Abstract
The aim of our study is to investigate the effects of ovarian steroid hormones on focal adhesion kinase (FAK) expression in ESCs and whether there is alteration in women with endometriosis. FAK expression was assessed by western blotting analysis. Elevated expression of FAK was seen in the cultured ESCs treated with estrogen (P < 0.05). Expression of FAK protein was not changed in ESCs after treated by progesterone or treated by estrogen and progesterone. The level of up-regulation by estrogen in endometriosis is significantly higher than that from women without endometriosis (P < 0.05). FAK expression in endometrial stromal cells from endometriosis was more sensitive to estrogen, which might contribute to the pathogenesis and progress of endometriosis.
Keywords: Endometriosis, focal adhesion kinase, endometrial stromal cells, ovarian steroid hormone
Introduction
Endometriosis is one of the most common causes of infertility and chronic pelvic pain. Its incidence is estimated to be as high as 30% in patients with infertility and 45% in patients with chronic pelvic pain [1]. Despite the fact that this disease has gained increasing interest in recent years, the pathogenesis still remains controversial. With over 10,000 scientific publications on the topic, Sampson’s theory of implantation of endometrial cells and fragments refluxed during the menstrual period is generally accepted among these hypotheses [2]. According to this theory, the primary defect in endometriosis may be located in the eutopic endometrium. Abnormalities inherent to the eutopic endometrium might therefore contribute to the ectopic growth outside the uterine cavity [3]. The adhesion of retrograde endometrial cells to the extracellular matrix is one of the first necessary stages in the framework of the implantation theory. As reported elsewhere, a number of factors are involved in the process of cell adhesion, and interaction with the extracellular matrix. Among them, the FAK gene encodes a non-receptor tyrosine kinase that localizes at focal adhesions, contact points of cells with extracellular matrix and is activated by integrin (cell surface receptor) signaling or by growth factor receptor (c-Met, EGFR, PDGFR) or by angiogenesis receptors [4].
In recent years, research on endometriosis has paid increased attention to the changes in endometrial stromal cells (ESCS) function. Recent evidence has demonstrated that estradiol regulate epithelial proliferation via paracrine mechanisms requiring the appropriate receptor in the stroma [5]. And a series of research has shown that active contraction and migration of ESCS in patients with endometriosis [6,7]. So to explore the mechanism changed in ESCS may be helpful to learn the pathogenesis of endometriosis.
In the previous study, we found that a significant increase of FAK expression in endometrial tissues of women with endometriosis, a relationship between FAK expression and disease stage, pelvic pain, and serum steroid hormones [8]. Furthermore, the endometrial FAK protein expression varied with the serum level of estrogen and the ratio of estrogen to progesterone [8]. These findings suggested that FAK might contribute to the pathogenesis of endometriosis and be regulated by ovarian steroid hormones. To the best of our knowledge, it is still unclear the relationship between FAK expression in ESCs and ovarian steroid hormones. So the purpose of our present study was to investigate the effects of ovarian steroid hormones on the expression of FAK in cultured ESCs and whether there is alteration in the reaction of ESCs in women with endometriosis.
Materials and methods
Patients and sample collection
Ethical approval for this project was granted by the Ethics Committee of School of Medicine, Zhejiang University. A total of 32 women in reproductive age volunteered for this study. All of them had normal menstrual cycles (28-32 days) and had not received any anti-inflammatory or hormonal treatment for at least 6 months before inclusion in the study and surgery. In all patients, 15 women (aged 25-39 years) consulting for ovarian endometriotic cyst had been identified as endometriosis by laparoscopy and succulent histology. Control subjects were 17 women (aged 28-38 years) undergoing surgery for hysterectomy for benign indications and having no visible evidence of endometriosis.
Tissue collection, cell isolation and culture
All the eutopic endometrial tissues were collected by curettage under sterile conditions and transported to the laboratory on ice in DMEM/F-12 (Gibco) with 10% FCS (Hyclone, Logan, UT, USA). The endometrial tissues were digested with collagenase type IV (0.1%; Sigma) for 30 min at 37.8°C with constant agitation for recovering ESCs. The ESCs were separated from epithelial cells by passing them over sterile gauzes (pore diameter sizes: 400 mesh). The ESCs were placed in a culture flask, and allowed to adhere for 20 min. The adherent stromal cells were cultured as monolayer in flasks with DMEM/F-12 containing 10% FCS and 20 mmol/l HEPES and incubated in 5% CO2 at 37.8°C. This method supplied a 95% purity of ESCs.
Immunocytochemistry
ESCS from different patients growing on coverslips were cultured for 48 h. The cover-slips were fixed in 4% paraformaldehyde for 20 min at room temperature, washed in PBS and permeabilized for 10 min with 0.25% Triton-100 in PBS. Nonspecific binding was blocked with normal nonimmune serum for 30 minutes. Slides were incubated with primary antibody at a 1:100 dilution in PBS for 2 hours at room temperature. The anti-human vimentin monoclonal antibody (sc-7558; Santa Cruz Biotechnology, Santa Cruz, CA, USA) as markers for ESCs, and cytokeratin-7 antibodies (sc-17118; Santa Cruz Biotechnology, Santa Cruz, CA, USA) as markers for glandular epithelial cells were then added. After three 5-minute washes with PBS, the slides were incubated for 30 minutes with a secondary antibody, anti-IgG horseradish peroxidase (P0488; DAKO Cyto-mation, Carpinteria, CA, USA). After another washing and following reaction with diaminobenzidine (DAKO Cytomation), the sections were counterstained with hematoxylin, dehydrated, and mounted in DPX. Slides incubated with rabbit immunoglobulins at the same dilution as the primary antibody were used as negative controls. The experiments were repeated three times from all endometrial tissues.
Treatment in vitro with ovarian steroid hormones
After starvation for 12 h, the ESCs (cells/well; FCS of cultured media was the charcoal stripped FCS) from women with or without endometriosis were treated, respectively, with estrogen, progesterone and both estrogen and progesterone (Sigma) for 24 h. The concentration of estrogen and progestin were 10-7 M and 10-8 M respectively.
Western blotting analysis
ESCs were lysed in lysis buffer. The protein concentration in the supernate was determined by Bradford Assay (Bio-Rad Laboratories, Hercules, CA, USA). 50 μg of proteins were added into 2 × SDS buffer and heated at 95°C for 5 min. Samples at 50 μg/lane were separated on a 8% SDS-polyacrylamide gel and transferred to Immun-Blot Nitrocellulose Transfer membrane (PROTRAN, Schleicher & Schuell, Bioscience, Dassel, Germany).
After blocking for 1 h with 5% non-fat milk powder in Tween-20 TBS (TBST), the membranes were incubated with 1:200 dilution of FAK (sc-932, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and ERα (sc-73479, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and 1:400 dilution of β-actin primary antibody (Santa Cruz) at 4°C overnight. After several washes with TBST, the membranes were incubated for 2 h at room temperature with 1:5000 dilution of the second antibody-labeled horseradish peroxidase (HRP) (Santa Cruz). The bound antibody was detected using an ECL detection reagent (Santa Cruz). The bands were scanned by Quantity One software (Bio-Rad). Normalized densities were determined with ratio of band density of FAK to band density of β-actin. This experiment was performed by using ESCS from different individuals.
Statistical analysis
All data were in normal distribution and statistical analysis of ratios of FAK to endoreference was performed using one-way ANOVA by SPSS 11.5 software. And results were expressed as means ± SD. Single comparisons using Student’s t test. Probability values less than 0.05 were regarded as significant.
Results
Immunocytochemistry
Immunocytochemistry showed more than 95% vimentin-positive and cytokeratin-negative ESCs (Figure 1).
Figure 1.
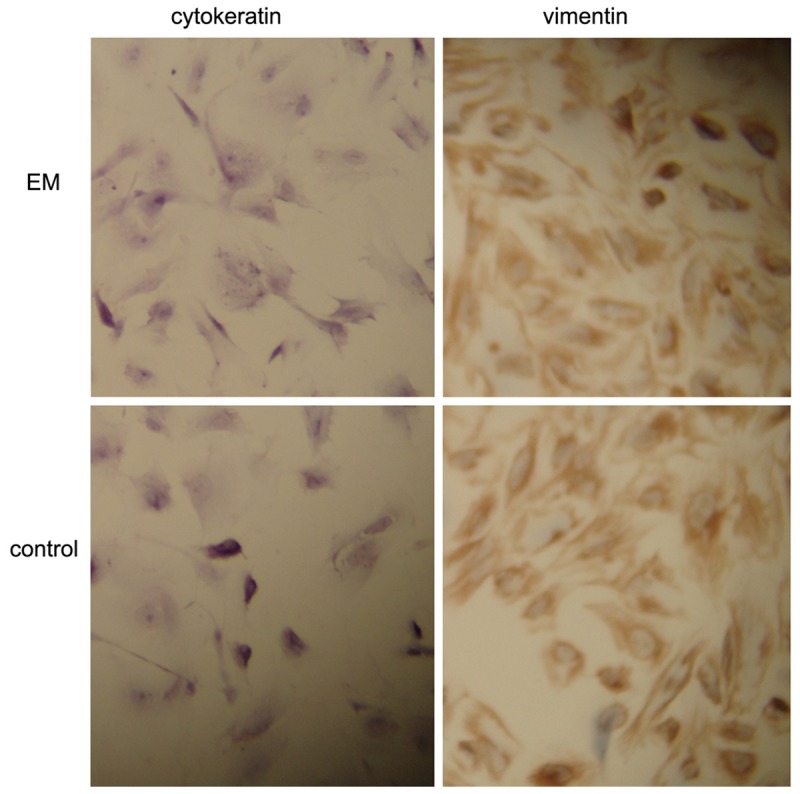
Immunohistochemical staining of ESCS with different antibody. Original magnification 300 ×.
Expression of FAK protein was elevated in ESCs after treated by estrogen
The anti-FAK antibody detected a band at 125 kDa in protein extracts from all cultured ESCs (Figure 2). After normalizing each band of FAK with β-actin from different samples, we found that FAK protein expression in the cultured ESCs from endometriosis (0.221 ± 0.009) was higher than that from women without (0.191 ± 0.011) (P < 0.05). After treated by estrogen, FAK protein expression in the cultured ESCs from endometriosis (0.421 ± 0.014) was also higher than that from women without (0.321 ± 0.011) (P < 0.05) (Figure 3).
Figure 2.
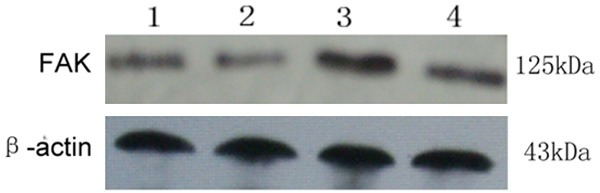
FAK protein expression in endometrial tissues was assessed by Western blotting. The anti-FAK antibody detected a band at 125 kDa. 1: ESCs from women with endometriosis; 2: ESCs from women without endometriosis; 3: ESCs from women with endometriosis treated by estrogen; 4: ESCs from women without endometriosis treated by estrogen.
Figure 3.
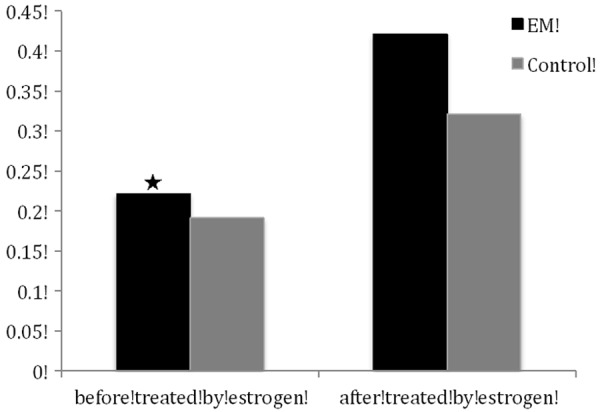
Normalized density was analyzed using the internal β-actin as reference (means ± SD). ★: P < 0.05, compared with the normalized density of FAK proteins in endometrial tissues of controls. ✻: P < 0.05, compared with the normalized density of FAK proteins in endometrial tissues of controls.
ESCs from endometriosis was more sensitive to estrogen
Elevated expression of FAK protein was seen in the cultured ESCs treated with estrogen. The level of up-regulation by estrogen in ESCs from endometriosis (0.201 ± 0.007) was significantly higher than that from women without endometriosis (0.130 ± 0.008) (P < 0.05) (Figure 3).
Expression of FAK protein was not changed in ESCs after treated by progesterone or treated by estrogen and progesterone
After normalizing each band of FAK with β-actin from different samples, we found that FAK protein expression in the cultured ESCs was not changed after treated by progesterone or treated by estrogen and progesterone (P > 0.05) (Table 1).
Table 1.
FAK expression in ESCS before and after treated by ovarian steroid hormones
| Group | n | None | E + P | P |
|---|---|---|---|---|
| EM | 15 | 0.221 ± 0.009 | 0.226 ± 0.006a | 0.022 ± 0.011b |
| Control | 17 | 0.191 ± 0.011 | 0.196 ± 0.012c | 0.193 ± 0.007d |
P > 0.05, compared with FAK expression before treated by ovarian steroid hormones;
P > 0.05, compared with FAK expression before treated by ovarian steroid hormones;
P > 0.05, compared with FAK expression before treated by ovarian steroid hormones.
P > 0.05, compared with FAK expression before treated by ovarian steroid hormones.
Discussion
Focal adhesion kinase (FAK), a non-receptor tyrosine kinase involved in the formation and turnover of focal adhesion sites [9,10], acts as a key regulator in cell migration and cell invasion involving proteolytic degradation of the extracellular matrix [11]. Overexpression of FAK has been demonstrated to indicate invasive potential and poor prognosis in various human cancers [12]. Results from the previous study also indicated that the FAK pathway played an important role in mediating cell migration induced by estrogen [13]. One of the results of our study is that elevated expression of FAK was seen in the cultured ESCs treated with estrogen. This finding is in line with our founding that the endometrial FAK protein expression varied with the serum estrogen [5]. Also another research had showed that estrogen receptor-alpha promotes breast cancer cell motility and invasion via focal adhesion kinase [14]. But some studies demonstrated that ER gene transfection significantly inhibited FGF-stimulated tyrosine phosphorylation of FAK [15] and estrogen treatment of MCF-7 cells resulted in a decrease in the tyrosine phosphorylation of FAK [16]. We speculate that this result from different cells. Estrogen action is mediated by two receptors, ERα and ERβ. ERα is the receptor responsible for 17β-estradiol-induced signaling, whereas function of ERβ is opposed to that of ERα [17]. In our current study, estrogen stimulated expression of FAK in ESCS. However, whether these effects depend on ERβ and or ERα remains unclear, which need further exploration.
In this research we also found that FAK expression was not changed significantly after treated by progesterone. The relationship between progesterone and FAK was studied in the several researches. Zheng et al had reported that progesterone promoted endothelial cell movement via the rapid regulation of FAK [18]. And PR has been reported to facilitate metastasis evolution by increasing invasiveness of primary cancer cells through transcriptional regulation of key proteins such as FAK which involved in cellular migration and adhesion [19]. The researches above had not focus on the expression of FAK but the phosphorylation of FAK. So we speculated that progesterone might regulate the phosphorylation of FAK not the expression which need further research in the future. At the same time we found that FAK expression was also not changed significantly after treated by both estrogen and progesterone. As we know estrogen can stimulate the expression of FAK, progesterone might play the contrary role in the regulation of FAK expression according to estrogen. Progesterone just took part in the role of this regulation based on existence of estrogen because of estrogen induction of PR expression via ERα. Progesterone antagonizes estrogen-driven growth in the endometrium.
In addition we revealed that the elevation of FAK expression in cultured ESCs from women with endometriosis was higher than that from women without. As we know endometriosis is also an estrogen-dependent disease [20]. Estrogen enhanced the proliferation and invasiveness of ESCs, and promotes angiogenesis in the endometriotic milieu [21,22]. Estrogen enhances cytoskeletal and membrane remodeling in ESC and Ishikawa cells by controlling FAK, thus resulting in enhanced cell motility and invasion [23]. Although the morphology of eutopic endometrium from women with endometriosis is similar to that from normal women, its physiology and biochemistry are different. And to this point, this difference found in our work was the ESCs were more sensitive to the estrogen. So we can speculate that the reaction to estrogen lead the ESCs from women with endometriosis have a higher expression of FAK which might make the endometrial cells has the predisposition to adhere and grow outside of the uterus.
In conclusion, our study suggested that endometrial stromal cells from endometriosis were more sensitive to estrogen, which up-regulated the expression of FAK and might contribute to the pathogenesis and progress of endometriosis.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (grant number: 81100407).
Disclosure of conflict of interest
None.
References
- 1.Strathy JH, Molgaard CA, Coulman CB. Endometriosis and infertility: a laparoscopic study of endometriosis among fertile and infertile women. Fertil Steril. 1982;38:667–672. doi: 10.1016/s0015-0282(16)46691-4. [DOI] [PubMed] [Google Scholar]
- 2.Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 3.Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investig. 2006;13:467–476. doi: 10.1016/j.jsgi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Cox BD, Natarajan M, Stettner MR, Gladson CL. New concepts regarding focal adhesion kinase: promotion of cell migration and proliferation. J Cell Biochem. 2006;99:36–52. doi: 10.1002/jcb.20956. [DOI] [PubMed] [Google Scholar]
- 5.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 6.Gentilini D, Vigano P, Somigliana E, Vicentini LM, Vignali M, Busacca M, Di Blasio AM. Endometrial stromal cells from women with endometriosis reveal peculiar migratory behavior in response to ovarian steroids. Fertil Steril. 2010;93:706–715. doi: 10.1016/j.fertnstert.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Yuge A, Nasu K, Matsumoto H, Nishida M, Narahara H. Collagen gel contractility is enhanced in human endometriotic stromal cells: a possible mechanism underlying the pathogenesis of endometriosis-associated fibrosis. Hum Reprod. 2007;22:938–944. doi: 10.1093/humrep/del485. [DOI] [PubMed] [Google Scholar]
- 8.Mu L, Zheng W, Wang L, Chen XJ, Zhang X, Yang JH. Alteration of focal adhesion kinase expression in eutopic endometrium of women with endometriosis. Fertil Steril. 2007;88:1700–1712. doi: 10.1016/j.fertnstert.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 9.Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin mediated adhesion controls axon pathfinding. Nat Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- 10.Van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Andò S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki T, Kato H, Nakajima M, Sohda M, Fukai Y, Masuda N, Manda R, Fukuchi M, Tsukada K, Kuwano H. FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 2003;89:140–145. doi: 10.1038/sj.bjc.6601050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang YL, Hsu YK, Wu TF, Huang CM, Liou LY, Chiu YW, Hsiao YH, Luo FJ, Yuan TC. Regulation of estrogen receptor α function in oral squamous cell carcinoma cells by FAK signaling. Endocr Relat Cancer. 2014;21:555–565. doi: 10.1530/ERC-14-0102. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez AM, Flamini MI, Baldacci C, Goglia L, Genazzani AR, Simoncini T. Estrogen receptor-alpha promotes breast cancer cell motility and invasion via focal adhesion kinase and N- WASP. Mol Endocrinol. 2010;24:2114–2125. doi: 10.1210/me.2010-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan E, Gurjar MV, Sharma RV, Bhalla RC. Estrogen receptor-alpha gene transfer into bovine aortic endothelial cells induces eNOS gene expression and inhibits cell migration. Cardiovasc Res. 1999;43:788–797. doi: 10.1016/s0008-6363(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 16.Bartholomew PJ, Vinci JM, DePasquale JA. Decreased tyrosine phosphorylation of focal adhesion kinase after estradiol treatment of MCF-7 human breast carcinoma cells. J Steroid Biochem Mol Biol. 1998;67:241–249. doi: 10.1016/s0960-0760(98)00098-3. [DOI] [PubMed] [Google Scholar]
- 17.Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci U S A. 2004;101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng S, Huang J, Zhou K, Xiang Q, Zhang Y, Tan Z, Simoncini T, Fu X, Wang T. Progesterone enhances vascular endothelial cell migration via activation of focal adhesion kinase. J Cell Mol Med. 2012;16:296–305. doi: 10.1111/j.1582-4934.2011.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu XD, Goglia L, Sanchez AM, Flamini M, Giretti MS, Tosi V, Genazzani AR, Simoncini T. Progesterone receptor enhances breast cancer cell motility and invasion via extranuclear activation of focal adhesion kinase. Endocr Relat Cancer. 2010;17:431–443. doi: 10.1677/ERC-09-0258. [DOI] [PubMed] [Google Scholar]
- 20.Rizner TL. Estrogen metabolism and action in endometriosis. Mol Cell Endocrinol. 2009;307:8–18. doi: 10.1016/j.mce.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Li MQ, Shao J, Meng YH, Mei J, Wang Y, Li H, Zhang L, Chang KK, Wang XQ, Zhu XY, Li DJ. NME1 suppression promotes growth, adhesion and implantation of endometrial stromal cells via Akt and MAPK/Erk1/2 signal pathways in the endometriotic milieu. Hum Reprod. 2013;28:2822–2831. doi: 10.1093/humrep/det248. [DOI] [PubMed] [Google Scholar]
- 22.Chang KK, Liu LB, Jin LP, Meng YH, Shao J, Wang Y, Mei J, Li MQ, Li DJ. NME1 suppression of endometrial stromal cells promotes angio- genesis in the endometriotic milieu via stimulating the secretion of IL-8 and VEGF. Int J Clin Exp Pathol. 2013;6:2030–2038. [PMC free article] [PubMed] [Google Scholar]
- 23.Flamini MI, Sanchez AM, Genazzani AR, Simoncini T. Estrogen regulates endometrial cell cytoskeletal remodeling and motility via focal adhesion kinase. Fertil Steril. 2011;95:722–726. doi: 10.1016/j.fertnstert.2010.08.039. [DOI] [PubMed] [Google Scholar]


