Abstract
Objective: To investigate whether celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, can attenuate proliferation, migration, invasion and MMP-14 expression in pancreatic cancer cells PANC-1 and the possible anti-tumor mechanism of celecoxib. Methods: Human pancreatic cancer cell line PANC-1 cells were treated with diverse concentrations of celecoxib (20, 60, 100 μmol/L). Cell proliferation, invasion and migration capabilities were measured by MTT colorimetry, transwell invasion assay, and scratch assay separately. At the same time, the protein expression of COX-2 and MMP-14 was assessed by ELISA. Results: The capabilities of proliferation, invasion and migration in PANC-1 cells were attenuated in a concentration-dependent manner after treated with celecoxib, followed by the down-regulation of the protein expression of COX-2 and MMP-14. In addition, MMP-14 expression was significantly positively correlated with COX-2 expression. Conclusions: COX-2 inhibitor celecoxib can inhibit the proliferation, invasion and migration of PANC-1 cells via down-regulating the expression of MMP-14 in a concentration-dependent manner, thus contributing to its anti-tumor effect in pancreatic cancer.
Keywords: Pancreatic cancer, celecoxib, matrix metalloproteinase-14, cyclooxygenase-2
Introduction
Pancreatic cancer (PC) is one of the highly lethal malignant tumors, of which the most important biological characteristics are invasion and metastasis [1]. Majority of patients with pancreatic cancer were died of tumor invasion and metastasis. The key event in this complicated process is degradation of the extracellular matrix (ECM). Studies demonstrated that matrix metalloproteinases (MMPs) plays a key role in inducing protein degradation of ECM [2]. When migrating away from a primary tumor, cancer cells interact with the ECM and remodel its structure. The transmembrane matrix metalloproteinase-14 (MMP-14), also known as MT1-MMP, is key enzyme in tumor-cell invasion [3,4]. High cyclooxygenase-2 (COX-2) expression is another condition associated with pancreatic cancer and other clinically aggressive tumors. COX-2 is a rate limited enzyme in prostanoid synthesis and has been shown to have a close relationship with invasion and metastasis in the development and progression of cancer [5]. However, Studies focusing on the associations between COX-2 and MMP-14 in PC are rarely reported. The involvement of their relationship in the invasion and metastasis of PC remains undetermined. In this study, we treated human pancreatic cancer cells with different concentrations of the selective COX-2 inhibitor celecoxib, determining the protein expression of COX-2 and MMP-14, and the proliferation, migration and invasion abilities in pancreatic cancer cells. The purpose of which is to gain a further understanding of the possible mechanism of invasion and metastasis of PC, and provide research prospects for effective therapeutic intervention strategies for patients with PC.
Materials and methods
Cell culture
PANC-1 human pancreatic cancer cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and 1% ampicillin/streptomycin (all from Gibco, San Diego, CA, USA) in a 37°C humidified incubator with 5% CO2 and 95% air. Celecoxib (Sigma, St Louis, MO) was prepared into stock solutions at a concentration of 100 mmol/L in anhydrous dimethyl sulfoxide (Sigma), and stored at -20°C. Confluent monolayers of cells were incubated with different concentrations of celecoxib and solvent control for 24, 48 and 72 hrs.
MTT assay
Cancer cells were cultured in 96-well plates at 104 cells per well. After overnight growth, the cells were treated with celecoxib or solvent. At the end of the experimental period, 1 mg/mL of freshly prepared MTT (Sigma) was added to each well and incubated for an additional 4 h. A 150 μL aliquot of DMSO was added and allowed to react for 15 min, after which the spectrometric absorbance was measured at 490 nm. All experiments were done with 6-8 wells per experiment and repeated at least three times.
Matrigel invasion assay
Six-well transwell plates (Costar, Cambridge, MA) with an 8 μm pore size were coated with Matrigel (Becton Dickinson, Heidelberg, Germany) diluted at a 1:2 ratio with medium. The lower chamber was filled with DMEM containing 10% FBS. Cancer cells were treated with celecoxib or solvent. Homogeneous single cell suspensions (5 × 105 cells/well) were added to the upper chambers, and allowed to invade for 24 hour at 37°C in a CO2 incubator. Migrated cells were stained with 0.1% crystal violet for 10 min at room temperature and examined by light microscopy. Migrated cells were counted from 10 microscopic fields of each membrane, and the average value of each experiment was calculated and compared.
Scratch migration assay
Cancer cells were cultured in 12-well plates (105 cells/well), treated with celecoxib or solvent, and scraped with the fine end of 1-ml pipette tips (time 0). Plates were washed twice with PBS to remove detached cells, and incubated with the complete growth medium. Cell migration was photographed using 10 high-power fields, at 0 and 24 hour post-induction of injury. Remodeling was measured as diminishing distance across the induced injury, normalized to the 0 hr control, and expressed as outgrowth.
ELISA assay
Cancer cells were cultured in 6-well plates at 105 cells per well for 48 hour at 37°C in a CO2 incubator. Then the cells were treated with celecoxib or solvent for 24 hour. Through repeated freezing and thawing, the intracellular proteins were released and detected by Enzyme-linked immunosorbent assay (ELISA) system carried out using the kit (R&D Systems, MN, USA) according to the manufacturer’s protocol. Briefly, 100 μl of Standard or Sample was added per well for 40 min at 37°C, washed and incubated with 50 μl of primary antibody working solution for 20 min. Then samples were incubated with enzyme-labeled antibody working solution for 10 min, washed and added with substrate solution and stop solution. The optical density of each well was determined at once, using a microplate reader set to 450 nm.
Statistical analysis
All data were shown as mean ± standard error of the mean (SEM). The SPSS 17.0 statistical software (SPSS Inc., Chicago, IL) was applied for statistical analysis. Pearson’s coefficient correlation was applied for analyzing the relationship between COX-2 expression and MMP-14 expression levels. Difference of cancer cells was determined by t test or analysis of variance (ANOVA).
Results
Effects of cyclooxygenase-2 inhibitor on proliferation ability of PANC-1 cell
In this study, we treated PANC-1 cells with diverse concentrations of celecoxib (20, 60, 100 μmol/L) and solvent control for 24 h, 48 h and 72 h by using MTT assay. The levels of proliferation rates were 94.146 ± 4.612, 85.599 ± 4.862, 60.333 ± 4.554, 98.187 ± 4.173 after 24 h, 86.758 ± 3.787, 74.316 ± 3.967, 47.151 ± 4.450, 98.050 ± 3.491 after 48 h, 79.605 ± 2.871, 66.081 ± 3.430, 34.776 ± 2.515, 97.985 ± 2.325 after 72 h, respectively (all, P<0.05 vs. the control group). The proliferation capabilities were attenuated in a concentration-dependent manner after treating PANC-1 cells with celecoxib (P<0.05, Figure 1).
Figure 1.

Effects of Celebrex on proliferation ability of PANC-1 cell.
Effects of cyclooxygenase-2 inhibitor on invasive and migration ability of PANC-1 cell
We next assessed the invasive and migration capabilities of cells after celecoxib treatment using matrigel invasion assay and scratch migration assay. Cells were treated with increasing concentrations of celecoxib (20, 60, 100 μmol/L) and solvent control for 24 h. The number of PANC-1 cells invaded through matrigel barriers were 271.041 ± 10.569, 184.003 ± 10.865 and 92.667 ± 7.567 (P<0.05 vs. the control group 300.654 ± 12.558). The relative migration distance were 9.511 ± 1.110, 5.856 ± 0.778 and 2.844 ± 0.416 (P<0.05 vs. the control group 11.400 ± 0.959). These findings were also recapitulated in PANC-1 cells where celecoxib treatment attenuated cell invasion and migration in a concentration-dependent manner (P<0.05, Figure 2).
Figure 2.
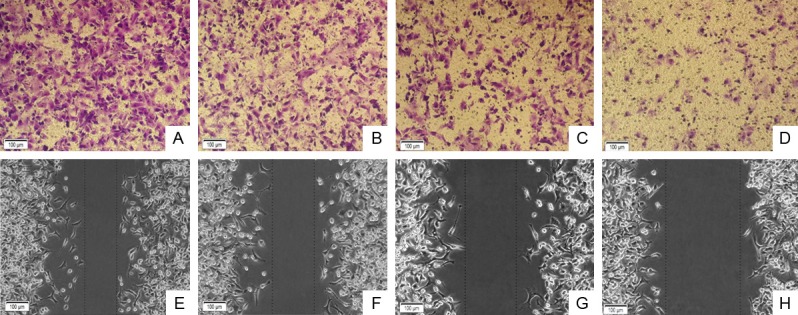
Effects of Celebrex on invasive and migration ability of PANC-1 cell. A, E: Solvent control; B, F: Celecoxib 20 μmol/L; C, G: Celecoxib 60 μmol/L; D, H: Celecoxib 100 μmol/L.
Effects of cyclooxygenase-2 inhibitor on morphology of PANC-1 cell
The normal morphology of PANC-1 cells were spindle or polygonal, accompanied with large nucleus and short protrusions. But this morphology of PANC-1 was changed after treated with increasing concentrations of celecoxib for 24 h. Compared with normal cells; PANC-1 became round, shrinking and aging. Moreover, the cell number became decreased after the appearance of dead cells along with increasing concentrations of celecoxib (Figure 3).
Figure 3.

Morphology of PANC-1 cell. A: solvent control; B: 60 μmol/L celecoxib group; C: 100 μmol/L celecoxib group.
Effects of cyclooxygenase-2 inhibitor on COX-2 and MMP-4 expressions of PANC-1 cell
We at the same time determined the protein expressions of COX-2 and MMP-14 after celecoxib treatment using ELISA assay. Cells were treated with increasing concentrations of celecoxib (20, 60, 100 μmol/L) and solvent control for 24 h. The protein expressions of COX-2 were 0.682 ± 0.027, 0.505 ± 0.022 and 0.384 ± 0.019 (P<0.05 vs. the control group 0.876 ± 0.023). The protein expressions of MMP-14 were 7.031 ± 0.178, 6.072 ± 0.209 and 5.047 ± 0.216 (P<0.05 vs. the control group 7.955 ± 0.297). These data further validated the protein expression of COX-2 and MMP-14 was decreased in a concentration-dependent manner, further attenuating celecoxib-caused cell proliferation, invasion and migration abilities (P<0.05, Figure 4). Pearson’s coefficient correlation indicated that COX-2 was expressed negatively correlated with MMP-14 expression (r=0.873, P<0.05).
Figure 4.

The standard curve and protein expression levels of COX-2 and MMP-14. *P<0.05.
Discussion
Pancreatic cancer, the fourth leading cause of cancer mortality in the world, is characterized by advanced clinical stages at diagnosis and extremely poor prognosis. Difficulty of early detection contributes to the poor prognosis, with most patients going undiagnosed until emergency admission. In 2010, the estimated incidence of PC in China was 40,394 males, and an estimated 34,509 cases died from the disease. The incidence and mortality rates are almost equal, clearly reflecting the malignant degree of PC [6,7]. Selective COX-2 inhibitor celecoxib is commonly used as one of the non-steroidal anti-inflammatory drugs (NSAIDs) in clinical. In fact, the anti-tumor effect of selective COX-2 inhibitor celecoxib has been reported in a variety of tumor cells. The anti-tumor mechanism mainly involved in inducing cell apoptosis, regulating cell cycle and impacting tumor angiogenesis [8,9], however, effectiveness of COX-2 inhibitor in pancreatic cancer cells remain poorly investigated, and the possible action mechanism is also unclear.
In the current study, we first observed the effect of celecoxib on cell proliferation in PANC-1 cells. Our data demonstrated that the inhibition rates were gradually increased in a concentration-dependent manner by treating PANC-1 cells with celecoxib after 24 h, 48 h and 72 h. This result is consistent with the latest literature reported [10]. Moreover, celecoxib can apparently inhibit cell proliferation after 48 h and 72 h, leading to inhibition rate elevates more than 50%. Thus, the processing time of invasion and scratch assay next is defined within 24 h, so as to eliminate interference from celecoxib significantly suppressing cell number and status. Then we determined the effects of celecoxib on PANC-1 cell migration and invasion capacities. Invasion assay results suggested that increasing concentration of celecoxib was able to significantly inhibit the ability of cell invading and passing the basal membrane that contains rich levels of ECM components. Thus, the ability and capacity of tumor cell invasion to cross the Matrigel membrane was decreased. Furthermore, our scratch data also prompted that after treating PANC-1 cells with celecoxib, the speed of cell migration to central scratches was obviously slowed down, and the inhibitory effect on cell migration was amplified along with elevation of celecoxib concentration, corresponded with our invasion assay results. Collectively, according to our experimental results and cell morphology images, we found that celecoxib effectively inhibited cell proliferation, invasion and migration in a concentration-dependent manner, and this anti-tumor effect was mostly evident when the concentration was 100 μmol/L. The latest reports suggested that celecoxib inhibited cell invasion and migration in nasopharyngeal carcinoma and lung cancer [11,12]. Our data indicated that celecoxib also has anti-pancreatic cancer effect in PC progression.
MMP-14, key enzymes in tumor-cell invasion, is implicated both in the breaching of basement membranes by tumor cells and in cell invasion and migration through interstitial type-I collagen tissues. Remarkably, MMP-14 accumulates at invadopodia, which are specialized ECM-degrading membrane protrusions of invasive cells, leading to increased pericellular degradation of the matrix [13].
In order to further investigate the roles of MMP-14 and COX-2 protein in cell invasion and metastasis, we detected the protein expression of COX-2 and MMP-14 after treating PANC-1 cells with various concentrations of celecoxib after 24 h. Our data demonstrated that COX-2 was decreasingly expressed in a gradual declining trend, as compared to the normal PANC-1 cells, indicating that COX-2 expression was suppressed indeed. At the same time, the protein level of MMP-14 was correspondingly reduced compared with control group. Moreover, when the concentration of elecoxib was 20, 60, 100 μmol/L, the declining range of COX-2 and MMP-14 expression was more distinguished, corresponded with the previous result of invasion and migration assay. Taken together, these findings may indicate that COX-2 inhibitor celecoxib may down-regulate MMP-14 expression via inhibiting COX-2 expression, then attenuating the invasion and migration of human pancreatic cancer cell.
Increasing evidence supports the importance of COX-2 and MMP-14 in tumor progression, invasion, and growth. Studies have found that in breast cancer cells up-regulation of COX-2 resulted in increased MMP-14 expression provoked by their interaction, however, inhibition COX-2 activity and down-regulation of COX-2 expression attenuated the invasion-promoting effects compared with the control group [14]. In human glioblastoma cells, it has been reported that NF-κB-mediated COX-2 expression performs as a checkpoint controller in regulation of MMP-14 pathway [15]. In addition, MMP-14 regulation of COX-2 is involved in inflammatory response on mesenchymal stromal cells (MSC) contribution to tumor angiogenesis, and is believed to play a crucial role in prevention and therapy of cancer [16]. Our data also shows that in PC cells, MMP-14 expression was significantly positively correlated with COX-2 expression, which may play a crucial role in development of PC. Despite the importance of this association in PC cells, the underlying molecular mechanisms are poorly characterized, in part because of their complexity and redundancy.
In summary, we demonstrate that COX-2 inhibitor celecoxib attenuates the expression of MMP-14, thus inhibiting the migration, invasion and proliferation of PC cells. These findings support the notion that COX-2 inhibitor celecoxib exerts anti-metastatic and anti-invasion functions in a concentration-dependent manner as well. These results lay the groundwork for further investigation into the mechanisms of celecoxib-mediated anti-cancer functions, which is of potential values as a novel therapeutic approach for PC.
Acknowledgements
This work was supported by the funding from the International Cooperation Fund of The Xinjiang Production and Construction Corps (2011BC005) and the Program for Postgraduate Innovative Research in Xinjiang Uygur Autonomous Region (XJGRI2014063).
Disclosure of conflict of interest
None.
References
- 1.Mihaljevic AL, Michalski CW, Friess H, Kleeff J. Molecular mechanism of pancreatic cancer--understanding proliferation, invasion, and metastasis. Langenbecks Arch Surg. 2010;395:295–308. doi: 10.1007/s00423-010-0622-5. [DOI] [PubMed] [Google Scholar]
- 2.Haage A, Schneider IC. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. FASEB J. 2014;28:1–11. doi: 10.1096/fj.13-245613. [DOI] [PubMed] [Google Scholar]
- 3.Pahwa S, Stawikowski MJ, Fields GB. Monitoring and Inhibiting MT1-MMP during Cancer Initiation and Progression. Cancers. 2014;6:416–435. doi: 10.3390/cancers6010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haage A, Nam DH, Ge X, Schneider IC. Matrix metalloproteinase-14 is a mechanically regulated activator of secreted MMPs and invasion. Biochem Biophys Res Commun. 2014;450:213–8. doi: 10.1016/j.bbrc.2014.05.086. [DOI] [PubMed] [Google Scholar]
- 5.Hill R, Li Y, Tran LM, Dry S, Calvopina JH, Garcia A, Kim C, Wang Y, Donahue TR, Herschman HR, Wu H. Cell intrinsic role of COX-2 in pancreatic cancer development. Mol Cancer Ther. 2012;11:2127–2137. doi: 10.1158/1535-7163.MCT-12-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen WQ, Liang D, Zhang SW, Zheng RS, He YT. Pancreatic cancer incidence and mortality patterns in china, 2009. Asian Pac J Cancer Prev. 2013;14:7321–7324. doi: 10.7314/apjcp.2013.14.12.7321. [DOI] [PubMed] [Google Scholar]
- 7.Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M, Liu L, Liu C, Xu J, Gao YT, Zheng Y, Wu C, Ni QX, Li M, Yu X. Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett. 2014;346:273–277. doi: 10.1016/j.canlet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Sadeghi-Aliabadi H, Aliasgharluo M, Fattahi A, Mirian M, Ghannadian M. In vitro cytotoxic evaluation of some synthesized COX-2 inhibitor derivatives against a panel of human cancer cell lines. Res Pharm Sci. 2013;8:298–303. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ZL, Fan ZQ, Jiang HD, Qu JM. Selective Cox-2 inhibitor celecoxib induces epithelial-mesenchymal transition in human lung cancer cells via activating MEK-ERK signaling. Carcinogenesis. 2013;34:638–646. doi: 10.1093/carcin/bgs367. [DOI] [PubMed] [Google Scholar]
- 10.Ding N, Cui XX, Gao Z, Huang H, Wei X, Du Z, Lin Y, Shih WJ, Rabson AB, Conney AH, Hu C, Zheng X. A triple combination of atorvastatin, celecoxib and tipifarnib strongly inhibits pancreatic cancer cells and xenograft pancreatic tumors. Int J Oncol. 2014;44:2139–2145. doi: 10.3892/ijo.2014.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li WW, Long GX, Liu DB, Mei Q, Wang JF, Hu GY, Jiang JZ, Sun W, Gan L, Hu GQ. Cyclooxygenase-2 inhibitor celecoxib suppresses invasion and migration of nasopharyngeal carcinoma cell lines through a decrease in matrix metalloproteinase-2 and -9 activity. Pharmazie. 2014;69:132–137. [PubMed] [Google Scholar]
- 12.Zhang S, Da L, Yang X, Feng D, Yin R, Li M, Zhang Z, Jiang F, Xu L. Celecoxib potentially inhibits metastasis of lung cancer promoted by surgery in mice, via suppression of the PGE2-modulated beta-catenin pathway. Toxicol Lett. 2014;225:201–207. doi: 10.1016/j.toxlet.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 14.Hu M, Peluffo G, Chen H, Gelman R, Schnitt S, Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci U S A. 2009;106:3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annabi B, Laflamme C, Sina A, Lachambre MP, Béliveau R. A MT1-MMP/NF-kappaB signaling axis as a checkpoint controller of COX-2 expression in CD133+ U87 glioblastoma cells. J Neuroinflammation. 2009;6:8. doi: 10.1186/1742-2094-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akla N, Pratt J, Annabi B. Concanavalin-A triggers inflammatory response through JAK/STAT3 signalling and modulates MT1-MMP regulation of COX-2 in mesenchymal stromal cells. Exp Cell Res. 2012;318:2498–2506. doi: 10.1016/j.yexcr.2012.08.003. [DOI] [PubMed] [Google Scholar]


