Abstract
Background: There are a few reports regarding the comparison of these anesthetic agents, but previous studies mainly focus on the veterinary anesthesiology. Less attention has been focused comparing the effectiveness of these inhalational anesthetic agents in neurosurgery. This lack of interest is regretful particularly considering the fact that anesthetics during neurosurgery are an issue of extreme sensitivity and subtlety, where the cerebral oxygenation process plays a significant role in the neuroprotective mechanisms. Objective: The purpose of this retrospective study is to contribute to the existing knowledge of the comparative studies of the volatile anesthetic agents such as isoflurane, sevoflurane and desflurane by evaluating the maintenance and emergence characteristics after volatile anesthetics-induced preconditioning with isoflurane, sevoflurane or desflurane for inpatient ischemia/reperfusion cerebral injury during cerebral or neural surgeries. Methods: The aim was to investigate their neuroprotective mechanisms and effects by analyzing and comparing the superiority of each agent in a Chinese patient population, in terms of faster emergence, and early and intermediate recovery. The intraoperative haemodynamic profiles and postoperative adverse effects of these three agents were also systematically analyzed. Results: We found that sevoflurane, when compared with isoflurane and desflurane, provided anesthesia with similar hemodynamic stability but allowed for a smoother, more rapid emergence and better quality of induction and recovery to surgical patients under clinical conditions, particularly to those who were experiencing substantial cerebral vasodilation. Conclusion: Sevoflurane offers several advantages, including a relative lack of airway irritation, a more rapid onset and recovery, and greater hemodynamic stability than other potent inhaled agents. These properties would appear to afford sevoflurane significant clinical potential.
Keywords: Isoflurane, sevoflurane, desflurane, recovery, cerebral oxygenation
Introduction
Ischemia is a medical condition briefly described as the deprival of sufficient blood supply to an organ or tissue, which will incur severe damage to the body and thus critical clinical outcomes, such as hemorrhagic shock or myocardial infarction. Although restoration of blood flow to an ischemic organ is essential to prevent irreversible tissue injury, reperfusion perse may result in a local and systemic inflammatory response that may augment tissue injury in excess of that produced by ischemia alone. Cellular damage after reperfusion of previously viable ischemic tissues is defined as ischemia-reperfusion (I-R) injury. I-R injury is characterized by oxidant production, complement activation, leukocyte-endothelial cell adhesion, platelet-leukocyte aggregation, increased microvascular permeability and decreased endothelium-dependent relaxation. In its severest form, I-R injury can lead to multiorgan dysfunction or death [1].
Preconditioning refers to changes at the biomolecular level that enable specialized tissues to tolerate a major adverse event (e.g. ischemia) better if those tissues have already been exposed and adaptable to minor adverse events [2]. In other words, preconditioning is a phenomenon in which brief episodes of a sublethal insult induce robust protection against subsequent lethal injuries. Research conducted over the past few decades indicates that brain preconditioning is complex, involving multiple effectors such as metabolic inhibition, activation of extra- and intracellular defense mechanisms, a shift in the neuronal excitatory/inhibitory balance, and reduction in inflammatory sequelae [3]. Ischemic preconditioning can be mimicked pharmacologically by a variety of substances including the volatile anesthetic agents, which is termed as anesthetic preconditioning [2]. Various models have been proposed to unveil the molecular, cellular and neural mechanisms of inhaled anesthetic action. Numerous ligand- and voltage-gated channels might plausibly mediate the minimum alveolar anesthetic concentration (MAC) and specific amino acid sites in certain receptors present likely candidates for mediation, such as glycine, N-methyl-D-aspartate, sodium, gamma-aminobutyric acid A, acetylcholine, potassium, 5-hydroxytryptamine-3, opioids, and Alpha2-adrenergic [4].
Modern volatile anesthetic agents mainly include isoflurane (CHF2-O-CHCl-CF3), sevoflurane (CH2F-O-CH-(CF3)2) and desflurane (CHF2-O-CHF-CF3) (See Table 1 for the basic biophysical and biochemical properties of isoflurane, sevoflurane and desflurane). Compared with isoflurane, rapid emergence from desflurane and sevoflurane anesthesia has been observed in animals and humans, due to their lower blood-gas partition coefficients. Both agents allow more rapid emergence than traditional volatile anesthetics. Desflurane provided earlier recovery than sevoflurane, in patients undergoing total hip replacement surgery [5]. More rapid recovery from prolonged anesthesia may be an advantage in the elderly in whom cognitive impairment (e.g., delirium, confusion) is a problem during recovery [6]. In high-risk coronary surgery patients with documented impairment of myocardial function, anesthesia with desflurane and sevoflurane preserved cardiac function after CPB (cardiopulmonary bypass) with less evidence for myocardial damage than with propofol [7]. Isoflurane, sevoflurane and desflurane have neuroprotective effect against focal cerebral ischemia, more than halothane [8].
Table 1.
Basic biophysical and biochemical properties of isoflurane, sevoflurane and desflurane

|
Figure 1 shows some comparable properties of isoflurane, sevoflurane and desflurane with respect to the alveolar anesthetic uptake, verified by previous research [8]. In Figure 1A, the fractional anesthetic concentration that leaves the anesthetic circuit and enters the respiratory system is called the “fraction inspired” represented by FI. And the fractional anesthetic concentration that is present in the alveoli is represented as FA. The ratio FA/FI varies with time as a function of several considerations, including the intrinsic anesthetic blood: gas solubility. From the FA/FI ratio we may conclude that volatile anesthetics that have relatively low blood solubilities are associated with the most rapid rise in the FA/FI ratio. Within the first few minutes, significant anesthetic uptake occurs as organs which have significant blood flow (sometimes called vessel rich groups), such as the brain and the heart, contribute prominently. Later, the large muscle group becomes the more important compartment, while finally the relatively poorly perfused adipose tissue compartment dominates at a longer time. Figure 1B shows that there is an inverse trend between the blood gas solubility of the anesthetic and their MAC value. A rule of thumb about anesthetic uptake would in this case be that “anesthetic: blood solubility × cardiac output × the difference in alveolar: venous anesthetic partial pressures”. Figure 1C illustrates the ratio of delivered (FD) to alveolar (FD/FA) anesthetic concentration which would be required to maintain the alveolar anesthetic concentration at the 1 MAC level. The isoflurane curve shows the largest difference between the delivered concentration and alveolar concentration among the three anesthetics. At the one-hour., isoflurane vaporizers may have difficulty in meeting the anesthetic demand, indicating that if there is a need to maintain low flow and work within that constraint, then selection of a different anesthetic, i.e. not isoflurane, would be appropriate in order to position vaporizer outflow capacity in closer accord with physiological requirements [9].
Figure 1.
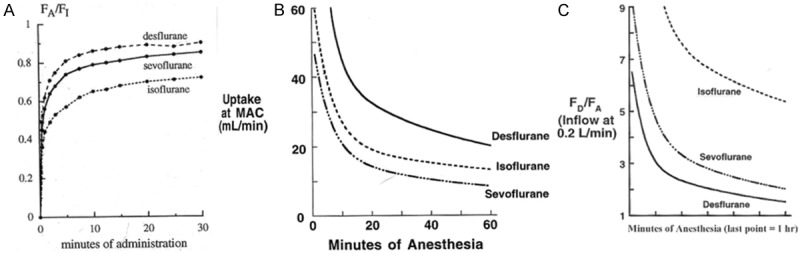
Comparisons of isoflurane sevoflurane and desflurane on the alveolar anesthetic uptake.
The purpose of this retrospective study was to contribute to the existing knowledge of the comparative studies of the volatile anesthetic agents such as isoflurane, sevoflurane and desflurane by evaluating the maintenance and emergence characteristics after volatile anesthetic-induced preconditioning with isoflurane, sevoflurane or desflurane for inpatient ischemia/reperfusion cerebral injury during cerebral or neural surgeries. The aim is to investigate their neuroprotective mechanisms and effects by analyzing and comparing the superiority of each agent in a Chinese patient population, in terms of faster emergence, and early and intermediate recovery. The intraoperative haemodynamic profiles and postoperative adverse effects of these three agents were also systematically analyzed. There are a few reports regarding the comparison of these anesthetic agents, but previous studies mainly focus on the veterinary anesthesiology. Less attention has been focused comparing the effectiveness of these inhalational anesthetic agents in neurosurgery. This lack of interest is regretful particularly considering the fact that anesthetics during neurosurgery is an issue of extreme sensitivity and subtlety, where the cerebral oxygenation process plays a significant role in the neuroprotective mechanisms.
Materials and methods
Participants
Ninety patients who registered at the inpatient section of our hospital from November 2012 to February 2014 to undergo acute cerebral and neurological surgeries were recruited in this study. Of these 90 participants, 57 were male and 33 were female, with mean age at (56 ± 7.3) years old (ranging from 48 to 79 years old). Patients were equally randomized into three groups, each receiving one of the three different anesthetic protocols: isoflurane (group A, n = 30), sevoflurane (group B, n = 30), or desflurane (group C, n = 30). In each group, anaesthesia was induced using propofol and maintained with 60% N2O in O2 and isoflurane, sevoflurane or desflurane, respectively. Cerebral and neurological surgeries included in this study were: acoustic neuroma, cerebellar tumor, meningioma, cerebral aneurysms, pineal tumors, trigeminal nerve transection, and craniopharyngioma. The anesthetic plan, I/R and surgery procedures were similar for the three groups. This study has been approved and endorsed by the institutional review committee, and all patients have signed an informed consent.
A pre-anesthetic examination comprising history, general physical and systemic examination of all the patients was conducted. Routine investigations including hemoglobin, total leukocyte count, blood sugar, serum creatinine and urine examination were carried out. All patients were kept fasting for at least 6 hours prior to surgery.
The study excluded the patients with significant cardiopulmonary disease, hepatic or renal dysfunction, endocrinal disturbances, neurological or psychiatric disorder, those with history of drug allergy or drug abuse, those on central nervous system (CNS) depressants, pregnant/breastfeeding females and those who had undergone recent anaesthesia (within the previous 7 days).
Anesthetic plan
Anesthetic plan was proceeded according to previously described [10,11]. Briefly, tablet alprazolam 0.25 mg and tablet pantoprazole 40 mg were given as premedication 60 min prior to induction to all the patients.
In the operating room, an intravenous (IV) line was secured on the non-dominant hand of the patient, monitors were attached and baseline heart rate (HR), mean arterial pressure (MAP), electrocardiogram (ECG), blood pressure (BP) and oxygen saturation (SpO2) were recorded.
All patients received fentanyl citrate 2 mcg/kg intravenously and were preoxygenated prior to induction of anaesthesia. Anaesthesia was induced with propofol 1.5 mg/kg IV. After loss of consciousness, ventilation of lungs was manually assisted. Neuromuscular blockade was achieved with vecuronium bromide 0.1 mg/kg IV and airway secured with an endotracheal tube.
Anaesthesia was maintained with oxygen (40%), nitrous oxide (60%) with isoflurane, sevoflurane or desflurane on spontaneous ventilation with closed circuit. The volatile agent was administered at approximately one ‘minimal alveolar concentration’ (MAC) for 3 min i.e. 1.2% for isoflurane, 2% for sevoflurane, and 2.4% for desflurane. Then it was continued at 0.75 MAC i.e. 0.8% for isoflurane, 1.5% for sevoflurane, and 1.6% for desflurane further increasing or decreasing by 0.6% for desflurane, 0.5% for sevoflurane and 0.2% for isoflurane (0.5 MAC) according to the clinical assessment of the depth of anaesthesia and to maintain blood pressure at ± 20% of base line values in response to surgical stimulation.
Clinical parameters recorded
Routine data
Monitoring was done using SpO2, non-invasive blood pressure (NIBP), electrocardiogram (ECG), HR and end-tidal carbon dioxide (EtCO2). The maintenance dose of anesthetics was titrated to maintain a bispectral index (BIS) value of 40-60. All the patients were ventilated to maintain an EtCO2 of 32-36 mm Hg.
Cerebral oxygen and glucose metabolism
At different time points (before anesthesia, before skin incision, immediately after the surgery, and 10 min after extubation), 1 mL of blood from the radial artery and jugular bulb was simultaneously extracted, with the exsanguinate velocity of the jugular bulb at 2 mL/min. The arterial and venous blood samples extracted were immediately tested separately using the 4+ and 8+ blood hemoglobin detection chip of the i-Star biochemical analyzer for detection of hemoglobin content (Hb), arterial or venous oxygen saturation (SaO2, SjvO2), arterial or venous blood oxygen partial pressure (PaO2, PjvO2), arterial or venous blood glucose (Glua, Glujv), and arterial or venous lactate (Laca, Lacjv). The arterial oxygen content (CaO2), internal jugular bulb oxygen content (CjvO2), cerebral arterio-venous oxygen content difference (Da-jvO2), cerebral oxygen uptake rate (OEr), cerebral arterio-venous blood glucose content difference (Da-jvGlu), brain glucose uptake (GluEr), cerebral arterio-venous lactate content difference (Da-jvLac), and lactate production rate (LacPr) were calculated according to the following formula:
CaO2 = 1.34 × Hb × SaO2 + 0.0031 × PaO2; CjvO2 = 1.34 × Hb × SjvO2 + 0.0031 × PjvO2; Da-jvO2 = CaO2 - CjvO2; OEr = Da-jvO2/CaO2; Da-jvGlu = Glua - Glujv; GluEr = Da-jvGlu/Glua; Da-jvLac = Laca - Lacjv; LacPr = Da-jvLac/Laca.
Statistical analysis
The observational results obtained in the three groups were recorded and tabulated. Data were represented as mean ± SD. Chi-square test and student’s t-test was used for the analysis of parametric data and Mann-Whitney for non parametric data. A probability value (P value) of < 0.05 was considered as statistically significant. The software package used in the statistical analysis was SPSS 17.0.
Results
General demographic data of the three groups showed no statistically significant difference (P > 0.05) (Table 2).
Table 2.
Demographic data of the 90 patients
| Group A (ISO) | Group B (SEV) | Group C (DES) | P value | |
|---|---|---|---|---|
| Age (years) | 55 ± 7.6 | 56 ± 7.2 | 57 ± 7.1 | 0.314 |
| Gender (male/female) | 20/12 | 18/11 | 19/10 | 0.286 |
| Weight (kg) | 71 ± 5.8 | 72 ± 5.3 | 72 ± 5.6 | 0.348 |
| Height (cm) | 172 ± 4.3 | 171 ± 4.7 | 173 ± 4.1 | 0.427 |
| Duration of anaesthesia (min) | 339 ± 86 | 341 ± 84 | 341 ± 80 | 0.392 |
| Duration of procedure (min) | 302 ± 92 | 304 ± 90 | 303 ± 89 | 0.381 |
| Dosage of propofol (mg) | 120 ± 28 | 118 ± 29 | 119 ± 30 | 0.413 |
| Dosage of inhalation (MAC· h) | 8.2 ± 0.9 | 8.4 ± 1.0 | 8.3 ± 1.1 | 0.369 |
| Blood loss (mL) | 801 ± 369 | 798 ± 366 | 792 ± 358 | 0.313 |
| Infusion volume (mL) | 3320 ± 830 | 3430 ± 850 | 3200 ± 790 | 0.297 |
| urinary volume (mL) | 1530 ± 480 | 1620 ± 530 | 1580 ± 500 | 0.283 |
Perioperative hemodynamics of the three groups showed the following characteristics: The sevoflurane group showed a rather smoother hemodynamics than the other two groups, with no statistically significant difference in data of within group comparison, and statistically significant difference in data of between group comparisons. There were considerably high hemodynamics in groups of isoflurane and desflurane, and difference between these two groups was not statistically significant (Table 3).
Table 3.
Hemodynamics at various time points in each group
| PREOP | 5’-AI | PD | 60’PD | POSTOP | |
|---|---|---|---|---|---|
| Group A (Isoflurane) | |||||
| HR (times/min) | 76 ± 11 | 66 ± 12 | 69 ± 10 | 81 ± 13 | 84 ± 11 |
| SBP (mmHg) | 128 ± 15 | 103 ± 13 | 115 ± 12 | 136 ± 18 | 147 ± 19 |
| DBP (mmHg) | 75 ± 10 | 63 ± 14 | 68 ± 13 | 73 ± 13 | 82 ± 11 |
| MAP (mmHg) | 93 ± 12 | 76 ± 15 | 82 ± 17 | 91 ± 14 | 104 ± 12 |
| Group B (Sevoflurane) | |||||
| HR (times/min) | 76 ± 12 | 69 ± 10 | 71 ± 13 | 79 ± 12 | 82 ± 10 |
| SBP (mmHg) | 129 ± 15 | 107 ± 12 | 118 ± 14 | 133 ± 19 | 142 ± 20 |
| DBP (mmHg) | 76 ± 9 | 66 ± 10 | 70 ± 12 | 75 ± 11 | 79 ± 12 |
| MAP (mmHg) | 93 ± 14 | 81 ± 13 | 87 ± 15 | 93 ± 13 | 99 ± 14 |
| Group C (Desflurane) | |||||
| HR (times/min) | 76 ± 11 | 65 ± 13 | 68 ± 12 | 82 ± 14 | 85 ± 12 |
| SBP (mmHg) | 128 ± 14 | 102 ± 15 | 114 ± 11 | 137 ± 16 | 149 ± 20 |
| DBP (mmHg) | 74 ± 9 | 64 ± 15 | 67 ± 15 | 72 ± 16 | 83 ± 14 |
| MAP (mmHg) | 92 ± 13 | 75 ± 14 | 81 ± 16 | 90 ± 15 | 103 ± 15 |
Cerebral oxygen and glucose metabolism of the three groups indicated that sevoflurane has allowed for a more stabilized perioperative cerebral homeostasis and better recovery profile than isoflurane (the difference was statistically significant), and desflurane has showed comparable characteristics with sevoflurane with slightly unfavorable trends, but the difference was not statistically significant (Table 4).
Table 4.
Cerebral oxygen and glucose metabolism at different time points in each group
| PREOP | 5’-AI | PD | 60’PD | POSTOP | |
|---|---|---|---|---|---|
| Group A (Isoflurane) | |||||
| Hb (g/dL) | 130 ± 16 | 101 ± 13 | 103 ± 16 | 106 ± 19 | 108 ± 12 |
| SjvO2 (%) | 58 ± 9 | 59 ± 7 | 62 ± 9 | 64 ± 12 | 67 ± 8 |
| CaO2 (mL/dL) | 165 ± 13 | 143 ± 15 | 147 ± 16 | 151 ± 12 | 139 ± 14 |
| CjvO2 (mL/dL) | 104 ± 14 | 87 ± 13 | 89 ± 17 | 92 ± 15 | 93 ± 18 |
| Da-jvO2 (mL/dL) | 58 ± 18 | 54 ± 16 | 52 ± 10 | 50 ± 13 | 47 ± 14 |
| OEr (%) | 35 ± 14 | 36 ± 12 | 35 ± 8 | 39 ± 12 | 31 ± 11 |
| Glua (mmol/L) | 59.2 ± 5.9 | 50.7 ± 3.2 | 54.9 ± 4.3 | 66.8 ± 7.7 | 68.4 ± 8.2 |
| Da-jvGlu (mmol/L) | 5.7 ± 1.6 | 5.1 ± 0.9 | 5.3 ± 1.2 | 5.4 ± 0.6 | 5.5 ± 0.8 |
| GluER (%) | 0.10 ± 0.05 | 0.11 ± 0.03 | 0.09 ± 0.03 | 0.07 ± 0.05 | 0.05 ± 0.04 |
| Laca (mmol/L) | 1.4 ± 0.6 | 1.3 ± 0.8 | 1.7 ± 0.9 | 2.1 ± 1.2 | 2.4 ± 0.8 |
| Da-jvLac (mmol/L) | -0.10 ± 0.09 | -0.13 ± 0.16 | -0.19 ± 0.20 | -0.23 ± 0.18 | -0.27 ± 0.14 |
| LacPR (%) | -0.08 ± 0.10 | -0.09 ± 0.12 | -0.10 ± 0.07 | -0.11 ± 0.09 | -0.11 ± 0.03 |
| Group B (Sevoflurane) | |||||
| Hb (g/dL) | 131 ± 14 | 104 ± 14 | 106 ± 15 | 108 ± 17 | 110 ± 16 |
| SjvO2 (%) | 57 ± 7 | 63 ± 8 | 66 ± 7 | 67 ± 9 | 68 ± 10 |
| CaO2 (mL/dL) | 164 ± 15 | 150 ± 13 | 153 ± 14 | 155 ± 16 | 142 ± 18 |
| CjvO2 (mL/dL) | 105 ± 17 | 92 ± 20 | 93 ± 20 | 94 ± 18 | 94 ± 20 |
| Da-jvO2 (mL/dL) | 60 ± 15 | 58 ± 17 | 54 ± 12 | 51 ± 11 | 49 ± 12 |
| OEr (%) | 37 ± 12 | 38 ± 13 | 39 ± 9 | 41 ± 10 | 33 ± 10 |
| Glua (mmol/L) | 58.1 ± 5.7 | 53.8 ± 4.6 | 56.9 ± 4.8 | 70.7 ± 9.2 | 71.5 ± 9.8 |
| Da-jvGlu (mmol/L) | 5.8 ± 1.4 | 5.5 ± 0.5 | 5.4 ± 1.6 | 5.3 ± 0.7 | 5.6 ± 0.9 |
| GluER (%) | 0.11 ± 0.03 | 0.12 ± 0.04 | 0.09 ± 0.05 | 0.08 ± 0.03 | 0.06 ± 0.03 |
| Laca (mmol/L) | 1.3 ± 0.7 | 1.6 ± 0.9 | 2.0 ± 0.8 | 2.4 ± 1.1 | 2.7 ± 0.9 |
| Da-jvLac (mmol/L) | -0.11 ± 0.08 | -0.16 ± 0.18 | -0.23 ± 0.15 | -0.27 ± 0.16 | -0.30 ± 0.19 |
| LacPR (%) | -0.07 ± 0.08 | -0.09 ± 0.06 | -0.10 ± 0.05 | -0.11 ± 0.02 | -0.12 ± 0.07 |
| Group C (Desflurane) | |||||
| Hb (g/dL) | 130 ± 13 | 102 ± 17 | 104 ± 16 | 107 ± 19 | 109 ± 15 |
| SjvO2 (%) | 57 ± 12 | 61 ± 9 | 63 ± 10 | 65 ± 8 | 67 ± 9 |
| CaO2 (mL/dL) | 163 ± 17 | 147 ± 12 | 150 ± 15 | 152 ± 14 | 141 ± 16 |
| CjvO2 (mL/dL) | 104 ± 21 | 90 ± 18 | 91 ± 19 | 92 ± 16 | 93 ± 19 |
| Da-jvO2 (mL/dL) | 59 ± 16 | 57 ± 15 | 52 ± 13 | 50 ± 12 | 48 ± 14 |
| OEr (%) | 36 ± 14 | 37 ± 11 | 38 ± 10 | 39 ± 12 | 32 ± 11 |
| Glua (mmol/L) | 58.4 ± 6.2 | 51.6 ± 3.2 | 54.7 ± 4.7 | 68.1 ± 10.2 | 70.7 ± 7.6 |
| Da-jvGlu (mmol/L) | 5.6 ± 1.7 | 5.3 ± 0.7 | 5.5 ± 1.2 | 5.1 ± 0.9 | 5.4 ± 0.7 |
| GluER (%) | 0.10 ± 0.07 | 0.11 ± 0.02 | 0.10 ± 0.03 | 0.09 ± 0.04 | 0.07 ± 0.01 |
| Laca (mmol/L) | 1.3 ± 0.9 | 1.5 ± 0.8 | 1.8 ± 0.7 | 2.2 ± 1.5 | 2.5 ± 0.7 |
| Da-jvLac (mmol/L) | -0.10 ± 0.13 | -0.14 ± 0.12 | -0.21 ± 0.17 | -0.26 ± 0.15 | -0.28 ± 0.13 |
| LacPR (%) | -0.07 ± 0.12 | -0.08 ± 0.07 | -0.09 ± 0.06 | -0.10 ± 0.04 | -0.11 ± 0.05 |
Discussion
Isoflurane is a potent volatile agent for clinical anaesthesia that has since long been used in the clinical practice, but is currently gradually being superseded by sevoflurane and desflurane due to their low blood: gas partition coefficient, which allows for relatively rapid anesthesia induction and recovery from anesthesia. Both sevoflurane and isoflurane are widely accepted as having the neuroprotective properties against adult and neonatal cerebral ischemic injury when used in preconditioning. Accumulating evidence indicates that isoflurane preconditioning (exposure to 1-2% isoflurane) provides a delayed protective effect (usually 24 h after preconditioning) against ischemic neuronal injury both in vitro models and in adult and neonate in vivo models. Sevoflurane is also a volatile anesthetic that is now replacing isoflurane in modern anesthesiology due to the reduction in mucosal membrane irritation and the faster onset and offset. A large body of evidence indicates that sevoflurane is able to protect the brain against ischemic injury in various models. In neonatal brain injury models, preconditioning with 1.5% sevoflurane alone or 0.75% sevoflurane in combination with 20% xenon significantly reduced infarct size in the model of neonatal asphyxia. Likewise, delayed preconditioning with 8.4% desflurane or 3.1% sevoflurane improved selective behavioral performance of adult mice previously subjected to neonatal HI injury [3]. Possible explanations for these protective effects of inhalation anesthetics are the preservation of ATP levels during ischemia, reduced adhesion of polymorphonuclear neutrophils, increased nitric oxide production, inhibition of free radical production, or a reduction in calcium overloading [12].
The investigation of cerebral oxygen and glucose metabolism advances our understanding and knowledge about cerebral physiology and the effects particular agents have on cerebral homeostasis. These include whether an agent maintains the coupling of cerebral blood flow to cerebral metabolic rate for oxygen, decreases intracranial pressure, maintains cerebral perfusion pressure and CO2 reactivity, is cerebroprotective and anti-convulsant, and does not compromise other organ systems. In neuroanaesthesia rapid awakening facilitating post-operative assessment of neurological function is desirable and the pharmacokinetics of modern agents may be advantageous. McKinlay & Moss have reviewed these pharmacological parameters by comparing popular anesthetic agents including isoflurane, sevoflurane and desflurane [13], and similar developments with our studies have been observed in the results. Previous studies have suggested that intraoperative cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction [14]. Cerebral blood flow is tightly coupled to the brain oxygen consumption. The neuronal mitochondria provide the energy for neuronal function and for neurotransmitter synthesis which are the two building blocks of brain oxygen consumption, via oxidative phosphorylation of glucose. Since the brain has minimal glycogen stores and low ATP concentrations, any decrease in the delivery of the oxygen and glucose substrates is followed by electrophysiological and neuronal changes. The extent of the decrease in blood flow and its duration determine the resulting neuronal outcome [15,16]. Virtually all commonly used anesthetic agents reduce cerebral metabolism, which decreases the brain’s requirements for oxygen. Under these circumstances, the brain’s tolerance for temporary ischemia may be enhanced. Brain blood oxygenation is reported to be higher under volatile anesthetics then under intravenous anesthetics [17].
The capacity of sevoflurane to increase cerebral blood flow while preserving cerebral autoregulation makes it an attractive agent for the preservation of neuronal function. However, the mechanism of sevoflurane preconditioning on neuroprotection is very complex. It occurs through complex signal transduction pathways that involve activation of canonical Notch signaling pathway, protein kinase C, protein kinase M, tyrosine kinase, extracellular signal-regulated protein kinase (MEK-ERK1/2, ERK1/2 MAPK), mitochondrial (mito) adenosine troposphere-regulated potassium (KATP) channels. Furthermore, the neuroprotection of sevoflurane preconditioning involves modification of glutamate transporter activity, altering the electrophysiological changes, inhibition of mitochondrial pore permeability transition opening, resistance against excitotoxicity, releasing reactive oxygen species, inhibition of inflammation, cerebral lipid peroxidation and apoptosis of the neurons, reduction of oxidative stress and maintenance of mitochondria membrane stability. Moreover, the favorable effects of sevoflurane on stabilizing cerebral blood flow and cerebral metabolism may also be a major cause for its preferential use as a neuroprotective agent compared to other anesthetics [18].
In conclusion, we found that sevoflurane, when compared with isoflurane and desflurane, provides anesthesia with similar hemodynamic stability but allows for a smoother, more rapid emergence and better quality of induction and recovery to surgical patients under clinical conditions, particularly to those who were experiencing substantial cerebral vasodilation. Sevoflurane offers several advantages, including a relative lack of airway irritation, a more rapid onset and recovery, and greater hemodynamic stability than other potent inhaled agents. These properties would appear to afford sevoflurane significant clinical potential.
Disclosure of conflict of interest
None.
References
- 1.Eltzschig HK, Collard CD. Vascular ischemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 2.Loveridge R, Schroeder F. Anesthetic preconditioning. Contin Educ Anaesth Crit Care Pain. 2010;10:38–42. [Google Scholar]
- 3.Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ, Gao Y. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol. 2014;114:58–83. doi: 10.1016/j.pneurobio.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonner JM, Antognini JF, Dutton RC, Flood P, Gray AT, Harris RA, Homanics GE, Kendig J, Orser B, Raines DE, Rampil IJ, Trudell J, Vissel B, Eger EI 2nd. Inhaled anesthetics and immobility: mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:718–40. doi: 10.1213/01.ANE.0000081063.76651.33. [DOI] [PubMed] [Google Scholar]
- 5.Dogru K, Dalgic H, Yildiz K, Sezer Z, Madenoglu H. The direct depressant effects of desflurane and sevoflurane on spontaneous contractions of isolated gravid rat myometrium. Int J Obstet Anesth. 2003;12:74–78. doi: 10.1016/S0959-289X(03)00010-4. [DOI] [PubMed] [Google Scholar]
- 6.Heavner JE, Kaye AD, Lin BK, King T. Recovery of elderly patients from tow or more hours of desflurane or sevoflurane anesthesia. Br J Anaesth. 2003;91:502–506. doi: 10.1093/bja/aeg221. [DOI] [PubMed] [Google Scholar]
- 7.De Hert SG, Cromheecke S, ten Broecke PW, Mertens E, De Blier IG, Stockman BA, Rodrigus IE, Van der Linden PJ. Effects of propofol, Desflurane, and sevoflurane on recovery of myocardial function after coronary surgery in elderly high-risk patients. Anesthesiology. 2003;99:314–323. doi: 10.1097/00000542-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Haelewyn B, Yvon A, Hanouz JL, Mackenzie ET, Ducouret P, Gerard JL, Roussel S. Desflurane affords greater protection than halothane against focal cerebral ischemia in the rat. Br J Anaesth. 2003;91:390. doi: 10.1093/bja/aeg186. [DOI] [PubMed] [Google Scholar]
- 9.Gordon M, editor. Anesthesia and Anesthesiology Teaching Site: (http://www.anesthesia2000.com/) 2011.
- 10.Jindal R, Kumra VP, Narani KK, Sood J. Comparison of maintenance and emergence characteristics after desflurane or sevoflurane in outpatient anaesthesia. Indian J Anaesth. 2011;55:36–42. doi: 10.4103/0019-5049.76604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahu DK, Kaul V, Parampill R. Comparison of isoflurane and sevoflurane in anaesthesia for day care surgeries using classical laryngeal mask airway. Indian J Anaesth. 2011;55:364–9. doi: 10.4103/0019-5049.84857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross S, Foëx P. Protective effects of anesthetics in reversible and irreversible ischemia-reperfusion injury. Br J Anaesth. 1999;82:622–32. doi: 10.1093/bja/82.4.622. [DOI] [PubMed] [Google Scholar]
- 13.McKinlay J, Moss E. Pharmacology of drugs used in neuroanaesthesia. Bailliere’s Clinical Anesthesiology. 1999;13:499–510. [Google Scholar]
- 14.Yao FS, Tseng CC, Ho CY, Levin SK, Illner P. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:552–8. doi: 10.1053/j.jvca.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Murdoch J, Hall R. Brain protection: physiological and pharmacological considerations. Part I: The physiology of brain injury. Can J Anaesth. 1990;37:663–71. doi: 10.1007/BF03006487. [DOI] [PubMed] [Google Scholar]
- 16.Lanier WL. The prevention and treatment of cerebral ischemia. Can J Anesth. 1999;46:46–51. doi: 10.1007/BF03013181. [DOI] [PubMed] [Google Scholar]
- 17.Uhrig L, Dehaene S, Jarraya B. Cerebral mechanisms of general anesthesia: Effects des agents d’anesthesie. Ann Fr Anesth. 2014;33:72–82. doi: 10.1016/j.annfar.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Hu XW, Zhang Y, Li WY, Liu J, Lia Y. Preconditioning with sevoflurane ameliorates spatial learning and memory deficit after focal cerebral ischemia-reperfusion in rats. Int J Dev Neurosci. 2013;31:328–333. doi: 10.1016/j.ijdevneu.2013.04.004. [DOI] [PubMed] [Google Scholar]


