Abstract
Introduction: MicroRNA-124 (miR-124) has been proven dysregulated in several human malignancies and correlated with tumor progression. However, its expression and clinical significance in non-small cell lung cancer (NSCLC) is still unclear. Thus, the aim of this study was to investigate the clinical significance of miR-124 expression in NSCLC. Methods: Expression levels of miR-124 in 92 pairs of NSCLC and adjacent non-tumor tissues were detected by quantitative real-time PCR (qRT-PCR). In order to determine its prognostic value, overall survival (OS) and disease-free survival (DFS) were evaluated using the Kaplan-Meier method, and multivariate analysis was performed using the Cox proportional hazard analysis. Results: miR-124 expression level was significantly lower in NSCLC tissues compared with adjacent non-tumor tissues (P < 0.05). The 5-year OS of low miR-124 expression group was significantly shorter than that of high miR-124 expression group (P < 0.05). Moreover, the 5-year DFS of low miR-124 expression group was also significantly shorter than that of high miR-124 expression group (P < 0.05). In a multivariate Cox model, we found that miR-124 expression was an independent prognostic factor for both 5-year OS and 5-year DFS in NSCLC (P < 0.05). Conclusions: Our results offer the convincing evidence that miR-124 may play key roles in the progression of lung cancer and that the down-regulated expression of miR-124 may be independently associated with shorter OS and DFS of patients, suggesting that miR-124 might be a potential marker for further risk stratification in the treatment of lung cancer.
Keywords: Non-small cell lung cancer, miR-124, overall survival, disease-free survival
Introduction
Lung cancer is the most common cause of cancer-related death worldwide. Its incidence is rapidly increasing in developing countries, with non-small cell lung cancer (NSCLC) accounting for > 80% of all lung cancer cases [1]. The prognosis for NSCLC remains poor despite recent advances in the diagnosis of this cancer, and the 5-year overall survival rate of NSCLC is a dismal 11% [2]. Therefore, it is of great significance to investigate the molecular mechanisms involved in lung carcinogenesis, and to identify diagnostic and prognostic markers for early detection and targeted treatment of lung cancer.
MicroRNAs (miRNAs) are single stranded, small non-coding RNAs with 18-25 nucleotides in length [3]. They can negatively regulate gene expression through base pairing to the 3’ untranslational region (3’UTR) of target messenger RNA (mRNA), resulting in translation inhibition or mRNA degradation [4]. Beyond the involvement in diverse biological processes, including cell growth, apoptosis, development, differentiation and endocrine homeostasis [5], emerging evidence strongly suggests that the deregulation or dysfunction of miRNAs contributes to human carcinogenesis and cancer progression [6,7].
MiR-124, a brain-enriched miRNA, was first found to be involved in stem cell regulation and neuro-development [8,9]. Previous studies indicated that miR-124 is epigenetically silenced in various types of cancer, and play important roles in tumor development and progression. For example, Zhang et al showed that miR-124 is dramatically down-regulated in human colorectal cancer. MiR-124 promotes apoptosis of colorectal cancer cells by suppressing the expression of STAT3 [10]. Hu et al revealed that miR-124 was significantly reduced in both gastric cancer tissues and cell lines. Forced expression of miR-124 could suppress gastric cancer cell proliferation, migration, and invasion by targeting ROCK1 [11]. Zhao et al demonstrated that miR-124 was down-regulated in glioblastoma tissues, Over-expression of miR-124 inhibited proliferation, G1/S transition and invasiveness in glioblastoma cells which was partly attributed to increased PPP1R13L expression [12]. Li et al found that miR-124 is decreased and inversely associated with the lymph node metastasis in breast cancer. The ectopic expression of miR-124 inhibits cell proliferation and migration by down-regulating FLOT1 [13]. Those studies suggested that miR-124 play an important role in tumorigenesis and progression. However, the clinical significance of miR-124 in NSCLC has not yet been elucidated.
In the present study, we investigated the expression level of miR-124 in human NSCLC tissues and analyzed its correlation of miR-124 expression with clinicopathological features of patients. The prognostic value of miR-124 expression in NSCLC was also analyzed
Materials and methods
Patients and specimens
A total of 92 paired primary NSCLC tissues and corresponding non-tumor tissues were collected from patients with NSCLC undergoing surgery at the Department of respiration medicine, Huaihe Clinical College of Henan University. None of the patients received preoperative chemotherapy or radiotherapy. Written informed consent was obtained from all patients before surgery, and the study protocol was approved by the Institutional Review Board for the Use of Human Subjects at Huaihe Clinical College of Henan University. The clinicopathological findings were determined according to the classification of malignant tumors by the International Association for the Study of Lung Cancer international staging project on lung cancer [14]. All tumor tissues were diagnosed histopathologically by at least two trained pathologists. Surgically removed tumors and matched non-tumor tissue samples used for mRNA detection were immediately frozen in liquid nitrogen and kept at -80°C until extraction of RNA.
RNA extraction and quantitative real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription reaction was carried out starting from 100 ng of total RNA using the looped primers. Real-time PCR was performed using the standard Taqman MicroRNA assays protocol on ABI7900 real-time PCR detection system with cycling conditions of 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. U6 small nuclear RNA was used as an internal control. The PCR primers for mature miR-124 or U6 were designed as follows: miR-124 forward, 5’-GCGGCCGTGTTCACAGCGGACC-3’ and reverse, 5’-GTGCAGGGTCCGAGGT-3’. U6 forward, 5’-CTCGCTTCGGCAGCACA-3’ and reverse, 5’-AACGCTTCACGAATTTGCGT-3’. The threshold cycle (Ct) was defined as the fractional cycle number at which the fluorescence passed the fixed threshold. Each sample was measured in triplicate, and the relative amount of miR-124 to U6 was calculated using the equation 2−ΔCt, where ΔCT = (CTmiR-124 - CTU6).
Statistical analysis
All statistical analyses were performed using SPSS 18.0 statistical software (IBM). The Mann-Whitney test or Kruskal-Wallis was performed to determine the significance of miRNA levels. Survival curves were plotted using the Kaplan-Meier method and differences in survival rates were analyzed using the log-rank test. Prognostic relevance of each variable to overall survival (OS) and disease-free survival (DFS) were analyzed using the Cox regression model. Multivariate analysis of the prognostic factors was performed with Cox regression model. P < 0.05 was considered statistically significant.
Results
miR-124 was significantly down-regulated in NSCLC tissue
To determine whether its expression differed between NSCLC and adjacent non-tumor tissues, the expression levels of miR-124 were detected in 92 pairs of NSCLC tissues and adjacent non-tumor tissues normalized to U6. As shown in Figure 1, the expression levels of miR-124 were found to be distinctly reduced in NSCLC tissues compared to adjacent non-tumor tissues (P < 0.05).
Figure 1.
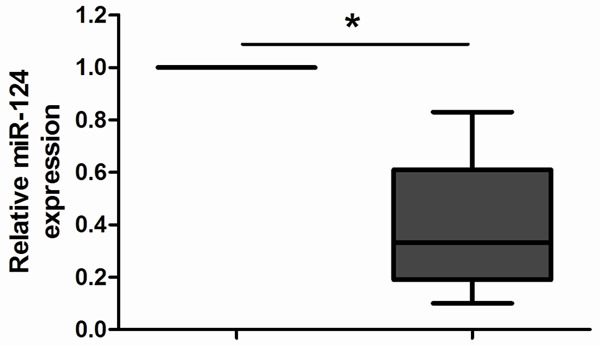
MiR-124 expression in 92 pairs of clinical NSCLC and adjacent non-tumor tissues were detected by qRT-PCR. After normalization to U6 expression levels, the expression level of miR-124 in NSCLC tissues was significantly lower than that in non-tumor tissues. *P < 0.05.
Association between miR-124 expression and the clinicopathological features of NSCLC
For better understanding of the clinical relevance of miR-124 expression in NSCLC, we divided the 92 NSCLC patients into a high expression group (n = 46) and a low expression group (n = 46), according to the median expression level of miR-124 (0.33) in all NSCLC samples. And, the relationships of the miR-124 with various clinical features of NSCLC were analyzed and summarized in Table 1. The results revealed that a high level of miR-124 expression was correlated with advanced clinical stage and positive lymph node metastasis (P < 0.05). However, there were no significant correlations of miR-124 expression with other clinical features such as age, gender, tumor size, and histologic type (P > 0.05).
Table 1.
Correlation between miR-124 expression and different clinicopathological features in NSCLC patients
| Variable | Number | miR-124 expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age (years) | 0.294 | |||
| < 60 | 51 | 23 | 28 | |
| ≥ 60 | 41 | 23 | 18 | |
| Gender | 0.662 | |||
| Male | 60 | 31 | 29 | |
| Female | 32 | 15 | 17 | |
| Tumor size (cm) | 0.672 | |||
| < 3 | 38 | 20 | 18 | |
| ≥ 3 | 54 | 26 | 28 | |
| Histologic type | 0.833 | |||
| SCC | 53 | 26 | 27 | |
| AD | 39 | 20 | 19 | |
| Clinical stage | 0.004 | |||
| I-II | 61 | 24 | 37 | |
| III | 31 | 22 | 9 | |
| Lymph nodes metastasis | 0.001 | |||
| No | 73 | 30 | 43 | |
| Yes | 19 | 16 | 3 | |
SCC: squamous cell carcinoma; AD: adenocarcinoma.
Prognostic values of miR-124 expression in NSCLC
Kaplan-Meier analyses were performed to investigate the association between the miR-124 expression and the prognosis of patients with NSCLC after pneumonectomy. From the Kaplan-Meier survival curves, we found that patients with low expression of miR-124 had shorter OS (P < 0.05; Figure 2A) and DFS (P < 0.05; Figure 2B) as compared with the miR-124-high group. Furthermore, in a multivariate Cox model, we found that miR-124 expression was an independent poor prognostic factor for both OS (RR = 2.942, P < 0.05; Table 2) and DFS (RR = 2.668, P < 0.05; Table 3) in NSCLC.
Figure 2.
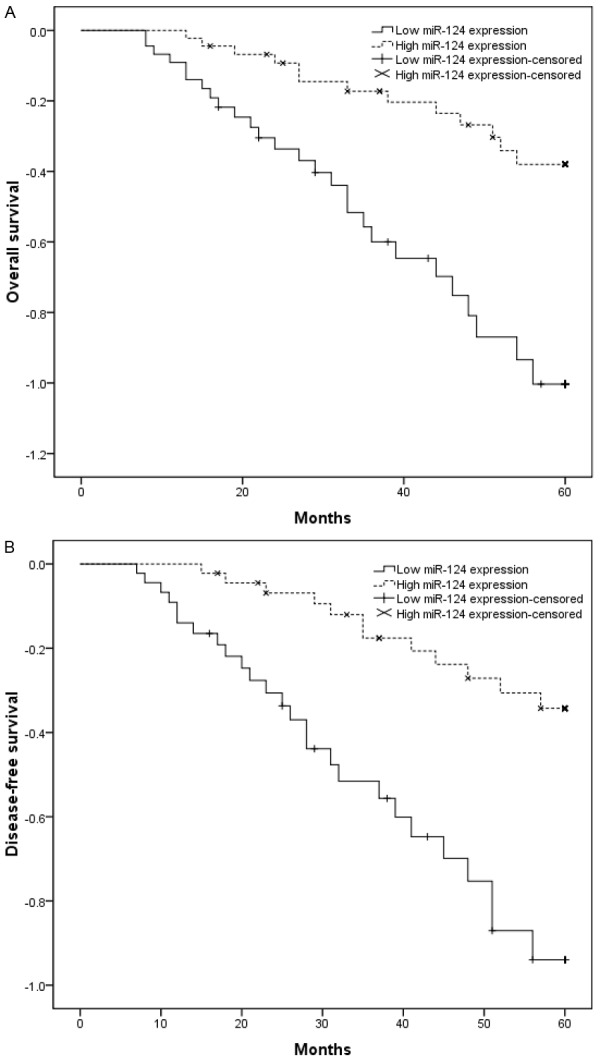
Kaplan-Meier curves for survival time in patients with NSCLC divided according to miR-124 expression. Overall survival and disease-free survival of patients with low vs. high miR-124 expression levels are shown. A. Overall survival rate of NSCLC patients with low miR-124 was significantly shorter compared to those patients with high miR-124 (P < 0.05). B. Disease-free survival rate of NSCLC patients with low miR-124 was significantly shorter compared to those patients with high miR-124 (P < 0.05).
Table 2.
Univariate analysis of prognostic parameters in patients with NSCLC by Cox regression analysis
| Variable | Overall survival | Disease-free survival | ||
|---|---|---|---|---|
|
| ||||
| RR | P | RR | P | |
| Age (years) | 1.417 | 0.297 | 1.213 | 0.336 |
| ≥ 60 vs. < 60 | ||||
| Gender | 1.043 | 0.218 | 0.983 | 0.297 |
| Male vs. Female | ||||
| Tumor size | 1.816 | 0.371 | 1.612 | 0.416 |
| ≥ 3 cm vs. < 3 cm | ||||
| Histologic type | 1.314 | 0.213 | 1.173 | 0.262 |
| SCC vs. AD | ||||
| Clinical stage | 2.917 | 0.008 | 2.635 | 0.012 |
| III vs. I-II | ||||
| Lymph nodes metastasis | 3.774 | 0.002 | 3.358 | 0.008 |
| Yes vs. No | ||||
| miR-124 | 3.271 | 0.003 | 3.019 | 0.007 |
| Low vs. High | ||||
RR: relative ratio; SCC: squamous cell carcinoma; AD: adenocarcinoma.
Table 3.
Multivariate analysis of prognostic parameters in patients with NSCLC by Cox regression analysis
| Variable | Overall survival | Disease-free survival | ||
|---|---|---|---|---|
|
| ||||
| RR | P | RR | P | |
| Clinical stage | 2.514 | 0.004 | 2.218 | 0.011 |
| III vs. I-II | ||||
| Lymph nodes metastasis | 3.281 | 0.001 | 3.015 | 0.006 |
| Yes vs. No | ||||
| miR-124 | 2.942 | 0.003 | 2.668 | 0.009 |
| Low vs. High | ||||
RR: relative ratio; SCC: squamous cell carcinoma; AD: adenocarcinoma.
Discussion
A growing number of novel treatment strategies have been developed for NSCLC, such as molecular targeted therapy and gene therapy, to our disappointment, satisfactory therapeutic outcomes have not been achieved [15]. Considering that the survival rate of NSCLC is still low, further identification of new prognostic markers remains important for the prevention and treatment of NSCLC.
The discovery that non-coding components of the genome, including microRNA, can contribute to the pathogenesis of cancer has led investigators to contemplate using these molecules to guide clinical decision making [16]. So far, there are more than 1000 microRNAs annotated by the latest version of miRBase. The expression of miRNAs is remarkably deregulated in NSCLC, strongly suggesting that miRNAs are involved in the initiation and progression of this disease. For example, Liu et al showed that miR-100 was significantly down-regulated in NSCLC tissues and correlated with higher clinical stage, advanced tumor classification and lymph node metastasis. The overall survival of NSCLC patients with low miR-100 was significantly lower than that of those patients with high miR-100 [17]. Zhang et al found that miR-10b was significantly up-regulated in NSCLC and correlated with TNM stage and lymph node involvement, in addition, patients with higher levels of miR-10b had significantly poorer survival than those with lower expression [18]. Xu et al indicated that miR-9 was up-regulated in NSCLC and correlated with adverse clinical features and unfavorable survival, indicating that miR-9 might be involved in NSCLC progression and could serve as a promising biomarker for further risk stratification in the treatment of this cancer [19]. However, to our knowledge, the clinical significance of miR-124 in NSCLC is still unknown. Therefore, we investigated the feasibility of miR-124 as a novel prognostic biomarker for NSCLC.
In the present study, our data showed that miR-124 expression was significantly lower in NSCLC tissues compared with adjacent non-tumor tissues. The relationships of the miR-124 with various clinical features of NSCLC were analyzed. We found that a low level of miR-124 expression was correlated with advanced clinical stage and positive lymph node metastasis, suggesting that miR-124 might be involved in the carcinogenesis and metastasis of NSCLC. Furthermore, the 5-year OS and DFS of low miR-124 expression group were significantly shorter than that of high miR-124 expression group. In a multivariate Cox model, we found that miR-124 expression was an independent poor prognostic factor for both OS and DFS, indicating that low miR-124 level might be a promising non-invasive biomarker for prognosis of patients with NSCLC.
In conclusion, our data offer the convincing evidence that miR-124 may play an important role in the progression of NSCLC and that the reduced expression of this miRNA may be independently associated with shorter OS and DFS of patients, suggesting that miR-124 might be a potential marker for further risk stratification in the treatment of NSCLC. Further studies are needed to elucidate the mechanisms of action of miR-124 in NSCLC.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 3.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osada H, Takahashi T. MicroRNAs in biological processes and carcinogenesis. Carcinogenesis. 2007;28:2–12. doi: 10.1093/carcin/bgl185. [DOI] [PubMed] [Google Scholar]
- 6.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nature Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 7.Mirnezami AH, Pickard K, Zhang L, Primrose JN, Packham G. MicroRNAs: key players in carcinogenesis and novel therapeutic targets. Eur J Surg Oncol. 2009;35:339–47. doi: 10.1016/j.ejso.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Lee MR, Kim JS, Kim KS. miR-124a is important for migratory cell fate transition during gastrulation of human embryonic stem cells. Stem Cells. 2010;28:1550–1559. doi: 10.1002/stem.490. [DOI] [PubMed] [Google Scholar]
- 9.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Lu Y, Yue X, Li H, Luo X, Wang Y, Wang K, Wan J. MiR-124 suppresses growth of human colorectal cancer by inhibiting STAT3. PLoS One. 2013;8:e70300. doi: 10.1371/journal.pone.0070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu CB, Li QL, Hu JF, Zhang Q, Xie JP, Deng L. miR-124 inhibits growth and invasion of gastric cancer by targeting ROCK1. Asian Pac J Cancer Prev. 2014;15:6543–6546. doi: 10.7314/apjcp.2014.15.16.6543. [DOI] [PubMed] [Google Scholar]
- 12.Zhao WH, Wu SQ, Zhang YD. Downregulation of miR-124 promotes the growth and invasiveness of glioblastoma cells involving upregulation of PPP1R13L. Int J Mol Med. 2013;32:101–107. doi: 10.3892/ijmm.2013.1365. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Luo J, Wang B, Wang D, Xie X, Yuan L, Guo J, Xi S, Gao J, Lin X, Kong Y, Xu X, Tang H, Liu M. Microrna-124 targets flotillin-1 to regulate proliferation and migration in breast cancer. Mol Cancer. 2013;12:163. doi: 10.1186/1476-4598-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstraw P, Crowley JJ. The International Association for the Study of Lung Cancer international staging project on lung cancer. J Thorac Oncol. 2006;1:281–286. [Google Scholar]
- 15.Haber DA, Bell DW, Sordella R, Kwak EL, Godin-Heymann N, Sharma SV, Lynch TJ, Settleman J. Molecular targeted therapy of lung cancer: EGFR mutations and response to EGFR inhibitors. Cold Spring Harb Symp Quant Biol. 2005;70:419–426. doi: 10.1101/sqb.2005.70.043. [DOI] [PubMed] [Google Scholar]
- 16.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Lu KH, Liu ZL, Sun M, De W, Wang ZX. MicroRNA-100 is a potential molecular marker of non-small cell lung cancer and functions as a tumor suppressor by targeting polo-like kinase 1. BMC Cancer. 2012;12:519. doi: 10.1186/1471-2407-12-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Xu L, Yang Z, Lu H, Hu D, Li W, Zhang Z, Liu B, Ma S. MicroRNA-10b indicates a poor prognosis of non-small cell lung cancer and targets E-cadherin. Clin Transl Oncol. 2015;17:209–14. doi: 10.1007/s12094-014-1213-7. [DOI] [PubMed] [Google Scholar]
- 19.Xu T, Liu X, Han L, Shen H, Liu L, Shu Y. Up-regulation of miR-9 expression as a poor prognostic biomarker in patients with non-small cell lung cancer. Clin Transl Oncol. 2014;16:469–475. doi: 10.1007/s12094-013-1106-1. [DOI] [PubMed] [Google Scholar]


