Abstract
In the present study, we investigated whether CVVH can reduce HMGB1, TLR4, NF-κB and other serum cytokine levels, preventing organ injury in a dog sepsis model. A total of 10 dogs were injected with LPS and treated with either CVVH group (n = 5) or nothing (Control, n = 5) for 24 h. EILSA was used for examining the concentration of TNF-α, IL-6, HMGB 1 and TLR4. The histological change of lung, liver and kidney tissues was determined. The mRNA expression of HMGB1, TLR4 and NF-κB was examined by RT-PCR. The protein of HMGB1 and phosphated NF-κB was examined by Western-blot. The levels of serum HMGB1 came to the peak at 8 h, 16 h and then declined. The LPS-induced increase in HMGB1 level was suppressed by CVVH compared with Control. Likewise, serum TNF-α and IL-6 levels decreased with CVVH along with a significant improvement in the function of main organs. Histologic examination revealed significant reduction in inflammation in lung; liver and kidney tissues harvested 24 h after CVVH compared with Control. The mRNA of HMGB1, TLR4 and NF-κB in the kidney was expressed at high level after LPS administration, which was significantly decreased by CVVH. The increased protein expression of HMGB1 and phosphated NF-κB was reduced after CVVH compared with control. CVVH by reducing the level of HMGB1, TLR4, NF-κB and other cytokines could weaken the cascade of cytokines and restore the immune system, and reduce the damage of important organs in sepsis.
Keywords: CVVH, sepsis, HMGB1, TLR4, NF-κB
Introduction
Sepsis-related mortality is a leading cause of death and is increasing worldwide. Systemic inflammatory response is the most frequent pathological picture of sepsis and leads to fatal multiple organ failure. Studies have confirmed the occurrence of sepsis is not necessarily dependent on the continued existence of bacteria and bacterial toxins but uncontrolled release of inflammatory mediators and immune dysfunction.
Recently, HMGB1, a DNA-binding protein, has been recognized as a late inflammatory cytokine during sepsis that is actively released by monocytes and macrophages [1]. HMGB1 is released into the cytoplasm and promotes inflammation by integrating the activities of members of the toll-like receptor (TLR) family and the receptor for advanced glycation end products (RAGE) [2]. The uncontrolled release of cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-10 which can be enhanced by TLR4 and its related pathway, such as NF-κB pathway play a major role in the development of organ dysfunction during sepsis. Importantly, NF-κB acts as a transcription factor that mediates the cellular responses to a wide variety of extracellular stress stimuli [3]. It also triggers the upregulation of cytokines such as TNF-α and IL-6 to initiate the inflammatory responses and process of apoptosis.
Antibodies against HMGB1 showed significant protection against endotoxin-induced lethality through decreasing the level of the early proinflammatory cytokine in mice [4]. Subsequently, patients with sepsis have elevated serum levels of HMGB1, and higher levels, in some studies are associated with an increased mortality, suggesting that clinical intervention by blocking or decreasing HMGB1 might be a viable treatment option [5,6]. The reduction of HMGB1/TLR4/NF-κB-mediated inflammatory response has been demonstrated as neuroprotective effect against acute brain injury [7].
To our knowledge, HMGB1/TLR4/NF-κB has not been studied in sepsis animal models treated by CVVH. The aim of our study was to evaluate the effects of continuous hemodiafiltration therapy on lung/liver/kidney injury in a lipopolysaccharide (LPS)-induced systemic inflammatory dog model. We hypothesized that continuous hemodiafiltration therapy can reduce HMGB1/TLR4/NF-κB levels, thus prevent those organs’ injury.
Materials and methods
Establishment of model with MODS and ARF
Healthy hybridization Beagle dogs (n = 10), weighing 14 to 19 kg, were bought from the Xingang group in Shanghai Animal Institute (Animal Production License No: SCXK (Shanghai) 2007-0009). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Shandong Provincial hospital. All dogs were put on fasting regimen and acclimated to the experimental circumstance for 17 to 20 hours. The animals received 3% pentobarbital sodium for anesthesia. The animals were prepared for study using a protocol previously reported in detail [8]. The right femoral vein was separated and a double-lumen catheter was inserted. Septic shock was induced by instillation of 1.0 mg·kg-1 of LPS by intravenous injection. All dogs received identical volume resuscitation protocols, and neither vasopressor nor inotropic agents.
Immediately after LPS administration, the dogs were divided into two groups randomly: the CVVH group (n = 5), which received treatment with CVVH; and the control group (n = 5), which did not receive any blood purification treatment.
The CVVH group had extracorporeal circulation established through the femoral vein double-lumen catheter. All access lines were secured, and the dialysis lines were connected to a continuous venvein hemofiltration (CVVH) machine (Asahi Kasei, Gambro Lundia AB, Sundsvall, Sweden) with an AV400 hollow-fiber dialyzer (Fresenius, Walnut Creek, CA). Extracorporeal blood flow was regulated at 80 ml/min. Anticoagulation was performed by heparin. The first dose was 2 mg, and 1 mg/h was used in the subsequent time. Net ultrafiltrate volume was monitored, and replacement solution was infused by predilution on a 1:1 volume replacement basis. The ingredients of the replacement solution were Na 135 mmol/L, K 2.0 mmol/L, Cl 108 mmol/L, Ca 1.75 mmol/L, Mg 0.75 mmol/L, HCO3 35.4 mmol/L, glucose 1.5 g/L. The osmotic pressure of the solution was 290.00 mOsm/L. No antibiotics were applied in any groups.
Indices of observation were 1) Vital signs: temperature, heart rate, respiratory rate, and mean artery pressure (MAP) were recorded every 15 minutes. 2) Measurement of organ function: blood biochemical markers, including electrolytes, urea nitrogen (BUN), creatinine (Cr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), white blood cell were examined at 0h, 2 h, 4 h, 8 h, 16 h, 24 h. Arterial blood gas was tested at 0 hours and 24 hours. 3) Cytokine assay: Serum samples were gathered at the same time point as above and stored in -80°C for later cytokines assay.
Dogs were sacrificed under pentobarbitone anesthesia at 24 h after LPS administration. Lung, livers and kidneys were quickly removed and stored for further analysis.
Histopathology
All the tissues were stained with hematoxylin and eosin. A pathologist blinded to each treatment protocol utilized the technique of Murakami et al to evaluate lung injury [9]. Briefly, 24 areas of lung parenchyma were graded on a scale of 0 to 4 for the degree of congestion, edema, inflammation, and hemorrhage (0, no abnormalities, normal tissue; 1, light; 2, moderate; 3, strong; 4, intense). Results were expressed as the mean score for each parameter. Likewise, a pathologist blinded to each treatment evaluated the extent of liver injury according to the technique of Heijnen et al [10]. Briefly, liver injury was reported as the sum of the individual scores for cytoplasmic color fading, vacuolization, nuclear condensation, nuclear fragmentation, nuclear fading, and erythrocyte stasis (0, no findings; 1, mild; 2, moderate; 3, severe). 20 visions were selected in each slice of renal external medulla; renal damage was assessed as tubular necrosis, atrophy, dilation, brush border loss, tubular formation, inflammatory cell infiltration and cell swollen. A pathologist blinded to each treatment protocol utilized the technique of Rabb et al to evaluate renal injury (0, normal; 1, minor; 2, mild; 3, moderate; 4, severe) [11].
RT-PCR
Total RNA was isolated from the collected liver tissues using TRIzol Reagent (Takara Japan, Shiga, Japan). The RNA was reverse transcribed into cDNA according to the manufacturer’s instructions. Equal quantities of cDNA were continuously amplified by PCR in a 10L reaction volume. The primers used for RT PCR (Table 1). PCR amplification was performed for 35 cycles of 30 seconds at 94°C, 50 seconds at 55°C, and 50 seconds at 72°C, followed by a final step of 7 minutes at 72°C. PCR products were detected by agarose gel electrophoresis in 2% NuSieve agarose gels and visualized by ethidium bromide staining. The intensity of the bands was quantified using ImageJ software, and the ratios of each gene product normalized to β-actin product were considered as the expression of each gene
Table 1.
The primers used for RT PCR
| Gene | Primer sequence (5’→3’) |
|---|---|
| NF-κB | Forward ATGGCAGACGATGATCCCTAC |
| Reverse CGGATCGAAATCCCCTCTGTT | |
| TLR4 | Forward GCCTTTCAGGGAATTAAGCTCC |
| Reverse GATCAACCGATGGACGTGTAAA | |
| HMGB1 | Forward GCATCCTGGCTTATCCATTGG |
| Reverse GGCTGCTTGTCATCTGCTG |
ELISA
The concentration of IL-6, HMGB1 and TNF-α were detected by enzyme-linked immunosorbent assay (ELISA), following the protocol of the specific kits (Biosource Co., Camarillo, CA, and R&D Systems Co., Minneapolis, MN). Serum levels of TLR4 were determined by ELISA according to the instructions provided with the kits (Beijing Jingmei Co, Beijing, China).
Western blotting
Proteins were extracted from kidney stored at -80°C for western blotting analysis. The proteins were then incubated in boiling water for 10 min, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a Polyvinylidene Fluoride Membrane (PVDF membrane), blocked with 5% milk for 1 h, and incubated overnight with anti-HMGB1 (Abcam, Cambridge, MA) and NF-κB antibodies (Abcam, Cambridge, MA) at 4°C. After the samples were incubated with a secondary antibody for 30 min at 37°C, the signal was detected with the Odyssey Two-Color Infrared Laser Imaging System.
Statistical analyses
All data are expressed as mean ± standard deviation. All analyses were performed by SPSS17.0 for Windows software. A value of P < 0.05 was considered statistically significant. All cytokines and vital sign data were analyzed by repeated measures ANOVA.
Results
General and indictors of major organs
After 2 h injection of LPS all dogs appeared to be chilling. At 4 h diffuse dry rales could be heard in the lungs. All the dogs pulled large yellow watery pus stool.
At 4 h the serum levels of SCr, BUN were significantly increased in the all dogs. But in the CVVH group kidney function was improved. The increasing levels of AST, ALT, WBC were significantly decreased by CVVH (P < 0.05) (Table 2).
Table 2.
Serum levels of AST, ALT, SCr, BUN and WBC (x̅ ± s)
| Group/n | Time | AST (U/L) | ALT (U/L) | SCr (μmol/L) | BUN (mmol/L) | WBC (× 109/L) |
|---|---|---|---|---|---|---|
| Control (n = 5) | 0 h | 26.00 ± 6.60 | 21.33 ± 5.92 | 55.74 ± 14.34 | 5.21 ± 0.98 | 5.58 ± 1.85 |
| 2 h | 76.00 ± 17.75 | 62.67 ± 12.56 | 68.11 ± 9.63 | 8.45 ± 2.00 | 11.07 ± 1.72 | |
| 4 h | 217.33 ± 42.26 | 147.33 ± 36.02 | 104.52 ± 13.07 | 13.44 ± 2.01 | 16.78 ± 2.27 | |
| 8 h | 414.00 ± 36.19 | 278.50 ± 50.82 | 215.46 ± 28.75 | 17.59 ± 1.48 | 25.32 ± 3.87 | |
| 16 h | 653.17 ± 88.25 | 494.33 ± 49.18 | 304.96 ± 41.07 | 22.30 ± 2.73 | 28.66 ± 1.42 | |
| 24 h | 525.67 ± 76.57 | 577.67 ± 52.55 | 438.75 ± 50.06 | 27.13 ± 3.09 | 26.91 ± 2.03 | |
| CVVH (n = 5) | 0 h | 32.17 ± 6.62 | 24.00 ± 8.10 | 53.48 ± 10.56 | 4.94 ± 1.36 | 5.36 ± 1.37 |
| 2 h | 67.67 ± 12.08 | 68.00 ± 16.49 | 66.79 ± 10.29 | 7.86 ± 1.61 | 9.50 ± 1.39 | |
| 4 h | 185.00 ± 34.96 | 127.50 ± 22.06 | 86.77 ± 8.46* | 10.26 ± 2.18* | 16.12 ± 1.49 | |
| 8 h | 333.5 ± 25.91* | 232.50 ± 45.52* | 168.02 ± 17.69* | 12.74 ± 3.19* | 19.22 ± 2.20* | |
| 16 h | 446.33 ± 48.13* | 380.50 ± 47.35* | 189.02 ± 26.97* | 14.97 ± 2.05* | 20.84 ± 3.03* | |
| 24 h | 375.50 ± 32.97* | 444.83 ± 47.39* | 230.76 ± 20.37* | 16.81 ± 2.20* | 19.65 ± 2.03* |
P < 0.05, compared with Control group.
Serum levels of AST, ALT, SCr, BUN and WBC induced by lipopolysaccharide (LPS) were reduced by CVVH. P < 0.05 compared with Control group.
After 24 h injection of LPS respiratory failure was showed in the two groups; arterial oxygen pressure in the treatment group by CVVH was significantly higher than that of control group; CVVH showed to reduce the high carbon dioxide pressure which induced by LPS, the difference was statistically significant (P < 0.05) (Table 3).
Table 3.
The change of arterial blood gas (x̅ ± s)
| Group | PaO2 (mmHg) | PaCO2 (mmHg) | ||
|---|---|---|---|---|
|
| ||||
| 0 h | 24 h | 0 h | 24 h | |
| Control (n = 5) | 96.70 ± 1.89 | 43.70 ± 4.31▲ | 30.09 ± 2.37 | 66.01 ± 4.27▲ |
| CVVH (n = 5) | 95.96 ± 1.94 | 55.70 ± 6.29▲,* | 32.23 ± 2.96 | 56.78 ± 2.89▲,* |
P < 0.05, compared with Control group at 24 h;
P < 0.05 compared with the same group at 0 h.
Change of serum cytokines
Two hours after LPS treatment, serum TNF-α level was increased in the control group, and significantly reduced by CVVH. Likewise, serum IL-6 was induced at 4 h after LPS administration in the control group. However, CVVH reduced IL-6 levels. Both of them increased to the first peak, and then decreased to the bottom which also significantly was higher than that of control group at 8 hours. At 16 hours Serum TNF-α and IL-6 increased to the second peak (Figure 1A, 1B).
Figure 1.
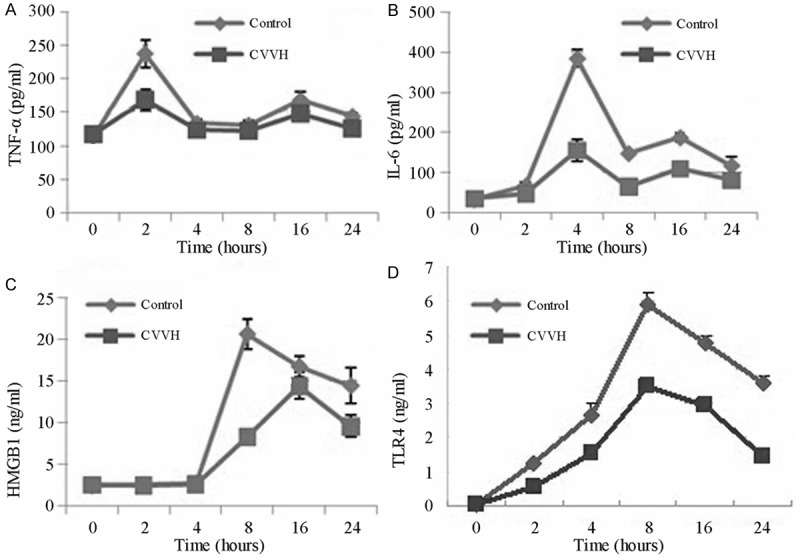
The level of Serum cytokines at 0 h, 2 h, 4 h, 8 h, 16 h, 24 h. The levels of HMGB1 and TLR4 of control group and CVVH group came to the peak at 8 h and 16 h and then declined (C, D). The LPS-induced increase in HMGB1 and TLR4 levels was suppressed in the CVVH group compared with the Control group. Likewise, LPS-induced serum TNF-α and IL-6 levels decreased with continuous hemodiafiltration (A, B).
Furthermore, the LPS-induced increase in HMGB1 levels was suppressed in the CVVH group compared with the control group (Figure 1C). The time point of HMGB1 peak was delayed from 8th to 16th hour by CVVH.
Results also showed that the expression of TLR4 was only significantly increased in control group. However, CVVH treatment resulted in the downregulation of TLR4 (Figure 1D).
Pathological changes of liver, lung, renal
Under light microscopy, liver morphology was observed in the Control group whereas marked interstitial bleeding and inflammatory cell infiltration were detected in liver tissue (Figure 2A, 2B). Interstitial bleeding and inflammatory cell infiltration were slightly reduced in the CVVH group.
Figure 2.
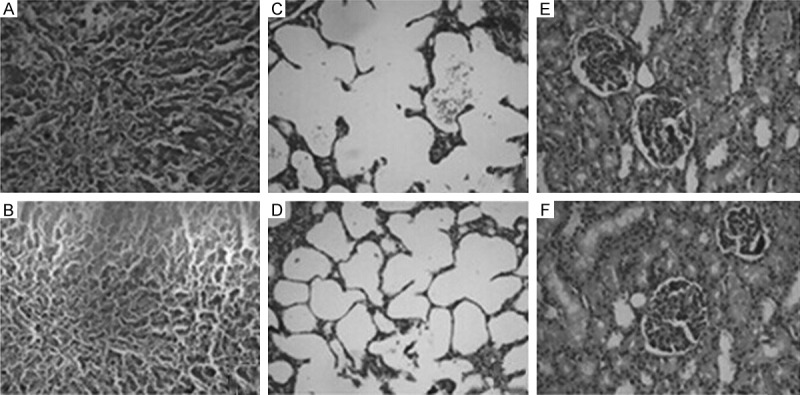
Lung, liver and kidney histology after LPS-induced systemic inflammation. Rats were no treated with CVVH (Control group), LPS followed by CVVH (CVVH group). Representative liver sections are shown for (A) Control, (B) CVVH group. Representative lung specimens are shown for (C) Control group, (D) CVVH group. Representative kidney specimens are shown for (E) Control group, (F) CVVH group. All sections were stained with hematoxylin and eosin, and magnified at 200.
Similarly, it showed alveolar rupture, shrinking, atrophy, substantial interstitial edema and inflammatory cell infiltration, interstitial and alveolar hyperemia, edema surrounding capillaries in the control group. Lung histology significantly was improved in the CVVH group compared with the control group (Figure 2C, 2D).
In the control group kidney glomerular appeared to be generally normal, tubular epithelial cell showed to be swelling, fatty degeneration and vacuolar degeneration, varied degrees of necrosis of renal tubular epithelial cells. Necrotic tubular epithelial cells shed into the lumen, and vague granular structure was showed. Interstitial edema and inflammatory cell infiltration was seen surrounding of distal tubule and collecting duct tubules. Under light microscopy in the CVVH group it showed that mild swelling, degeneration and necrosis of renal tubular epithelial cells, interstitial edema and inflammatory cell infiltration were improved (Figure 2E, 2F).
The CVVH group exhibited lower injury scores compared with the control group (P < 0.05). Liver and kidney histology scores showed a similar pattern (P < 0.05).
Change of cytokines in the supernatant of liver, lung, renal
We found that the expression of TLR4 in the supernatant of liver was significantly increased by LPS administration, and which was decreased by CVVH (P < 0.05). Compared with control HMGB1 also was downregulated by treatment of CVVH (P < 0.05). At same time CVVH showed to decrease the level of TNF-α and IL-6 (P < 0.05) (Table 4). Lung and kidney showed a similar pattern in those cytokines (P < 0.05) (Table 4).
Table 4.
The level of cytokines in supernatant of liver, lung, renal
| Tissue | Group | TNF-α (pg/ml) | IL-6 (pg/ml) | HMGB1 (ng/ml) | TLR4 (ng/ml) |
|---|---|---|---|---|---|
| liver | control | 567.9 ± 91.15 | 753.74 ± 131.31 | 59.82 ± 8.27 | 9.82 ± 1.27 |
| CVVH | 355.79 ± 71.21* | 447.11 ± 87.81* | 48.68 ± 4.56▲ | 5.68 ± 1.46▲ | |
| lung | control | 498.7 ± 79.68 | 610.3 ± 84.98 | 63.09 ± 5.02 | 8.09 ± 1.02 |
| CVVH | 345.6 ± 50.38* | 413.86 ± 89.02* | 47.81 ± 5.57* | 4.81 ± 1.17* | |
| kidney | control | 345.79 ± 47.17 | 480.38 ± 52.50 | 38.59 ± 3.06 | 8.59 ± 1.06 |
| CVVH | 284.95 ± 21.98▲ | 380.89 ± 32.29* | 34.05 ± 3.44▲ | 4.05 ± 1.14▲ |
P < 0.05, compared with control group;
P < 0.01, compared with control group.
Expression of HMGB1/TLR4/NF-κB in kidney
The mRNA levels of HMGB1, TLR4 and NF-κB were expressed at high levels in the dog kidney after LPS administration. But compared with the control group the mRNA levels of HMGB1, TLR4 and NF-κB were significantly decreased after CVVH treatment (P < 0.05) (Figure 3).
Figure 3.
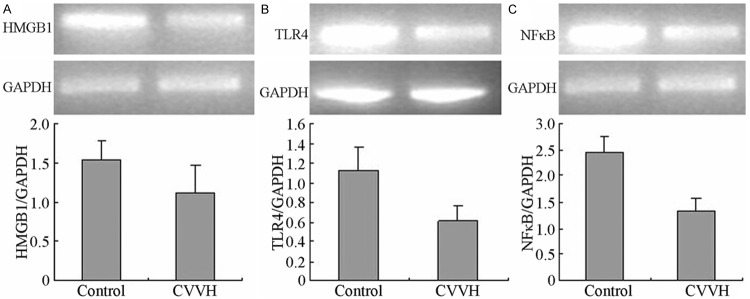
The mRNA level of HMGB1, TLR4 and NFκB in kidney. The LPS-induced increase in HMGB1, TLR4 and NFκB mRNA levels was suppressed in the CVVH group compared with the Control group (P < 0.05).
Because it was difficult to buy the antibody of TLR4 for dog, we only examined the protein expression of HMGB1 and phosphorylated NF-κB in kiney. The westen blot showed high levels of HMGB1 and phosphorylated NF-κB protein in the control group. But the protein levels of HMGB1 and phosphorylated NF-κB were significantly decreased in the kidney following 24 CVVH treatment compared with control group (P < 0.05) (Figure 4).
Figure 4.
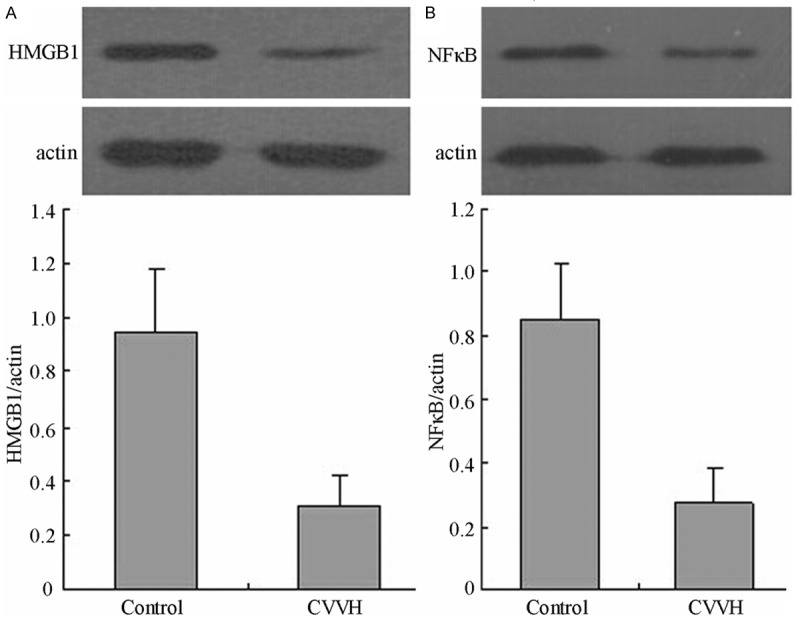
The protein level of HMGB1and NFκB in kidney. The LPS-induced increase in HMGB1 and NFκB protein levels was decreased in the CVVH group compared with the Control group (P < 0.05).
Discussion
In the present study, we demonstrated that CVVH exerts beneficial effects in a dog sepsis model. By treatment of CVVH, the increased inflammatory cytokines such as HMGB1, TLR4, NF-κB, other cytokines were well decreased, which result in the well improvement of damaged organs (liver, lung, kidney).
First, early after stimulation of LPS inflammatory cytokines (TNF-α, IL-6) increased, which stimulate monocytes and macrophages to secrete other inflammatory mediators such as IL-1, IL-8 and so on [12,13]. Interaction of increased inflammatory mediators lead B cell differentiation and produce antibodies, and promote tissue cell protein synthesis of acute phase response.
Elevated inflammatory mediators and damaged cells stimulate the release of nuclear HMGB1 to the extracellular, further mediates the occurrence of sepsis and the development of organ and tissue damage [14,15]. Some studies suggest that HMGB1 serves as a late mediator of lethality in sepsis [16,17]. As an intracellular protein HMGB1can translocate to the nucleus where it binds DNA and regulates gene expression. Importantly, HMGB1 induces migration of stem cells toward inflamed regions to promote repair and regeneration [18]. Furthermore, it results in increased mesangioblast and endothelial proliferation and migration to sites of inflammation and induces transit of these cells across the endothelial layer [19,20].
Sunden-Cullberg et al noted that HMGB1 levels were significantly higher in sepsis patients compared with healthy controls [5]. The increased level of HMGB1 likely reflects a systemic inflammatory response syndrome (SIRS) in patients following post-injury events [21,22].
In our study, we found the systemic serum and local kidney expression of HMGB1 was increased after LPS stimulation in a dog sepsis models. We also found that LPS-induced increasing of HMGB1 as a late metabolism of inflammatory cytokines in no treatment group reached the peak at 8 h, which was significantly later than that of TNF-α and IL-6. TNF-α, IL-6 levels decreased to the lowest value at 8 h, and then increased to a second peak at 16 h. The phenomenon indicated that the increase of HMGB1 further induced release of TNF-α, IL-6 lately, which reflected the co-activation between early inflammatory cytokines and HMGB1.
In vitro studies have demonstrated that the HMGB1-stimulated inflammatory response could be mediated mostly through receptors for advanced glycation end products (RAGE) [23] and TLR4 [24,25]. It has been reported that TLR4-deficient mice exhibited reduced cerbral ischemia-reperfusion injury as well as downregulation of inflammatory cytokines, suggesting that TLR4 plays an important role in inflammatory response [26]. Recent studies suggest that HMGB1 interacts with TLR4 and increases TLR-mediated NF-κB activation [27,28]. TLR4 receptors and NF-κB signaling pathway play a critical role in the inflammatory response [29-31]. The role of NF-κB activation in septic pathophysiology and the signal transduction pathways leading to NF-κB activation during sepsis/septic shock have been investigated extensively [32]. NF-κB acts as a transcription factor that triggers the upregulation of cytokines such as TNF-α and IL-6 to initiate the inflammatory responses and process of apoptosis. We observed that LPS stimulation increased the association between HMGB1 and TLR4, suggesting that HMGB1 could, in turn, activate the TLR4-mediated NF-κB signaling pathway.
In this study we showed that HMGB1/TLR4/NF-κB and proinflammatory mediates, such as TNF-α, IL-6 were significantly downregulated after CVVH treatment in a dog sepsis model.
HMGB1 is a 25 kd protein. TLR4 consists of 619 amino acids and predicts a molecular mass of 66 Kd. NF-κB molecular weight is 60 kd. It is widely known that CVVH could well remove the macromolecular protein especially inflammatory factors by convection. Compared with NF-κB and TLR4, HMGB1 was better cleared. The decreased TLR4 and NF-κB were resulted from the removement of CVVH and the reduction of HMGB1 activation.
Together these results indicate that inhibition of HMGB1/TLR4/NF-κB pathway by CVVH resulted in less apoptosis and better functional recovery. The indicators of major organ function (AST, ALT, Cr, BUN) were significantly decreased after CVVH treatment. At the same time, arterial oxygen pressure was increased significantly. The histological change of lung, liver and kidney was significantly improved.
We concluded that continuous blood purification therapy by reducing the level of HMGB1/TLR4/NF-κB and other cytokines could weaken the cascade of cytokines and restore the body’s immune system, and reduce the damage of important organs in sepsis.
The research was approved by the Ethical Committee of Shandong Provincial hospital regarding the animal experiment.
This article is subjected by the Young Scientists Fund of the Department of Science and Technology of Shandong Province.
Disclosure of conflict of interest
None.
References
- 1.Bae JS. Role of high mobility group box 1 in inflammatory disease: focus on sepsis. Arch Pharm Res. 2012;35:1511–1523. doi: 10.1007/s12272-012-0901-5. [DOI] [PubMed] [Google Scholar]
- 2.Tang D, Kang R, Zeh HJ 3rd, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14:1315–1335. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung HS, Joo JD, Kim DW, In JH, Roh M, Jeong JT, Noh SJ, Choi JW. Effect of milrinone on the inflammatory response and NF-kB activation in renal ischemia-reperfusion injury in mice. Korean J Anesthesiol. 2014;66:136–142. doi: 10.4097/kjae.2014.66.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uzawa A, Mori M, Taniguchi J, Masuda S, Muto M, Kuwabara S. Anti-high mobility group box 1 monoclonal antibody ameliorates experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2013;172:37–43. doi: 10.1111/cei.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 6.Gibot S, Massin F, Cravoisy A, Barraud D, Nace L, Levy B, Bollaert PE. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med. 2007;33:1347–1353. doi: 10.1007/s00134-007-0691-2. [DOI] [PubMed] [Google Scholar]
- 7.Shen M, Lu J, Dai W, Wang F, Xu L, Chen K, He L, Cheng P, Zhang Y, Wang C, Wu D, Yang J, Zhu R, Zhang H, Zhou Y, Guo C. Ethyl pyruvate ameliorates hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway of apoptosis and autophagy. Mediators Inflamm. 2013;2013:461536. doi: 10.1155/2013/461536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humes HD, Buffington DA, Lou L, Abrishami S, Wang M, Xia J, Fissell WH. Cell therapy with a tissue-engineered kidney reduces the multiple-organ consequences of septic shock. Crit Care Med. 2003;31:2421–2428. doi: 10.1097/01.CCM.0000089644.70597.C1. [DOI] [PubMed] [Google Scholar]
- 9.Murakami K, McGuire R, Cox RA, Jodoin JM, Bjertnaes LJ, Katahira J, Traber LD, Schmalstieg FC, Hawkins HK, Herndon DN, Traber DL. Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock. 2002;18:236–241. doi: 10.1097/00024382-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Heijnen BH, Straatsburg IH, Gouma DJ, van Gulik TM. Decrease in core liver temperature with 10 degrees C by in situ hypothermic perfusion under total hepatic vascular exclusion reduces liver ischemia and reperfusion injury during partial hepatectomy in pigs. Surgery. 2003;134:806–817. doi: 10.1016/s0039-6060(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 11.Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. doi: 10.1038/ki.1997.200. [DOI] [PubMed] [Google Scholar]
- 12.Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- 13.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 14.He GZ, Zhou KG, Zhang R, Wang YK, Chen XF. Impact of intestinal ischemia/reperfusion and lymph drainage on distant organs in rats. World J Gastroenterol. 2012;18:7271–7278. doi: 10.3748/wjg.v18.i48.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong Y, Tang Z, Yang T, Yang Y, Yang L, Shen W, Chen W. Ulinastatin preconditioning attenuates inflammatory reaction of hepatic ischemia reperfusion injury in rats via high mobility group box 1 (HMGB1) inhibition. Int J Med Sci. 2014;11:337–343. doi: 10.7150/ijms.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chagnon F, Metz CN, Bucala R, Lesur O. Endotoxin-induced myocardial dysfunction effects of macrophage migration inhibitory factor neutralization. Circ Res. 2005;96:1095–1102. doi: 10.1161/01.RES.0000168327.22888.4d. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, Bellussi LM, Passali D, Chen L. LPS may enhance expression and release of HMGB1 in human nasal epithelial cells in vitro. Acta Otorhinolaryngol Ital. 2013;33:398–404. [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiike S, Hiramatsu T, Shiraishi M, Ueda Y, Tsuchida H. Relationship between vascular reactivity and expression of HMGB1 in a rat model of septic aorta. J Anesth. 2013;27:684–692. doi: 10.1007/s00540-013-1584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176:12–15. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 20.Yanai H, Matsuda A, An J, Koshiba R, Nishio J, Negishi H, Ikushima H, Onoe T, Ohdan H, Yoshida N, Taniguchi T. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc Natl Acad Sci U S A. 2013;110:20699–20704. doi: 10.1073/pnas.1320808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aller MA, Arias JI, Alonso-Poza A, Arias J. A review of metabolic staging in severely injured patients. Scand J Trauma Resusc Emerg Med. 2010;18:27. doi: 10.1186/1757-7241-18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comstedt P, Storgaard M, Lassen AT. The Systemic Inflammatory Response Syndrome (SIRS) in acutely hospitalised medical patients: a cohort study. Scand J Trauma Resusc Emerg Med. 2009;17:67. doi: 10.1186/1757-7241-17-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu D, Young J, Song D, Esko JD. Heparan sulfate is essential for high mobility group protein 1 (HMGB1) signaling by the receptor for advanced glycation end products (RAGE) J Biol Chem. 2011;286:41736–41744. doi: 10.1074/jbc.M111.299685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 25.Velegraki M, Papakonstanti E, Mavroudi I, Psyllaki M, Tsatsanis C, Oulas A, Iliopoulos I, Katonis P, Papadaki HA. Impaired clearance of apoptotic cells leads to HMGB1 release in the bone marrow of patients with myelodysplastic syndromes and induces TLR4-mediated cytokine production. Haematologica. 2013;98:1206–1215. doi: 10.3324/haematol.2012.064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang QW, Lu FL, Zhou Y, Wang L, Zhong Q, Lin S, Xiang J, Li JC, Fang CQ, Wang JZ. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab. 2011;31:593–605. doi: 10.1038/jcbfm.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-κB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCauley MJ, Rueter EM, Rouzina I, Maher LJ 3rd, Williams MC. Single-molecule kinetics reveal microscopic mechanism by which High-Mobility Group B proteins alter DNA flexibility. Nucleic Acids Res. 2013;41:167–181. doi: 10.1093/nar/gks1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen G, Zhang S, Shi J, Ai J, Qi M, Hang C. Simvastatin reduces secondary brain injury caused by cortical contusion in rats: possible involvement of TLR4/NF-kappaB pathway. Exp Neurol. 2009;216:398–406. doi: 10.1016/j.expneurol.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Nace GW, Huang H, Klune JR, Eid RE, Rosborough BR, Korff S, Li S, Shapiro RA, Stolz DB, Sodhi CP, Hackam DJ, Geller DA, Billiar TR, Tsung A. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury. Hepatology. 2013;58:374–387. doi: 10.1002/hep.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabadi MM, Ghaly T, Goligorksy MS, Ratliff BB. HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol. 2012;303:F873–885. doi: 10.1152/ajprenal.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ha T, Xia Y, Liu X, Lu C, Liu L, Kelley J, Kalbfleisch J, Kao RL, Williams DL, Li C. Glucan phosphate attenuates myocardial HMGB1 translocation in severe sepsis through inhibiting NF-κB activation. Am J Physiol Heart Circ Physiol. 2011;301:H848–855. doi: 10.1152/ajpheart.01007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


