Wnt target genes in conjunction with the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors [39]. Nuclear β-catenin is significantly associated with the invasion and metastasis of human cancers, such as carcinomas of esophagus, stomach, colon and melanomas [40,41].
In the current research, as compared with the sub-cellular distribution of β-catenin in normal colonic mucosa, neoplastic cells demonstrated a distinct shift from a membranous localization to a more widespread distribution (membranous, cytoplasmic, and nuclear) in cancer lesions. This is in line with previous reports describing β-catenin expression in cancer cells with this type of altered pattern [42,43]. In agreement with Stanczak et al. [38], our study revealed abnormal membranous β-catenin expression in 36.11% of CRCs, aberrant nuclear expression in 31.94% and aberrant cytoplasmic expression in 52.78% of cases. Dilek et al. [44] detected lower levels of nuclear β-catenin expression; 26% of rectosigmoid cancer cases. However, Maruyama et al. [45] demonstrated higher levels of expression; 66% nuclear and 68% cytoplasmic accumulation in CRCs. A previous tissue microarray-based study has shown in a large series of CRC that the majority of cancers retained some degree of β-catenin membranous staining, whereas nuclear or cytoplasmic expression was seen in 50.15% and 81.53% of specimens, respectively [19].
There are multiple explanations for these different results. Such potential confounding factors might include type of antibody, site of tumor, differences in antigen retrieval and immunohistochemical staining procedures as well as lack of standardization in the evaluation of positive and negative results.
Previous studies demonstrated that nuclear β-catenin expression in CRC was related to poor prognosis [19,46], no effect [43] or even favorable prognosis [15]. In the current research, a statistical significant association was found between nuclear β-catenin expression and poor prognostic parameters as lymph node metastases, depth of tumour invasion, advanced stage according to TNM classification as well as advanced Dukes’ stage.
In line with the present series, previous studies found a significant relationship between nuclear β-catenin expression and advanced tumor stage [39,47,48]. Moreover, Peker et al. [47] and Wong et al. [49] revealed significant association between nuclear β-catenin expression and lymph node metastasis. Thus, β-catenin nuclear translocation can potentially be a valuable tool for the prognosis of colorectal cancer. Our data were supported by a previous study that showed a significant relationship between nuclear β-catenin and higher mortality rates in CRC patients [18]. In contrast, Chen et al. [39] observed no significant correlation between nuclear β-catenin expression and lymph node status or depth of tumor invasion.
In the current research, no significant relationship was revealed between nuclear β-catenin expression and tumor size and site, degree of tumor differentiation or distant metastases. Concomitant with our results, previous studies found no significant association between nuclear β-catenin expression and tumor size [38], tumor localization and grade [39] or distant metastases [50]. In contrast, Peker et al. [47] revealed a significant relationship between nuclear β-catenin expression and tumor size and high histologic grade. Moreover, Stanczak et al. [38] found that cancer localized in rectum displayed greater nuclear localization of β-catenin than tumors localized in sigmoid and colon. Whereas, Lee et al. [51] stated that nuclear β-catenin expression was higher in left CRCs than right CRCs.
Many factors could explain the discrepancies regarding the prognostic value of nuclear β-catenin expression, for example: sample size, patient population, stage of disease, site of tumor, different CRC subtypes and the existence of more than one type of CRC in a study.
There were contradictory results concerning the prognostic value of cytoplasmic β-catenin in CRC. Some researchers suggest that the accumulation of cytoplasmic β-catenin serves as a predictor of metastasis [45]. In contrast, Chen et al. [39] found no significant association between cytoplasmic β-catenin expression and adverse prognostic parameters as advanced Dukes’ stages and lymph node metastases. On the other hand, Lugli et al. [19] detected a significant relationship between increased cytoplasmic β-catenin expression and lower tumor grade. Our study revealed no significant association between cytoplasmic β-catenin and various prognostic parameters in CRC.
Up to our knowledge, this is the first study that reports a highly significant relationship between OPN and nuclear β-catenin co-expression and poor prognostic parameters.
In conclusion, OPN overexpression is strongly related to more aggressive behavior of CRC. It may be useful for predicting the risk of hematogenous metastasis in CRC patients. A direct relationship was observed between OPN and β-catenin expression supporting previous studies that reported possible regulation of OPN by aberrant activation of the Wnt pathway. Our study demonstrated disturbances in expression and localization of β-catenin in CRC. β-catenin expression in the nucleus, rather than in the cytoplasm, appeared to be associated with unfavorable prognostic factors. These findings support the hypothesis that Wnt/β-catenin pathway plays an important role in advanced colorectal carcinoma. OPN and β-catenin, when considered together, may be a novel prognostic indicator of CRC. Further understanding of the implications and roles of OPN and β-catenin in colorectal carcinogenesis as well as targeting the interaction between them might be effective in the future development of therapeutic agents for CRC patients. A limitation of our study is that patients were enrolled retrospectively. Large, well-designed prospective studies are required to investigate the precise prognostic significance of OPN and β-catenin in CRC.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Yang GZ, Zhu ZM, Zhou ZY, Li L. Osteopontin is overexpressed in colorectal carcinoma and is correlated with P53 by immunohistochemistry. Exp Ther Med. 2012;3:621–624. doi: 10.3892/etm.2012.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeatman TJ, Chambers AF. Osteopontin and colon cancer progression. Clin Exp Metastasis. 2003;20:85–90. doi: 10.1023/a:1022502805474. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J, Huo DH, Kuang DM, Yang J, Zheng L, Zhuang SM. Human macrophages promote the motility and invasiveness of osteopontin-knockdown tumor cells. Cancer Res. 2007;67:5141–5147. doi: 10.1158/0008-5472.CAN-06-4763. [DOI] [PubMed] [Google Scholar]
- 6.Senger DR, Perruzzi CA, Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989;9:1291–1299. [PubMed] [Google Scholar]
- 7.Young MF, Kerr JM, Termine JD, Wewer UM, Wang MG, McBride OW, Fisher LW. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN) Genomics. 1990;7:491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]
- 8.Johnston NI, Gunasekharan VK, Ravindranath A, O’Connell C, Johnston PG, El-Tanani MK. Osteopontin as a target for cancer therapy. Front Biosci. 2008;13:4361–72. doi: 10.2741/3009. [DOI] [PubMed] [Google Scholar]
- 9.Rohde F, Rimkus C, Friederichs J, Rosenberg R, Marthen C, Doll D, Holzmann B, Siewert JR, Janssen KP. Expression of osteopontin, a target gene of de-regulated Wnt signaling, predicts survival in colon cancer. Int J Cancer. 2007;121:1717–23. doi: 10.1002/ijc.22868. [DOI] [PubMed] [Google Scholar]
- 10.Boudjadi S, Bernatchez G, Beaulieu JF, Carrier JC. Control of the human osteopontin promoter by ERRα in colorectal cancer. Am J Pathol. 2013;183:266–76. doi: 10.1016/j.ajpath.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- 12.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 13.Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- 14.Fujimori M, Ikeda S, Shimizu Y, Okajima M, Asahara T. Accumulation of beta-catenin protein and mutations in exon 3 of beta-catenin gene in gastrointestinal carcinoid tumor. Cancer Res. 2001;61:6656–6659. [PubMed] [Google Scholar]
- 15.Wangefjord S, Brändstedt J, Lindquist KE, Nodin B, Jirström K, Eberhard J. Associations of beta-catenin alterations and MSI screening status with expression of key cell cycle regulating proteins and survival from colorectal cancer. Diagn Pathol. 2013;8:10. doi: 10.1186/1746-1596-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung GG, Provost E, Kielhorn EP, Charette LA, Smith BL, Rimm DL. Tissue microarray analysis of beta-catenin in colorectal cancer shows nuclear phospho-beta-catenin is associated with a better prognosis. Clin Cancer Res. 2001;7:4013–4020. [PubMed] [Google Scholar]
- 17.Norwood MG, Bailey N, Nanji M, Gillies RS, Nicholson A, Ubhi S, Darnton JJ, Steyn RS, Womack C, Hughes A, Hemingway D, Harrison R, Waters R, Jankowski JA. Cytoplasmic beta-catenin accumulation is a good prognostic marker in upper and lower gastrointestinal adenocarcinomas. Histopathology. 2010;57:101–1. doi: 10.1111/j.1365-2559.2010.03587.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheah PY, Choo PH, Yao J, Eu KW, Seow-Choen F. A survival-stratification model of human colorectal carcinomas with beta-catenin and p27kip1. Cancer. 2002;95:2479–2486. doi: 10.1002/cncr.10986. [DOI] [PubMed] [Google Scholar]
- 19.Lugli A, Zlobec I, Minoo P, Baker K, Tornillo L, Terracciano L, Jass JR. Prognostic significance of the wnt signalling pathway molecules APC, beta-catenin and E-cadherin in colorectal cancer: a tissue microarray based analysis. Histopathology. 2007;50:453–464. doi: 10.1111/j.1365-2559.2007.02620.x. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton SR, Aaltonen LA, editors. Tumors of digestive system organs. Lyon: IARC Press; 2000. WHO classification of tumors. Pathology and genetics. [Google Scholar]
- 21.Greene FL, Page DL, Fleming ID, editors. AJCC: Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 6th edition. New York: Springer Verlag; 2002. pp. 127–9. [Google Scholar]
- 22.Mole DJ, O’Neill C, Hamilton P, Olabi B, Robinson V, Williams L, Diamond T, El-Tanani M, Campbell FC. Expression of osteopontin coregulators in primary colorectal cancer and associated liver metastases. Br J Cancer. 2011;104:1007–12. doi: 10.1038/bjc.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 24.Zhao XQ, Dong JH, Zhang WZ, Liu Z. Prognosis of ampullary cancer based on immunohistochemical type and expression of osteopontin. Diagn Pathol. 2011;6:98. doi: 10.1186/1746-1596-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Chen F, Shi W, Qi L, Zhao Z, Zhang J. Prognostic impact of TAZ and β-catenin expression in adenocarcinoma of the esophagogastric junction. Diagn Pathol. 2014;9:125. doi: 10.1186/1746-1596-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 27.Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38:1023–1033. doi: 10.1016/s0959-8049(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 28.Siegel RL, Jemal A, Thun MJ, Hao Y, Ward EM. Trends in the incidence of colorectal cancer in relation to county-level poverty among blacks and whites. J Natl Med Assoc. 2008;100:1441–1444. doi: 10.1016/s0027-9684(15)31544-3. [DOI] [PubMed] [Google Scholar]
- 29.Goparaju CM, Pass HI, Blasberg JD, Hirsch N, Donington JS. Functional heterogeneity of osteopontin isoforms in non-small cell lung cancer. J Thorac Oncol. 2010;5:1516–1523. doi: 10.1097/JTO.0b013e3181eba6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, Yeatman TJ. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10:184–190. doi: 10.1158/1078-0432.ccr-1405-2. [DOI] [PubMed] [Google Scholar]
- 31.Senger DR, Perruzzi CA. Secreted phosphoprotein markers for neoplastic transformation of human epithelial and fibroblastic cells. Cancer Res. 1985;45:5818–23. [PubMed] [Google Scholar]
- 32.Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst. 2002;94:513–21. doi: 10.1093/jnci/94.7.513. [DOI] [PubMed] [Google Scholar]
- 33.Liotta LA. Cancer cell invasion and metastasis. Sci Am. 1992;266:54–59. 62–63. doi: 10.1038/scientificamerican0292-54. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Pan C, Hu H, Zheng S, Ding L. Osteopontin-enhanced hepatic metastasis of colorectal cancer cells. PLoS One. 2012;7:e47901. doi: 10.1371/journal.pone.0047901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 36.de Sousa E Melo F, Colak S, Buikhuisen J, Koster J, Cameron K, de Jong JH, Tuynman JB, Prasetyanti PR, Fessler E, van den Bergh SP, Rodermond H, Dekker E, van der Loos CM, Pals ST, van de Vijver MJ, Versteeg R, Richel DJ, Vermeulen L, Medema JP. Methylation of cancerstem-cell-associated Wnt target genes predicts poor prognosis in colorectal cancer patients. Cell Stem Cell. 2011;9:476–485. doi: 10.1016/j.stem.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 37.McCrea PD, Gumbiner BM. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell-cell adhesion molecule E-cadherin (uvomorulin). Characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem. 1991;266:4514–4520. [PubMed] [Google Scholar]
- 38.Stanczak A, Stec R, Bodnar L, Olszewski W, Cichowicz M, Kozlowski W, Szczylik C, Pietrucha T, Wieczorek M, Lamparska-Przybysz M. Prognostic significance of Wnt-1, β-catenin and E-cadherin expression in advanced colorectal carcinoma. Pathol Oncol Res. 2011;17:955–63. doi: 10.1007/s12253-011-9409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, He X, Jia M, Liu Y, Qu D, Wu D, Wu P, Ni C, Zhang Z, Ye J, Xu J, Huang J. β-catenin overexpression in the nucleus predicts progress disease and unfavorable survival in colorectal cancer: a meta-analysis. PLoS One. 2013;8:e63854. doi: 10.1371/journal.pone.0063854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osterheld MC, Bian YS, Bosman FT, Benhattar J, Fontolliet C. Beta-catenin expression and its association with prognostic factors in adenocarcinoma developed in Barrett esophagus. Am J Clin Pathol. 2002;117:451–456. doi: 10.1309/1db6-gfvh-ra6w-q07y. [DOI] [PubMed] [Google Scholar]
- 41.Kim HS, Hong EK, Park SY, Kim WH, Lee HS. Expression of beta-catenin and E-cadherin in the adenoma-carcinoma sequence of the stomach. Anticancer Res. 2003;23:2863–2868. [PubMed] [Google Scholar]
- 42.Mikami T, Mitomi H, Hara A, Yanagisawa N, Yoshida T, Tsuruta O, Okayasu I. Decreased expression of CD44, alpha catenin, and deleted colon carcinoma and altered expression of betacatenin in ulcerative colitis-associated dysplasia and carcinoma, as compared with sporadic colon neoplasms. Cancer. 2000;89:733–740. doi: 10.1002/1097-0142(20000815)89:4<733::aid-cncr3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Horkko TT, Klintrup K, Makinen JM, Napankangas JB, Tuominen HJ, Makela J, Karttunen TJ, Makinen MJ. Budding invasive margin and prognosis in colorectal cancer -- no direct association with beta-catenin expression. Eur J Cancer. 2006;42:964–971. doi: 10.1016/j.ejca.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Dılek FH, Topak N, Tokyol Ç, Akbulut G, Dılek ON. β-Catenin and its relation to VEGF and cyclin D1 expression in pT3 rectosigmoid cancers. Turk J Gastroenterol. 2010;21:365–71. doi: 10.4318/tjg.2010.0122. [DOI] [PubMed] [Google Scholar]
- 45.Maruyama K, Ochiai A, Akimoto S, Nakamura S, Baba S, Moriya Y, Hirohashi S. Cytoplasmic beta-catenin accumulation as a predictor of hematogenous metastasis in human colorectal cancer. Oncology. 2000;59:302–9. doi: 10.1159/000012187. [DOI] [PubMed] [Google Scholar]
- 46.Baldus SE, Mönig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Hölscher AH, Dienes HP. MUC1 and nuclear ß-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790–2796. doi: 10.1158/1078-0432.ccr-03-0163. [DOI] [PubMed] [Google Scholar]
- 47.Peker K, Başoğlu M, Gürsan N. Relationship between β-catenin expression and prognostic parameters of colorectal carcinomas. Turk Patoloji Derg. 2013;29:87–93. doi: 10.5146/tjpath.2013.01157. [DOI] [PubMed] [Google Scholar]
- 48.Cheng H, Liang H, Qin Y, Liu Y. Nuclear beta-catenin overexpression in metastatic sentinel lymph node is associated with synchronous liver metastasis in colorectal cancer. Diagn Pathol. 2011;6:109. doi: 10.1186/1746-1596-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong SC, Lo ES, Lee KC, Chan JK, Hsiao WL. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10:1401–8. doi: 10.1158/1078-0432.ccr-0157-03. [DOI] [PubMed] [Google Scholar]
- 50.Günther K, Brabletz T, Kraus C, Dworak O, Reymond MA, Jung A, Hohenberger W, Kirchner T, Köckerling F, Ballhausen WG. Predictive value of nuclear beta-catenin expression for the occurrence of distant metastases in rectal cancer. Dis Colon Rectum. 1998;41:1256–61. doi: 10.1007/BF02258226. [DOI] [PubMed] [Google Scholar]
- 51.Lee SJ, Choi SY, Kim WJ, Ji M, Lee TG, Son BR, Yoon SM, Sung R, Lee EJ, Youn SJ, Park SM. Combined aberrant expression of E-cadherin and S100A4, but not β-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn Pathol. 2013;8:99. doi: 10.1186/1746-1596-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]


