Abstract
Objective: This study investigated the biocompatibility of the small intestinal submucosa (SIS) and endothelial progenitor cells (EPCs) by co-cultivating EPCs and SIS in vitro and observing EPC growth on the SIS. Methods: The porcine SIS was prepared and bone marrow mononuclear cells (BMMNCs) were isolated from 3 or 4-week old male SD rats. Cellular morphology was observed by light microscopy and scanning electron microscopy (SEM) and viabilities by the MTT assays. Endothelial progenitor cells (EPCs) were phenotyped by immunocytochemistry, immunofluorescence microscopy and flow cytometry. Vascular lumen formation was evaluated by the Matrigel tube formation assays. EPCs were seeded onto the SIS and production of angiogenin-1 and endothelial cell growth factor (VEGF) by EPCs was examined by ELISA and immunoblotting assays. Results: Light microscopy and SEM showed that the mechanically and chemically treated small intestinal submucosa was composed of cell-free extracellular matrix. Immunohistochemistry, and flow cytometry revealed that the EPCs expressed appropriate surface markers including CD34, CD133, and VEGFR-2. Furthermore, the EPCs formed lumen-like structures and the SIS significantly enhanced the growth of EPCs in vitro. Conclusion: SIS has good biocompatibility with EPCs. SIS pre-seeded with EPCs can be potentially applied as an alternative scaffold material in artificial blood vessel prosthesis.
Keywords: Biocompatibility, porcine, small intestinal submucosa, endothelial progenitor cells
Introduction
Autologous great saphenous vein, commonly used as artificial blood vessel, is often associated with the occurrence of phlebitis despite it has a higher patency rate than vascular prosthesis [1]. Vascular prosthesis, on the other hand, has a low long-term patency rate, especially small-diameter synthetic grafts crossing the knee joints [2]. Vascular surgeons have has long been in pursuit of a safe and effective small-diameter vascular graft. Small intestinal submucosa (SIS) tissue has been used as a cell-free natural extracellular matrix (ECM) material and preliminary studies have demonstrated a satisfactory outcome [3-5]. However, the use of SIS graft itself may induce inflammatory response and cause thrombosis [6].
Endothelial progenitor cells are capable of directed differentiation into vascular endothelial cells, and exhibit good adhesion and robust proliferation in vitro, allowing them to be used as ideal seed cells for vascular endothelial cell production. The biocompatibility of endothelial progenitor cells is essential for their application in vascular tissue engineering. However, the biocompatibility of porcine SIS with endothelial progenitor cells has not been evaluated in vitro so far, and the suitability of porcine SIS for endothelial progenitor cell adhesion and growth has not been confirmed either. In the current study, we sought to characterize the SIS preparations and rat endothelial progenitor cells and examine the biocompatibility of the endothelial progenitor cells with SIS.
Materials and methods
SIS preparation
The experimental protocol for the animal study was approved by the Institutional Animal Care and Use Committee, which has been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care Institutions and animal experiments were conducted in accordance with the USA National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
The porcine SIS was prepared as described previously [7,8]. Briefly, the jejunum was freshly prepared from a healthy swine (weight > 100 kg). After gentle cleansing in water, one segment of the jejunum was everted, and the tunica mucosa was abraded from the jejunum in a longitudinal wiping motion by using a moistened gauze-wrapped scalpel handle. The jejunal segment was everted again, and the tunica serosa and tunica muscularis were gently removed using the same abrasion procedure. Upon completion of mechanical cleaning, the intestine was split longitudinally and divided into a set of 15-cm sections. The tissue specimens were incubated in 100 mM EDTA and 10 mM NaOH (pH 11-12) for 16 h. Then, they were incubated in 1 M HCl and NaCl (pH 0-1) for 6-8 h, followed by incubation in 1 M NaCl and 10 mM phosphate-buffered saline (PBS) (pH 7-7.4) for 16 h. After final incubation in 10 mM PBS for 2 h, the tissue specimens were rinsed in sterile water (pH 5.8-7.0) for at least 2 h. The porcine SIS was rinsed extensively in 0.1% peracetic acid for 2 h, vacuum-sealed into hermetic packaging, and terminally sterilized by gamma irradiation (25-35 kGy).
Culture of endothelial progenitor cells
Bone marrow-derived mononuclear cells were isolated from the bone marrow of 3 or 4-week old male SD rats as previously described [9] and purified by density gradient centrifugation. The cells were then cultured in endothelial growth medium-2 microvascular (EGM-2MV) supplemented with 5% fetal bovine serum. Cellular morphology was observed by light microscopy.
The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium (MTT) assays
Cell viabilities were examined at the indicated time points by MTT assays as instructed by the manufacturer (Sigma, St. Louis, MO). Absorbance was measured by a multimode microplate reader (Infinite M200, Tecan) at 450 nm. Viability (%) was calculated with the following formula: [(Absorbance of treated cells-Absorbance of blanks)/(Absorbance of control cells-Absorbance of blanks)] × 100%. The experiment was performed three times independently in sextuplicates.
Matrigel tube formation assays
For Matrigel™ tube formation assays, 96 well plates were coated with Matrigel according to the manufacturer’s instructions (BD Biosciences). Endothelial progenitor cells were seeded on a layer of previously polymerized and growth factor reduced Matrigel™. After 6-h incubation at 37°C in 5% CO2, network-like structures of endothelial cells were examined under an inverted microscope (Olympus). The assay was performed three times independently.
Immunocytochemistry
Immunocytochemical staining was performed by the standard streptavidin-peroxidase (S-P) method. Briefly, endothelial progenitor cells were seeded in fibronectin-coated glass coverslips immersed in 35-mm Petri dish. They were then fixed by 4% paraformaldehyde. After rinsing with PBS, 0.3% H2O2 was used to block endogenous peroxidase activity by incubating with the cells for 15 min. Nonspecific binding was blocked by incubation with 5% normal goat serum and 2% bovine serum albumin (BSA). Then, cells were incubated with rabbit anti-human vWF and VEGFR-2 antibodies (all from Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight followed by incubation with biotinylated goat anti-rabbit antibodies at 37°C for 20 min and visualized with diaminobenzidine. Brown staining in the cytoplasm was determined as positive.
Immunofluorescence
Immunofluorescence microscopy was done as previously described [10]. For immunofluorescence microscopy, endothelial progenitor cells were stained with anti-CD34 and anti-CD133 antibodies (Beijing Biosynthesis Biotechnology Co., Beijing, China). For confocal immunofluorescence microscopy, endothelial progenitor cells were double stained for FITC-labeled Ulex europaeus agglutinin-1 (UEA-1) and Dil-labeled acetylated low-density lipoprotein (ac-LDL) (Molecular Probe). Confocal images were taken using a Leica scanning confocal microscope (Leica Camera AG, Solms, Hessen, Germany) and 20 × and 63 × objective lenses for low-magnification and high-magnification analysis, respectively. Pictures were cropped, labeled, and spaced using the Adobe Photoshop software.
Flow cytometric analysis
To verify the presentation of endothelial progenitor cell markers, phenotyping was performed by flow cytometry on culture-expanded endothelial progenitor cells. Cells were collected from confluent layers after incubation with 0.25% trypsin-EDTA for 5 minutes. Single-cell suspensions were washed with PBS/0.5% BSA before staining. For direct staining, cells were centrifuged (250 g, 5 min) and re-suspended in cold PBS/0.5% BSA. Totally, 1 × 106 cells were incubated for 15 minutes on ice in the dark in cold PBS/0.5% BSA with monoclonal fluorescein isothiocyanate (FITC) conjugated mouse anti-human CD34, CD133, and VEGFR-2 antibodies. Cells were then washed twice by centrifugation (250 g, 5 min) and re-suspended with cold PBS/0.5% BSA before proceeding with flow cytometry. Dead cells and debris were stained with propidium iodide (100 μg/mL) and excluded from measurements. Acquisitions were performed on a FACS Calibur flow cytometer (Becton Dickinson Bioscience) data analysis was conducted with FCS Express V2 software after gating for the designated population.
Scanning electron microscopy
For scanning electron microscopy, endothelial progenitor cells (2 × 105) on day 1 to 14 post seeding were harvested and fixed with 2% electron microscopy-grade glutaraldehyde for 2 h, postfixed in 1% osmium tetroxide with 0.1% potassium ferricyanide, dehydrated in gradient ethanol (30-90%), and embedded in Epon. Ultrathin sections (65 nm) were cut, stained with 2% uranyl acetate and examined under a scanning electron microscope (PhilipsQUANTA-200, Holland) with the surface coated with a gold layer.
Seeding of endothelial progenitor cells on the SIS
The 1 cm × 1 cm SIS tiles were placed in 24-well plates with Hanks solution and stored for 24 h, and then dried under aseptic condition (ultraviolet disinfection for 2 h). Afterwards, the wells were rinsed extensively with PBS, followed by seeding endothelial progenitor cells onto the SIS pieces at a density of 5 × 105 cells/cm2. All plates were then incubated in EGM-2MV inside a thermostatic incubator for 1 to 2 h at 37°C.
Enzyme-linked immunosorbent assay (ELISA) and Western blotting assays
Angiopoietin-1 and VEGF in the cellular supernatant was analyzed by ELISA using commercially available kits as instructed by the manufacturer (Boster, China). Briefly, the cell samples were added to each well of 96-well plates pre-coated with specific antibody and incubated for 90 min at 37°C. The plates were then washed and incubated with biotinylated rabbit anti-rat isotype-specific antibody for 90 min at 37°C, followed by wash and incubation with avidin-biotin complex (ABC) working dilutions for 30 min at 37°C. Then, the plates were rewashed, and incubated with TMB solution for 25 min at 37°C. Finally, with the stop solution added into the wells, and the absorbance was read at 450 nm on a microplate reader (Bio-Tek Instruments, Winooski, VT). For Western blotting assays, the procedure was performed as previously described. Anti-angiopoietin-1, anti-VEGF and anti-b-actin antibodies (Santa Cruz Biotechnology) were used. b-Actin was used as a loading control.
Results
The mechanically and chemically treated small intestinal submucosa is composed of cell-free extracellular matrix
After mechanical and chemical treatment, the semi-wet SIS became translucent membranes and were milky white (Figure 1A), and the lyophilized SIS showed paper-like appearance (Figure 1B). In addition, light microscopy showed absence of any cells on the SIS membrane (Figure 1C). SEM further revealed that the SIS was composed mainly of the ECM with structural integrity. In addition, the mucosal surface appeared smooth while the surface of the muscular layer was slightly rough (Figure 1D).
Figure 1.
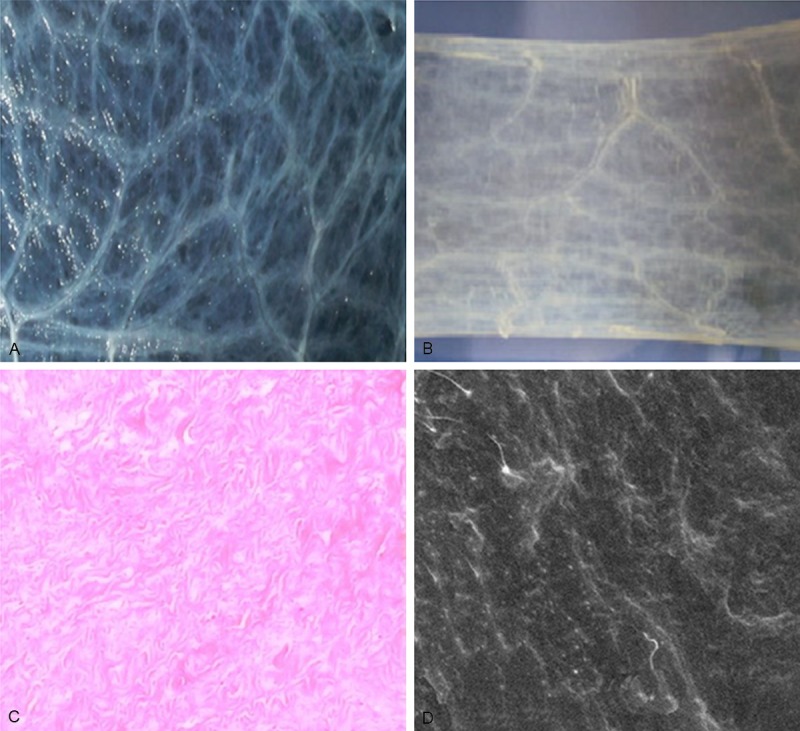
The mechanically and chemically treated small intestinal submucosa is composed of cell-free extracellular matrix. The small intestinal submucosa (SIS) was mechanically and chemically treated as detailed in Methods. A. Gross anatomy of the SIS in the semi-wet state, magnification: × 40. B. Gross anatomy of the SIS in the dry state after lyophilization, magnification: × 40. C. H&E staining of the SIS specimen, magnification: × 200. D. SEM image with a smooth mucosal surface, magnification: × 700.
Morphological characteristics of endothelial progenitor cells derived from bone marrow mononuclear cells
We purified BMMNCs from SD rats. Light microscopy of the primary culture showed that the freshly isolated BMMNCs grew in suspension and were spherical in shape and exhibited varied sizes (Figure 2A). By day 7, the cells were fusiform, triangular, or irregular in shape and formed colonies (Figure 2B). By week 3, the cells exhibited a typical cobblestone-like pavement appearance (Figure 2C). TEM showed abundant finger-like, spherical, or villous protrusions on the surface of the endothelial progenitor cells. The rough endoplasmic reticula, mitochondria, free ribosomes, and plasma membrane vesicles were observed (Figure 2D). The Weible-Palade (W-P) corpuscle, a characteristic structure of endothelial cells, was also detected (Figure 2E).
Figure 2.
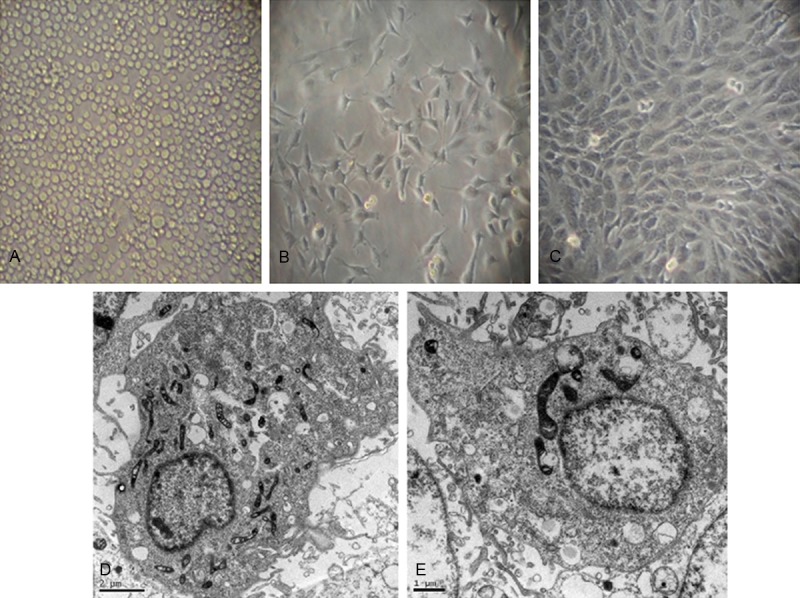
Morphological characteristics of endothelial progenitor cells derived from bone marrow mononuclear cells. Light microscopy of bone marrow mononuclear cells isolatd from SD rats. (A) Freshly isolated bone marrow mononuclear cells. (B) Early outgrowth of endothelial progenitor cells by day 7 of primary culture. The cells are fusiform, triangular, or irregular in shape. (C) Endothelial progenitor cells exhibit a typical cobblestone-like appearance by day 21. Transmission electron microscopy shows abundant finger-like, spherical, or villous protrusions on the surface of the endothelial progenitor cells (D) and Weible-Palade (W-P) corpuscle (E).
Phenotypic characteristics of endothelial progenitor cells derived from bone marrow mononuclear cells
Immunohistochemistry showed that vWF and VEGFR-2 were positively expressed in the BMMNCs by day 7 (Figure 3A and 3B) and immunofluorescent microscopy revealed that the BMMNCs were positive for CD34 (Figure 3C) and CD133 (Figure 3D). In addition, laser confocal microscopy showed that the endothelial progenitor cells took up the DiL-Ac-LDL and FITC-Lectin-UEA-1 (Figure 3E-G). Flow cytometric analysis additionally revealed that surface markers CD34, CD133, and VEGFR-2 were expressed on the endothelial progenitor cells at variable levels. CD34 positive cells increased from 30.6% on day 7 to 82.8% on day 21, whereas CD133 decreased from 96.6% on day 7 to 38% on day 21, and VEGFR-2 positive cells increased from 59.8% to 92.7%.
Figure 3.
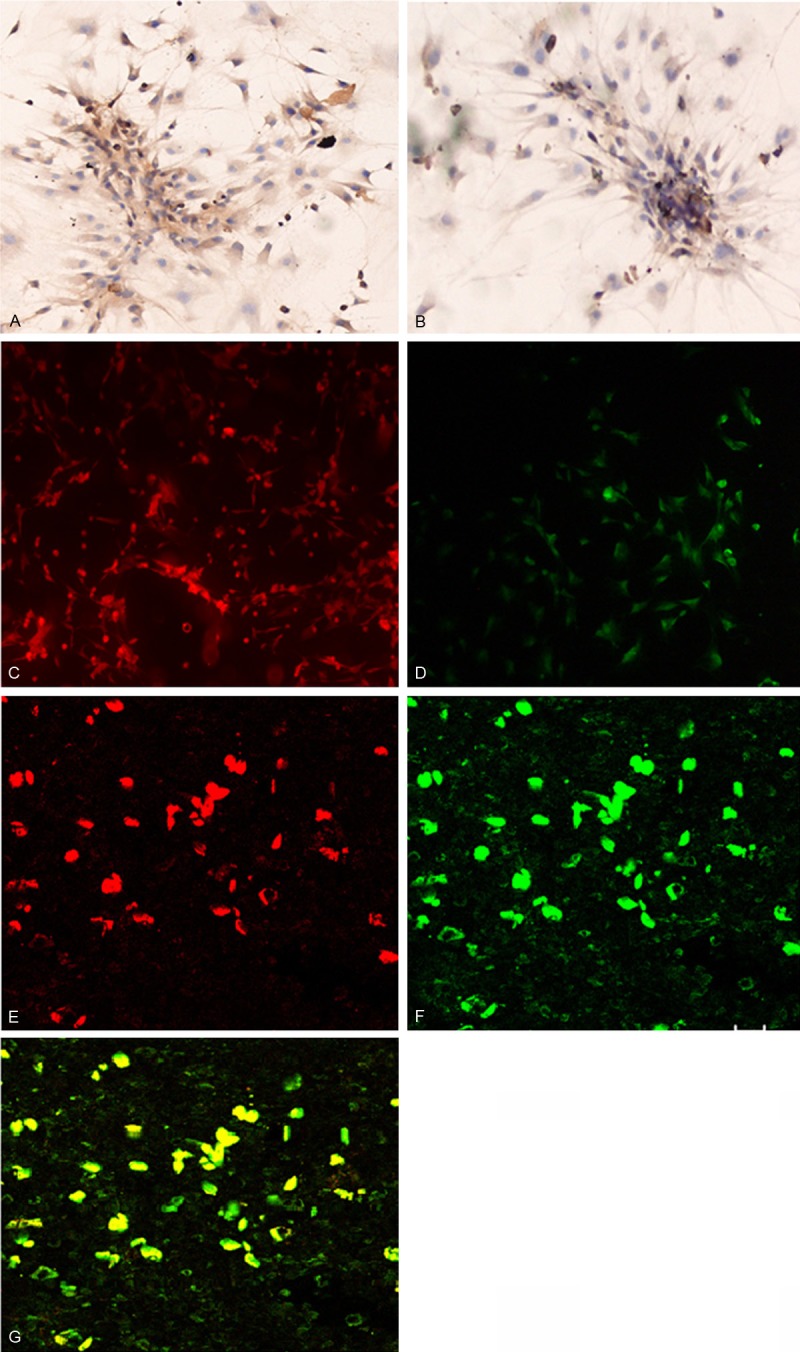
Immunohistochemical staining of cultured bone marrow mononuclear cells at day 7 for vWF (A) and VEGFR-2 (B). Immunofluorescence staining for CD34 (C) and CD133 (D). Magnification × 200 Confocal microscopy showed (E) EPCs after the cytoplasm of EPCs took up the DiL-Ac-LDL stain (shown in red), (F) EPCs stained positive for FITC-UEA-1 (shown in green), and (G) EPCs with double positive staining for phagocytic DiL-Ac-LDL and FITC-Lectin-UEA-1 (shown in yellow). Magnification: × 200.
Endothelial progenitor cells derived from bone marrow mononuclear cells form lumen-like structures
Matrigel gel assays showed that by day 12 to 21 of culture, the endothelial progenitor cells formed lumen-like structures (Figure 4A and 4B).
Figure 4.
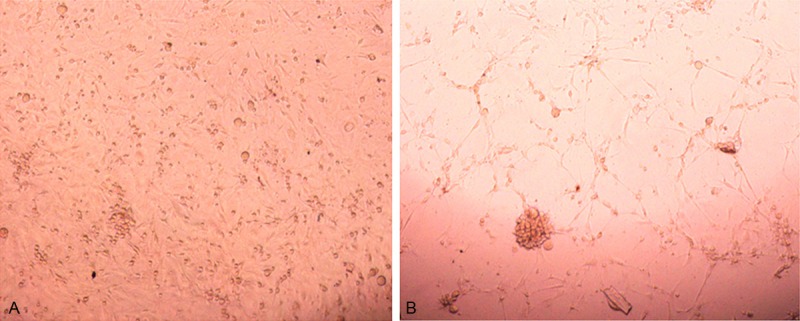
Endothelial progenitor cells derived from bone marrow mononuclear cells form lumen-like structures. Matrigel gel assays were performed as in described in Methods. Showed that by day 12 to 21 of culture, the Endothelial progenitor cells form lumen-like structures at day 12 (A) and day 21 (B).
SIS enhances the growth of endothelial progenitor cells
Endothelial progenitor cells were seeded on the SIS, and after 24 h the cells were spindle-shaped and started attaching to the surface of the SIS (Figure 5A). SEM showed that the endothelial progenitor cells showed robust growth on the surface of the SIS 3 to 5 days post seeding. SEM showed that by day 14, the cells were assembled into sheets and secretory granules were observed (Figure 5B). The MTT assays revealed that by week 1 post seeding onto the SIS, the endothelial progenitor cells exhibited a significantly more rapid growth than the control cells (Figure 5C).
Figure 5.
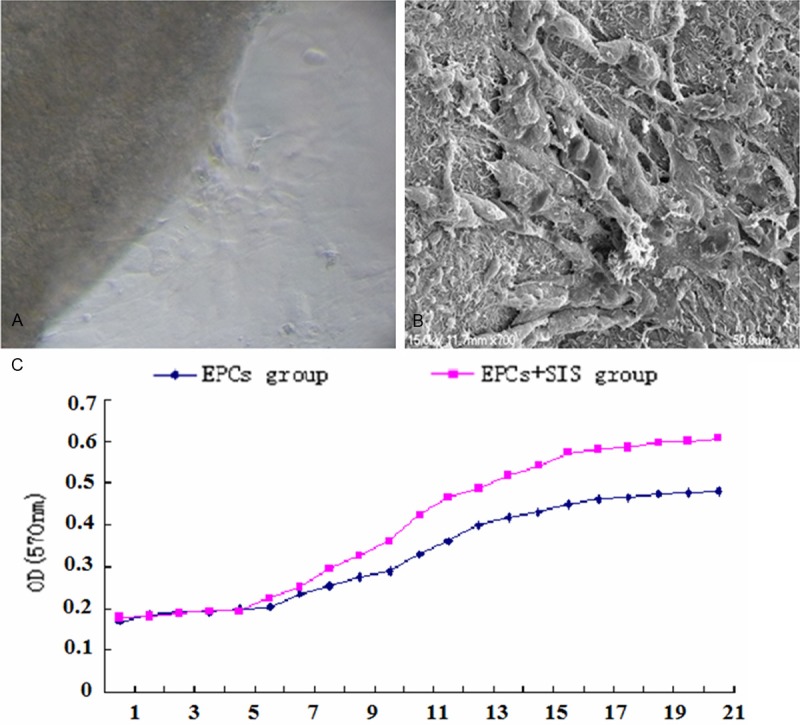
SIS enhances the growth of endothelial progenitor cells. (A) SEM. (B) Proliferaiton of endothelial progenitor cells was assessed by the MTT assays (C).
SIS potentiates the production of angiopoietin-1 and VEGF by endothelial progenitor cells
We further examined the production of angiopoietin-1 and VEGF by the endothelial progenitor cells by ELISA and immunoblotting assays. ELISA showed that angiopoietin-1 levels increased after seeding on the surface of the SIS and peaked at day 7 post seeding (288.636 pg/mL), which was significantly higher than that of the control cells (P < 0.05) (Figure 6A). Similarly, the level of VEGF was markedly higher in the endothelial progenitor cells starting 5 days (5601.1 pg/mL) after seeding onto the surface of the SIS than that of the control cells (P < 0.05) (Figure 6B). The immunoblotting assays further showed that apparently higher levels of both angiopoietin-1 and VEGF upon seeding of the EPS onto the surface of the SIS (Figure 6C).
Figure 6.
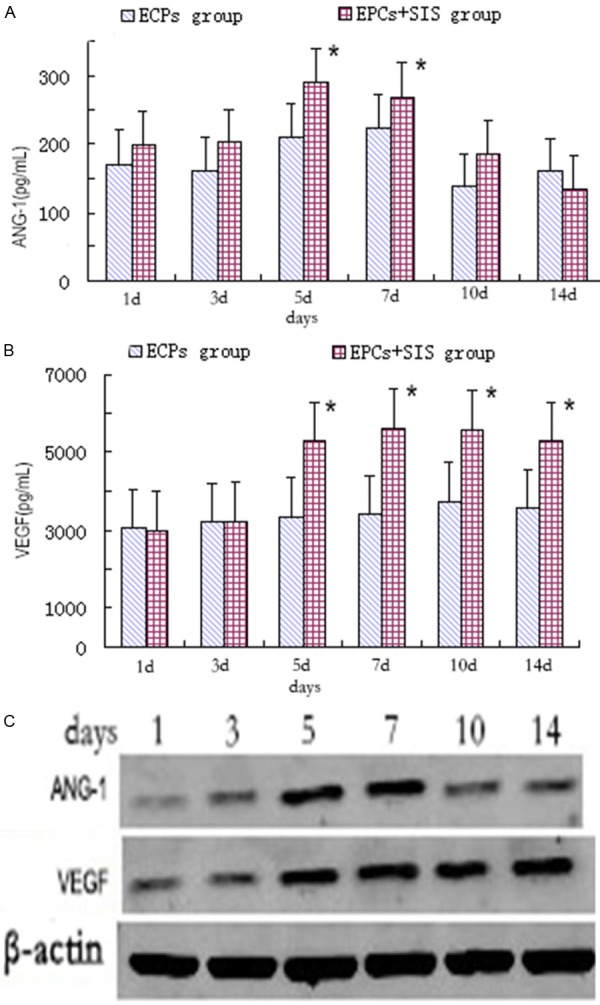
SIS potentiates the production of angiopoietin-1 and VEGF by endothelial progenitor cells. A-C. Error bars represent means ± SE. *P < 0.05 vs. EPCs group.
Discussion
The key element of success in vascular tissue engineering is the development of blood vessels with a complete layer of endothelial cells. In 1997, Asahara et al. [11] were the first to report that human peripheral blood CD34+ mononuclear cells are capable of differentiating into mature endothelial cells, and therefore, they can be mobilized onto damaged blood vessels and participate in embryonic angiogenesis and post-birth angiogenesis. In this study, we purified BMMNCs from SD rats and Immunocytochemistry, immunofluorescence microscopy and phenotyping by flow cytometry confirmed successful cultivation of endothelial progenitor cells which expressed the endothelial progenitor cells markers such as CD34, CD133, and VEGFR-2. Furthermore, Weibel-Palade corpuscle, which is characteristic of endothelial cells, was observed by scanning electron microscopy. The endothelial progenitor cells exhibited active proliferation capacity in vitro as demonstrated by the MTT assays, and can be obtained in large quantities, on the order of 109-1011 cells per liter of the medium (data not shown). These results have established a solid theoretical and empirical foundation for future exploration of endothelial progenitor cells for tissue engineering.
A large variety of ECM materials are available for vascular tissue engineering. SIS is a naturally obtained acellular ECM with fibrous collagen constituting 40% of its dry weight. Previous studies have demonstrated that SIS contains sugar glycosaminoglycans and glycoprotein such as hyaluronic acid, heparin, heparan sulfate, and chondroitin sulfate A [12]. Furthermore, SIS can release natural growth factors such as fibroblast growth factor 2 (FGF2), transforming growth factor beta (TGFβ), and VEGF [13]. Host immunological response should always be considered for xenogenic grafts. Allman [14] has reported that porcine SIS can elicit an Th2 immune response, which is consistent with a remodeling reaction rather than rejection. Acellular SIS can be used as an allogeneic biological scaffold for repair of tissue defects and it does not cause immune rejection, as demonstrated through cross-species transplantation experiments and direct immune challenge experiments [15,16].
Overall, SIS has demonstrated favorable characteristics, including the possibility of being used as a xenograft without eliciting an immune response, beneficial biological effects on tissue remodeling and microenvironment, and capability of accelerating cell differentiation, release of growth factors, and ease of harvesting cells [17]. Therefore, the application of SIS as a carrier for endothelial progenitor cells in the present study has suggested good usability and great potential for future applications.
Preliminary studies have shown that SIS can be used as an alternative vascular graft material. After the tubular SIS was transplanted into the canine carotid artery, it gradually evolved into the internal carotid artery and exhibited natural mechanical properties of the matrix [3]. Other studies reported that SIS can be used as a basement membrane (BM) to support the adhesion of epidermal cells to fibroblasts and deposition of differentiated cells [18]. The results obtained from this study showed that the growth of endothelial progenitor cells was promoted, rather than inhibited, by SIS. Endothelial progenitor cells seeded on SIS attained good cell morphology and proliferated rapidly on the SIS surface. Scanning electron microscopy confirmed that the endothelial progenitor cells could adhere, grow, and differentiate on the surface of the SIS, which, as revealed by the MTT assays, did not exhibit any cytotoxicity.
Glycoproteins and cytokines present in the SIS played important roles in supporting endothelial progenitor cell growth. The proteoglycan components in the SIS bind to specific receptors on cell surfaces and link ECM components such as collagen type IV, which facilitate adherence and migration of endothelial progenitor cells. Moreover, SIS growth factor activity is present after sterilization [19,20]. Endothelial progenitor cell growth is dependent on the presence of VEGF in the SIS, and cell infiltration and angiogenesis could benefit from the microporous structure of SIS [21]. In consideration of these, SIS has created a favorable environment for the growth of endothelial progenitor cells, and in turn, the seeding of endothelial progenitor cells could promote quick SIS endothelialization, enabling the use of SIS as an alternative vascular material.
In summary, as a natural biological material, SIS exhibits good cell affinity; SIS seeded with endothelial progenitor cells shows great promise as vascular graft for vascular tissue engineering, and our future research will focus on their transplantation in vivo.
Disclosure of conflict of interest
None.
References
- 1.Aracil-Sanus E, Mendieta-Azcona C, Cuesta-Gimeno C, Chinchilla-Molina A. Infragenicular bypass graft for limb salvage using polytetrafluoroethylene and distal vein cuff as the first alternative in patients without ipsilateral greater saphenous vein. Ann Vasc Surg. 2005;19:379–385. doi: 10.1007/s10016-004-0130-6. [DOI] [PubMed] [Google Scholar]
- 2.Kim YW, Lee JH, Kim HG, Huh S. Factors affecting the long-term patency of crossover femorofemoral bypass graft. Eur J Vasc Endovasc Surg. 2005;30:376–380. doi: 10.1016/j.ejvs.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Roeder R, Wolfe J, Lianakis N, Hinson T, Geddes LA, Obermiller J. Compliance, elastic modulus, and burst pressure of small-intestine submucosa (SIS), small-diameter vascular grafts. J Biomed Mater Res. 1999;47:65–70. doi: 10.1002/(sici)1097-4636(199910)47:1<65::aid-jbm9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Andrée B, Bär A, Haverich A, Hilfiker A. Small intestinal submucosa segments as matrix for tissue engineering: review. Tissue Eng Part B Rev. 2013;19:279–291. doi: 10.1089/ten.TEB.2012.0583. [DOI] [PubMed] [Google Scholar]
- 5.John TT, Aggarwal N, Singla AK, Santucci RA. Intense inflammatory reaction with porcine small intestine submucosa pubovaginal sling or tape for stress urinary incontinence. Urology. 2008;72:1036–9. doi: 10.1016/j.urology.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DK, Hara H, Ezzelarab M, Bottino R, Trucco M, Phelps C, Ayares D, Dai Y. The potential of genetically-engineered pigs in providing an alternative source of organs and cells for transplantation. J Biomed Res. 2013;27:249. doi: 10.7555/JBR.27.20130063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham GA, Murray J, Billiar K, Sullivan SJ. Evaluation of the porcine intestinal collagen layer as a biomaterial. J Biomed Mater Res. 2000;51:442–52. doi: 10.1002/1097-4636(20000905)51:3<442::aid-jbm19>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Luo JC, Chen W, Chen XH, Qin TW, Huang YC, Xie HQ, Li XQ, Qian ZY, Yang ZM. A multi-step method for preparation of porcine small intestinal submucosa (SIS) Biomaterials. 2011;32:706–713. doi: 10.1016/j.biomaterials.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 10.Cui B, Johnson SP, Bullock N, Ali-Osman F, Bigner DD, Friedman HS. Decoupling of DNA damage response signaling from DNA damages underlies temozolomide resistance in glioblastoma cells. J Biomed Res. 2010;24:424–435. doi: 10.1016/S1674-8301(10)60057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 12.Hurst RE, Bonner RB. Mapping of the distribution of significant proteins and proteoglycans in small intestinal submucosa by fluorescence microscopy. J Biomater Sci Polym Ed. 2001;12:1267–1279. doi: 10.1163/156856201753395798. [DOI] [PubMed] [Google Scholar]
- 13.Musahl V, Abramowitch SD, Gilbert TW, Tsuda E, Wang JH, Badylak SF, Woo SL. The use of porcine small intestinal submucosa to enhance the healing of the medial collateral ligament--a functional tissue engineering study in rabbits. J Orthop Res. 2004;22:214–220. doi: 10.1016/S0736-0266(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 14.Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, Raeder RH, Metzger DW. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe JS, Ginsberg PC, Yanoshak SJ, Costa LE Jr, Ogbolu FN, Moyer CP, Greene CH, Finkelstein LH, Harkaway RC. Ureteral segment replacement using a circumferential small-intestinal submucosa xenogenic graft. J Invest Surg. 2001;14:259–265. doi: 10.1080/089419301753170039. [DOI] [PubMed] [Google Scholar]
- 16.Cobb MA, Badylak SF, Janas W, Simmons-Byrd A, Boop FA. Porcine small intestinal submucosa as a dural substitute. Surg Neurol. 1999;51:99–104. doi: 10.1016/s0090-3019(97)00475-8. [DOI] [PubMed] [Google Scholar]
- 17.Campodonico F, Benelli R, Michelazzi A, Ognio E, Toncini C, Maffezzini M. Bladder cell culture on small intestinal submucosa as bioscaffold: experimental study on engineered urothelial grafts. Eur Urol. 2004;46:531–537. doi: 10.1016/j.eururo.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Lindberg K, Badylak SF. Porcine small intestinal submucosa (SIS): a bioscaffold supporting in vitro primary human epidermal cell differentiation and synthesis of basement membrane proteins. Burns. 2001;27:254–266. doi: 10.1016/s0305-4179(00)00113-3. [DOI] [PubMed] [Google Scholar]
- 19.Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478–491. [PubMed] [Google Scholar]
- 20.Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11–24. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 21.Badylak S, Liang A, Record R, Tullius R, Hodde J. Endothelial cell adherence to small intestinal submucosa: an acellular bioscaffold. Biomaterials. 1999;20:2257–2263. doi: 10.1016/s0142-9612(99)00156-8. [DOI] [PubMed] [Google Scholar]


