Abstract
Pulmonary fibrosis is a respiratory disease with a high mortality rate and its pathogenesis involves multiple mechanisms including epithelial cell injury, fibroblast proliferation, inflammation, and collagen coagulation. The treatment regimens still fail to recover this disease. We have previously found that dihydroartemisinin inhibits the development of pulmonary fibrosis in rats. This study aimed to determine the mechanisms of dihydroartemisinin in bleomycin-induced pulmonary fibrosis. The experimental rats were divided into six groups as normal saline control group (NS group), bleomycin group (BLM group), dihydroartemisinin-1, -2, or -3 group (DHA-1, DHA-2 and DHA-3 group) and dexamethasone group (DXM group). In BLM group, rats were treated with intratracheal instillation of bleomycin. NS group received the same volume of saline instead of bleomycin. In DHA-1, DHA-2 and DHA-3 group, in addition to intratracheal instillation of bleomycin, respectively, dihydroartemisinin (25 mg/kg, 50 mg/kg, 100 mg/kg daily) was administrated by intraperitoneal instillation. In DXM group, rats were treated with intraperitoneal instillation of dexamethasone as control. Immunocytochemical assay, reverse transcription PCR and western blot were used for detecting the expression of TGF-β1, TNF-α, α-SMA and NF-κB in lung tissues. What’s more, morphological change and collagen deposition were analyzed by hematoxylin-eosin staining and Masson staining. Collagen synthesis was detected by hydroxyproline chromatometry. Results showed that dihydroartemisinin significantly decreased the amount of inflammatory cytokines and collagen synthesis, and inhibited fibroblast proliferation in bleomycin-induced pulmonary fibrosis (P < 0.001). This study provides experimental evidence that dihydroartemisinin could decrease cytokines, alveolar inflammation and attenuates lung injury and fibrosis.
Keywords: Dihydroartemisinin, pulmonary fibrosis, collagen, bleomycin, rats
Introduction
Pulmonary fibrosis develops after viral infections or after exposure to radiotherapy, chemotherapeutic drugs, or aerosolized environmental toxins. It also occurs as a secondary effect of rheumatoid arthritis and systemic sclerosis. Pulmonary fibrosis mainly results in inflammation injury and fibroblast proliferation that consequently leads to abnormal deposition of extracellular collagen [1]. Patients with pulmonary fibrosis are treated with immune suppressive agents such as corticosteroids in combination with cytotoxic agents such as azathioprine and cyclophosphamide. However, these treatments cause numerous side effects including myelotoxicity and diffuse alveolar hemorrhage, etc [2]. The anti-fibrotic efficacy of immunosuppressive agents such as dexamethasone (DXM) is limited and the long term survival rates are extremely poor with 3- and 5-year mortality rates approximately 50% and 80%, respectively. To date, no effective treatments have been developed and lung fibrosis remains to be a fatal disorder. The only effective treatment available for progressive lung fibrosis is lung transplantation [3]. Therefore, there is an urgent need to develop new drugs with better efficacy and tolerability against pulmonary fibrosis. Dihydroartemisinin is mainly used to treat malaria. However, recent studies showed that dihydroartemisinin not only has an anti-malarial effect, but also plays roles in anti-inflammation, immune regulation, and restraining skin scar hyperplasia [4-6] which opens the possibility of using it in treatment of pulmonary fibrosis.
Intratracheal administration of bleomycin is the most extensively used experimental model of pulmonary fibrosis [7], which stimulates an inflammatory response with increased infiltration of macrophages, granulocytes and lymphocytes in lung tissues. These inflammatory cells cause fibroblast proliferations and production of extracellular matrix components in the alveolar interstitial space, leading to pulmonary fibrosis. A variety of cytokines including TGF-β1 and TNF-α have been implicated in the development of bleomycin-induced pulmonary inflammation and fibrosis, which are likely due to their induced transcription by NF-κB. Additional factors associated with the disease progression include enhanced expression of SM α-actin (α-SMA) in pulmonary fibroblasts, differentiation of fibroblasts into myofibroblasts, fibroblasts migration, and protein synthesis of cytokines and collagen.
We aimed in this study to investigate the anti-inflammatory and anti-fibrotic effects of dihydroartemisinin in bleomycin-induced pulmonary fibrosis in rats by determining the contents of TGF-β1, TNF-α, α-SMA and NF-κB and collagen levels in lung tissues.
Materials and methods
Animals and reagents
Male Sprague-Dawley rats aged 4-6 weeks and weighing 180-220 g were provided by the Experimental Animal Center of Binzhou Medical University. Rats were kept in an air-conditioned room with 12 h light cycle at 20-25°C and 45-55% humidity, and were fed with standard laboratory chow and tap water ad libitum.
Bleomycin was bought from Taihe Co, Tianjin, China. Anti-TGF-β1 antibody, anti-TNF-α antibody, anti-α-SMA antibody, and anti-NF-κBp65 antibody were bought from Beyotime, Shanghai, China. All other analytical grade reagents for histology and biochemical assays were bought from Sigma Chemical Co, Nanjing, China.
Induction of pulmonary fibrosis by bleomycin
This study was conducted according to the published method [8]. Briefly, rats were anaesthetized with ether, and then were cut in the middle of the neck skin. Trachea was exposed after separation of subcutaneous tissue, muscle, and fat, and was punctured with a needle of 1 ml syringe. Bleomycin (4 mg/m1) in saline solution was delivered into the trachea with a modified syringe needle in a dosage of 5 mg/kg body weight. The rats were rotated immediately after receiving bleomycin to ensure an even drug distribution in the lung. Then the neck skin incision was sewn. After recovery from anesthesia, the rats were returned to their cages and allowed free access to food and water as normal. Control rats received an intratracheal instillation of the same volume of sterile saline. Their food intake, respiration, and activity were observed in a daily base.
Experimental model and study groups
The animals were divided into the following groups (n = 6 rats): 1. only receiving intratracheal instillation of bleomycin as “BLM group”; 2. receiving the same volume of normal saline instead of bleomycin as “NS group”; 3. receiving intraperitoneal instillation of dihydroartemisinin 25 mg/kg/day, 50 mg/kg/day and 100 mg/kg/day, as “DHA-1 group”, “DHA-2 group” and “DHA-3 group” (dihydroartemisinin) respectively, 28 days after administration of single-dose bleomycin; 4. receiving dexamethasone (3 mg/kg/day) after single-dose bleomycin as “DXM group”. All rats treated with either dihydroartemisinin or dexamethasone was sacrificed 14 days after intraperitoneal instillation. “BLM group” and “NS group” were sacrificed on day 29 after bleomycin instillation.
Morphological analysis and histochemical staining
The grades of pulmonary inflammation and fibrosis were analyzed by Hematoxylin-Eosin staining and Masson staining. Biopsies of lung tissues were fixed with 10% paraformaldehyde in sterile phosphate-buffered saline and embedded in paraffin wax. Sections were cut at 5 μm thickness using a microtome and deparaffinized tissue sections were subjected to staining for histological examination.
Sections were stained with both haematoxylin and eosin to observe morphology changes or light green by Masson staining to identify collagen fibers [9]. The slides were examined by light microscopy and photographed. Lung inflammation and fibrosis were evaluated by Szapiel scoring method, a standard four point scoring scale for intensity, with each slide scored as 1, 2, 3 or 4.
Determination of collagen and hydroxyproline content of lung tissue
For collagen determination we employed a hydroxyproline assay technique [10]. Total content of tissue collagen was calculated with the assumption that 12.5% of collagen is constituted of hydroxyproline. Hydroxyproline extracted from collagen can be oxidized to pyrrole by chloramine-T, and then it can produce color with addition of para-dimethylbenzaldehyde. Briefly after death, lung tissues were removed and weighed, 5 g lung tissues cut into pieces, and added with 6 M hydrochloric acid 20 ml, and then sealed and placed in an oven overnight at 110°C. Excess acid was removed by evaporation and hydrolyzed samples were dissolved in 1 ml of PBS. The samples were adding Chloramine T reagent 1 ml. After 20 minutes of mixing, 1 ml of p-dimethyl-amino-benzaldehyde reagent was added and the mixture incubated at 60°C. Absorbance was measured with a spectrophotometer at 550 nm, and compared to a standard curve. The hydroxyproline value is divided by 0.125 to index the collagen content of the tissue (mg/g tissue).
Assessment of TGF-β1, TNF-α, α-SMA and NF-κB by immunohistochemical staining
For immunohistochemical staining, rabbit polyclonal antibodies against TGF-β1, TNF-α, α-SMA and mouse polyclonal antibody against NF-κBp65 were used as primary antibodies. Sections were deparaffinized and permeabilized in citrate buffer solution by microwaving for proteolysis. Sections were then washed in phosphate buffer solution (PBS) and endogenous peroxidase activities were blocked by 3% H2O2 for 15 min. After washing with PBS three times, the sections were blocked with rabbit serum for 30 min, followed by incubation with primary antibodies against the TGF-β1 (1:100), TNF-α (1:500), α-SMA (1:100), and NF-κB antigen (1:100) at 4°C, overnight. After washing with PBS, sections were incubated with biotinylated secondary antibody for 30 min at 37°C. Then, sections were washed in PBS, and incubated in streptavidin-biotin-complex. Antibody binding was visualized with a 3, 3’-diaminobenzidine kit. Histological pictures were taken by using Nikon digital-sight imaging system attached to Nikon E-600 microscope (Nikon Corporation, Japan).
TGF-β1, TNF-α, α-SMA and NF-κB mRNA expression by RT-PCR
Total RNA was extracted from the lung tissues using Trizol reagent according to the manufacturer’s instructions. RNA was reverse-transcribed into cDNA using reverse transcriptase. PCR was then performed in a final volume of 20 μL using a PCR reagent kit (Takara, Japan). ß-actin was used as internal control. The cycling program involved preliminary denaturation at 95°C for 10 min, followed by 35 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 1 min, followed by a final elongation step at 72°C for 5 min. The sequences of the primers are listed in Table 1.
Table 1.
PCR primers used in this study
| Gene Name | Sequence |
|---|---|
| TGF-β1 | forward: 5’-GTCATAGATTGCATTGTTGC-3’ |
| reverse: 5’-AAGGAGACGGAATACAGGG-3’ | |
| TNF-α | forward: 5’-TCTCAAAACTCGAGTGACAAG-3’ |
| reverse: 5’-AGTTGGTTGTCT TTGAGATCC-3’ | |
| α-SMA | forward: 5’-CGAGAAGCTGCTCCAGCTATGTG-3’ |
| reverse: 5’-CTCTCTTGCTCTGCGCTTCGT-3’ | |
| NF-κB | forward: 5’-GAAGAACGAGACCTGGAG-3’ |
| reverse: 5’-TCCGGAACACAATGGCCAC-3’ | |
| β-actin | forward: 5’-GGAGATTACTGCCCTGGCTCCTA-3’ |
| reverse: 5’-GACTCATCGTACTCCTGCTTGCTG-3’ |
TGF-β1, TNF-α, α-SMA and NF-κB protein expression by Western Blot
The lung tissues were cut with scissors into fragments of 1.0 mm3 and centrifuged, then with RIPA lysis buffer for 30 min. The protein concentration was determined with the Bradford protein assay reagent. Total protein for each sample (50 μg) was resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. After blocking with 5% skim milk in Tween 20 (Beyotime, China) at room temperature for 1 h, the membrane was incubated with primary antibodies against the TGF-β1 antigen (1:100), TNF-α antigen (1:500), α-SMA (1:100), NF-κBp65 antigen (1:100) and at 4°C, overnight. After washing with Tris-buffered saline (TBS), the membrane was incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit or anti-mouse IgG antibody (1:10,000) for 1 h at room temperature. After washing with TBS, the bound antibody was visualized according to standard protocols for the electrochemiluminescence detection system (Beyotime, China).
Statistical analysis
Data will be presented as the mean ± SD. Statistical analyses will be performed using one-way ANOVA and also Kruskal-Wallis by SPSS software. Values of P < 0.05 will be regard as statistically significant.
Results
Dihydroartemisinin relieved bleomycin-induced pulmonary fibrosis
Pulmonary fibrosis was induced by intratracheal administration of bleomycin. In contrast to the normal alveolar morphology in saline-treated control rats, bleomycin stimulation induced obvious alveolar wall thickening, massive infiltration of leukocytes, and excessive deposition of mature collagen in the interstitium on day 28 after bleomycin infusion as visualized by Hematoxylin-eosin and Masson staining on lung tissues (Figure 1A, 1B). Moreover, an induction in cellularity of alveolar septal, intra alveolar fibrosis and moderate infiltration of lymphocytes with collagenous bands accompanying great septal thickness and diffuse damage to lung architecture were also observed in belomycin-treated rats; (Figure 1C) Interstitial fibrosis with scattered septal thickness but slight infiltration of lymphocytes in comparison to the previous section in DHA-3 group; (Figure 1D) Decreased fibrosis although it can be seen focally somewhere with cellularity of alveolar septal and intra alveolar fibrosis in DXM group; (Figure 1E) A large number of collagen fibers deposition (Masson staining appears light green) in pulmonary interstitial, severely damagedalveolar structure, obvious changes of fibrosis in BLM group; (Figure 1F) Collagen fibers deposition in pulmonary interstitial in DHA-3 group.
Figure 1.
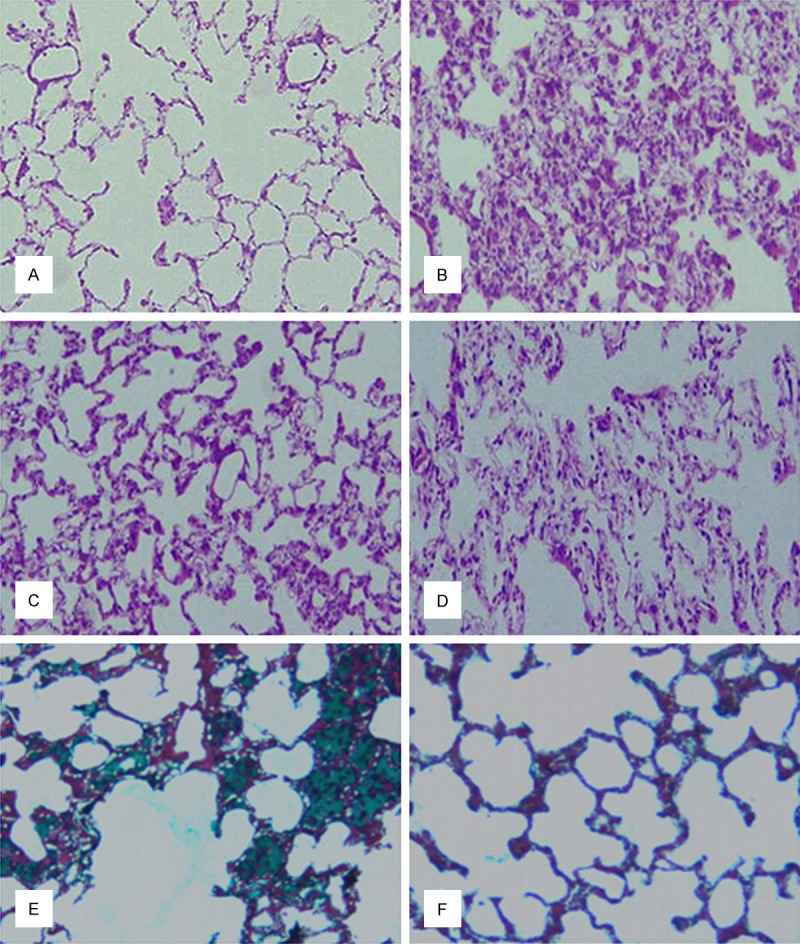
Effects of dihydroartemisinin on bleomycin-induced pulmonary fibrosis. Lung morphology of saline-treated control rats (A), bleomycin-treated rats (B), and bleomycin-treated rats after treatment with high dose dihydroartemisinin (C) or DXM (D) was evaluated by hematoxylin-eosin staining. Collagen fibers deposition in pulmonary interstitium was evaluated by Masson’s staining in lungs in bleomycin-treated rats (E), and bleomycin-treated rats after treatment with high dose dihydroartemisinin (F). (Magnifications × 100).
To study the effect of dihydroartemisinin on bleomycin-induced pulmonary fibrosis, we treated diseased rats with various doses of dihydroartemisinin and dexamethasone and examined the lung morphology after 14 days of treatement. Dexamethasone treatment, as a positive control, decreased fibrosis showing cellularity of alveolar septal and intra alveolar fibrosis. (Figure 1) Although fibrotic lesions were observed in the dihydroartemisinin-treat group, both the extent and intensity of the lesions were less than those of the Bleomycin model group. High dose dihydroartemisinin treatment showed a similar effect as compared to DXM treatment.
The overall grades of fibrotic changes in the lungs were determined using the Szapiel scoring method (Figure 2). Scores of the NS group, BLM group, DHA-1, DHA-2, DHA-3 and DXM group were 1.0 ± 0.00, 5.90 ± 0.21, 3.63 ± 0.32, 3.25 ± 0.18, 2.65 ± 0.33, and 2.33 ± 0.26, respectively. Bleomycin administration induced a significant increase in the fibrotic scores compared to controls (P < 0.05), and the scores of the rats treated by dihydroartemisinin were significantly suppressed (P < 0.05). Importantly, dihydroartemisinin displayed a dose-dependent response and the highest dose of dihydroartemisinin was associated with a significant reduction in the scores (P < 0.05).
Figure 2.
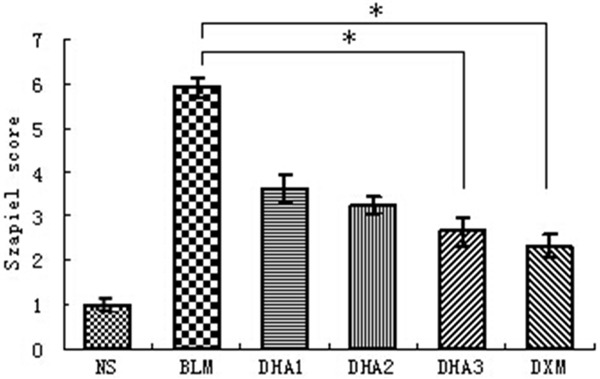
Effect of dihydroartemisinin on fibrotic changes as evaluated by Szapiel scoring method. Results are expressed as mean ± SD (n = 6). Statistical analyses was performed using one-way ANOVA and Kruskal-Wallis test (*P < 0.05).
Dihydroartemisinin reduced hydroxyproline content of collagen
We measured the hydroxyproline content of collagen to quantitatively assess the difference in the extent of pulmonary fibrosis (Figure 3). The hydroxyproline content of lung tissue in the NS group was 30.24 ± 1.12 mg/lung. The hydroxyproline content in the BLM, DHA-1, DHA-2, DHA-3-treated groups and DXM treated groups was 82.75 ± 2.43, 76.35 ± 2.46, 54.87 ± 2.63, 50.18 ± 2.75, and 48.86 ± 1.92 mg/lung, respectively. Bleomycin administration induced a significant increase in the level of hydroxyproline compared to NS group (P < 0.05), but amount of hydroxyproline was significantly decreased in rats treated with dihydroartemisinin (P < 0.05). Dihydroartemisinin again showed a dose-dependent effect with high dose associated with a significant reduction in hydroxyproline content (P < 0.05). Compared to DXM treatment, high dose dihydroartemisinin also showed similar effect in inhibiting hydroxyproline content.
Figure 3.
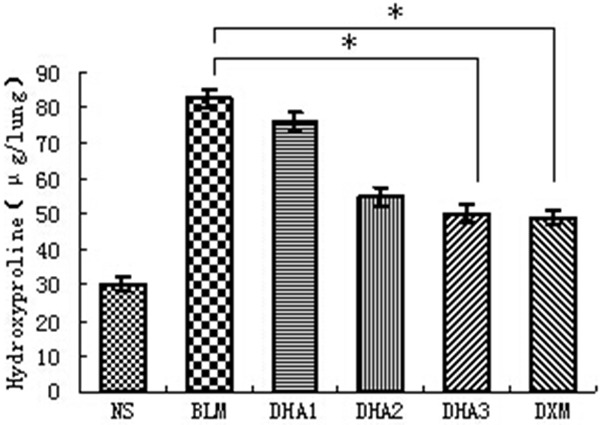
Effect of dihydroartemisinin on the hydroxyproline content of collagen. Results are expressed as mean ± SD (n = 6). Statistical analyses was performed using one-way ANOVA and Kruskal-Wallis test (*P < 0.05).
Dihydroartemisinin repressed fibrosis by downregulating the TGF-β1, TNF-α, α-SMA, and NF-κB expression in lung tissues
To study the effect of dihydroartemisinin on inflammatory response, we evaluated the expression of TGF-β1, TNF-α, α-SMA, and NF-κB in lung tissues on day 14 after dihydroartemisinin infusion by immunocytochemical staining (Figure 4). It is examined that bleomycin can upregulate the lung tissues TGF-β1, TNF-α, α-SMA, and NF-κB expression of BLM group compared to NS group (Figure 4A, 4B), nevertheless, Treating with dihydroartemisinin 25 mg/kg/day, 50 mg/kg/day and 100 mg/kg/day for 14 d resulted in significantly lower expression level with less and weak staining. The higher dose of dihydroartemisinin was associated with a more significant reduction in the level of TGF-β1, TNF-α, α-SMA, and NF-κB expression (Figure 4C-F). It is suggested that the inhibitory effects of dihydroartemisinin on these increasing cytokines expression is effective.
Figure 4.
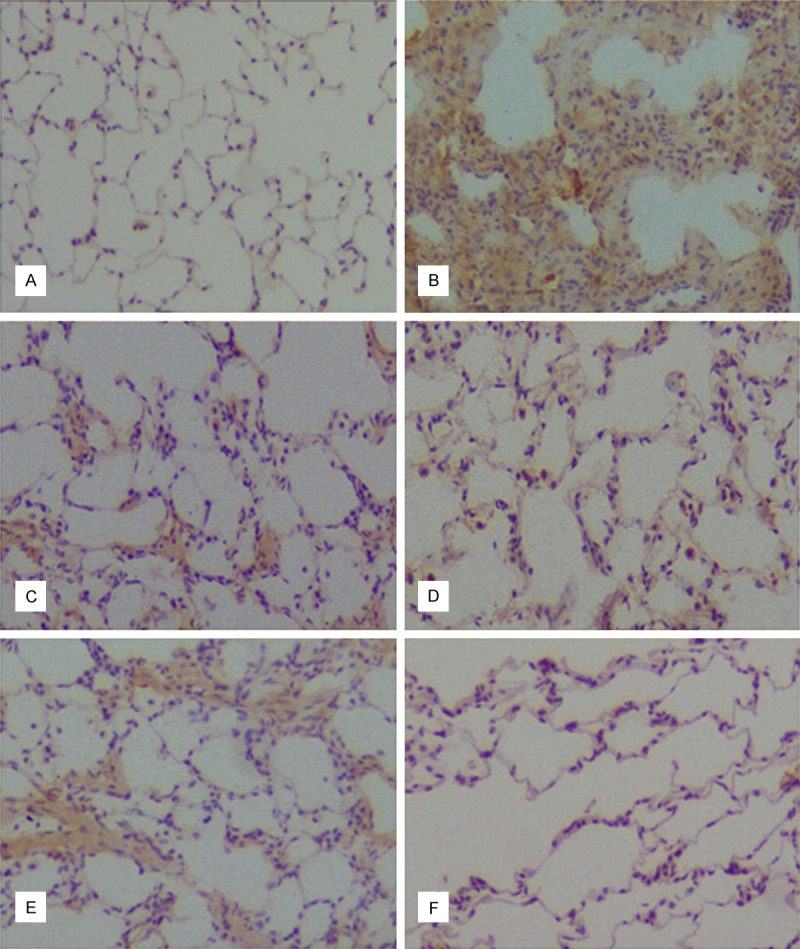
Comparing the expression of TGF-β1, TNF-α, α-SMA and NF-κB in every group by immunohistochemical assay. Intracellular TGF-β1 expression in NS group (A), BLM group (B) and DHA-3 group (C); (D) cell membrane TNF-α expression in DHA-3 group; (E) intracellular α-SMA (a characteristic marker of myofibroblasts) expression in DHA-3 group, (F) cell nuclear NF-κBp65 staining in DHA-3 group.
Dihydroartemisinin inhibited the mRNA and protein expression of TGF-β1, TNF-α, α-SMA, and NF-κB
To assess the effects of dihydroartemisinin on TGF-β1, TNF-α, α-SMA, and NF-κB production, the gene expression of TGF-β1, TNF-α, α-SMA, and NF-κB were examined in lung tissue by semi quantitative RT-PCR, and the protein level of TGF-β1, TNF-α, α-SMA, and NF-κB by western blotting (Figures 5, 6). Intratracheal bleomycin administration induced a significant increase in the levels of TGF-β1, TNF-α, α-SMA, and NF-κB mRNA and protein compared to NS group (P < 0.05). Treatment with 25, 50 and 100 mg/kg dihydroartemisinin significantly decreased the levels of TGF-β1, TNF-α, α-SMA, and NF-κB mRNA and protein compared to the bleomycin alone group (P < 0.05). The higher dose of dihydroartemisinin was associated with a more significant reduction in the level of mRNA and protein expression (P < 0.05). These data demonstrated that dihydroartemisinin can down regulate TGF-β1, TNF-α, α-SMA, and NF-κB expression at transcriptional and translational levels.
Figure 5.
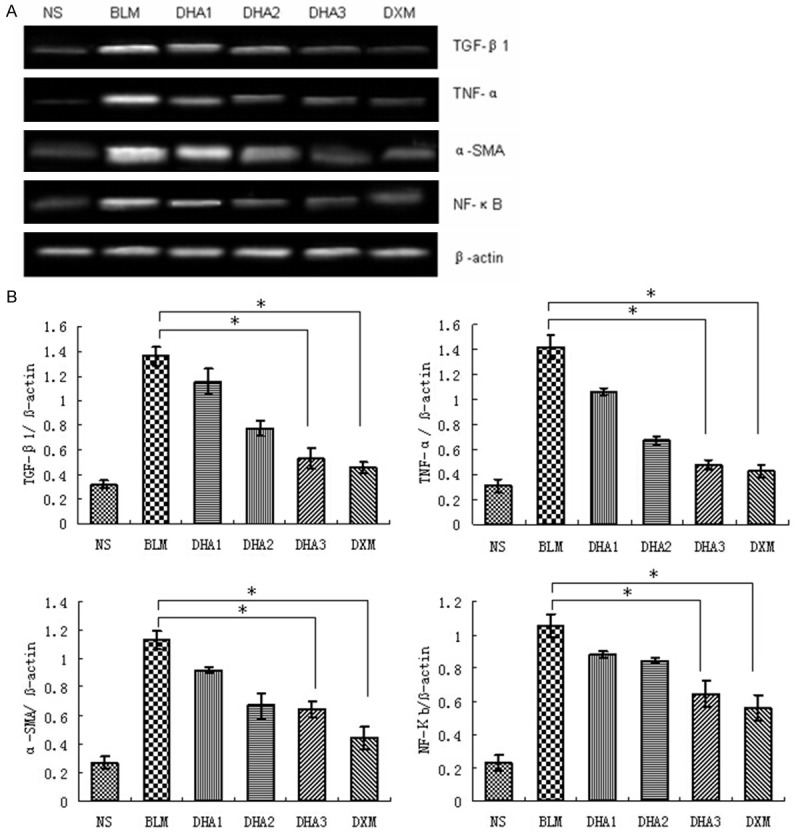
Effects of dihydroartemisinin on mRNA expression of TGF-β1, TNF-α, α-SMA, and NF-κB. A. Representative images of significant differences among groups are indicated by RT-PCR. B. Quantitative analysis of TGF-β1, TNF-α, α-SMA, and NF-κB. Averages of at least six independent experiments (n = 6). Bars indicate the mean ± SD. β-actin was used as internal control. Statistical analysis was performed using one-way ANOVA followed by multiple comparison test (*P < 0.05).
Figure 6.
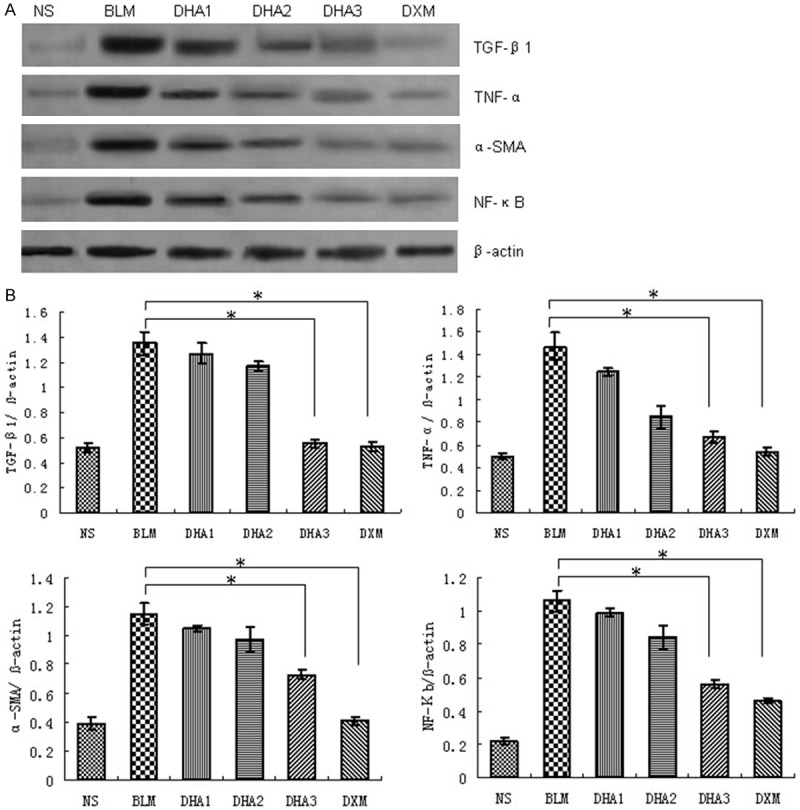
Effects of dihydroartemisinin on protein expression of TGF-β1, TNF-α, α-SMA, and NF-κB. A. Representative images of significant differences among groups are indicated by Western Blot. B. Quantitative analysis of TGF-β1, TNF-α, α-SMA, and NF-κB. Averages of at least six independent experiments. β-actin was used as internal control. Results are expressed as means ± SD (n = 6). Statistical analysis was performed using one-way ANOVA followed by multiple comparison test (*P < 0.05).
Discussion
The present study shows that dihydroartemisinin treatment may attenuate bleomycin-induced pulmonary fibrosis with the following mechanisms: (I) repressing the proliferation of bronchiolar fibroblasts; (II) decreasing the production of inflammatory and fibrotic cytokines and the infiltration of inflammatory cells; (III) reducing interalveolar septal thickening and alveolar damage; (IIII) dihydroartemisinin can down regulate TGF-β1, TNF-α, α-SMA, and NF-κB expression at transcriptional and translational levels.
Although several models of experimental pulmonary fibrosis were induced in animals by drug, radiation, or bronchiolitis obliterans, it remains unclear whether any of the experimental models exactly simulate the pulmonary fibrosis that is commonly seen in humans. However, the mouse model of bleomycin-induced pulmonary fibrosis has garnered most attention, perhaps due to its good characaterizaiotn and clinical relevance to human pulmonary fibrosis. Human lung fibrosis is characterized by alveolar epithelial cell injury, accumulation of fibroblasts and myofibroblasts, and deposition of extracellular matrix proteins. The result is a progressive loss of normal lung architecture and gas exchange. Intratracheal instillation of bleomycin induces acute lung inflammation with infiltration of inflammatory cells [11]. It has been shown that activated inflammatory cells and fibroblasts can synthesize and secrete reactive oxygen species, various chemokines and cytokines, and proteases, which can lead to aberrant fibroproliferation and collagen production in mice [12]. As demonstrated by our study, bleomycin treatment results in components of diffuse interalveolar septal thickening, accumulation of exudative fluids in alveolar space, diffuse infiltration of lymphocytes and macrophages in peribronchiolar connective tissue as seen in human pulmonary fibrosis.
A variety of pharmacological agents have been proposed as therapies for pulmonary fibrosis; however, no effective treatments are currently available [13]. This study aims to investigate the mechanisms of dihydroartemisinin-repressed pulmonary fibrosis in rat model of bleomycin-induced lung injury. The effectiveness and dosage of dihydroartemisinin in pulmonary fibrosis are unknown and it should be investigated in different dihydroartemisinin treatment groups of this study. In the present study, the number of neutrophils and macrophages in the pulmonary was significantly decreased by administration of dihydroartemisinin to bleomycin-treated rats. Intratracheal instillation of bleomycin induces acute lung inflammation with infiltration of inflammatory cells [14]. Inflammatory mediators play important roles in both the initiation and progression of pulmonary fibrosis, and biopsy and serum samples obtained from pulmonary fibrosis patients display elevated levels of TNF-α [15-17]. Experimental studies also showed that overexpression of TNF-α causes development of pulmonary fibrosis [18,19]. Macrophages and the other inflammatory cells produce TNF-α in lung after exposure to particulate matters of silica or bleomycin [20]. Interleukin-1 (IL-1) is another pivotal inflammatory cytokine that can activate RhoA and in turn evokes Rho-dependent cytoskeletal reorganization in vitro [21]. Importantly, in this study we determined that dihydroartemisinin decreases the levels of TNF-α, IL-1 in lung, which is correlated with reduced pulmonary fibrosis as evaluated by histopathological methods.
TGF-β1 is an important mediator with a broad spectrum of activities in pulmonary inflammation, tissue repair, and fibrosis [22-24]. Up-regulation of TGF-β1 plays critical roles in the pathogenesis of bleomycin-induced pulmonary fibrosis [25-27]. Moreover, TGF-β1 has been shown to induce differentiation of pulmonary fibroblasts into myofibroblasts, which is characterized by α-SMA, expression and active synthesis of extracellular matrix proteins [28,29]. Consistent with reduced fibrosis, the increased TGF-β1 production and α-SMA expression were reduced by dihydroartemisinin treatment.
The promoter parts of most genes encoding cytokines contain the binding sites of transcription factor NF-κB [30-32], such as TGF-β1 and TNF-α, and both are involved in the pathogenesis of pulmonary inflammation and fibrosis. In the current study, we determined the expression of p65 was significantly elevated upon bleomycin treatment, while the administration of dihydroartemisinin repressed its expression. These findings suggest that the inhibitory effects of dihydroartemisinin on the inflammation response and proliferation of bronchiolar fibroblast are likely mediated by repressed NF-κB signaling.
Collectively, our findings indicate that attenuation of pulmonary fibrosis by dihydroartemisinin maybe at least partially attributed to its inhibitory effect on fibrogenic cytokine production, which thereby modulates collagen synthesis. Thus, dihydroartemisinin holds promise as a novel drug to treat pulmonary fibrosis. In spite of these important findings, there are some limitations in experimental lung studies. The pulmonary fibrosis is evaluated only by biochemical and histopathological endpoints which can only partly reflect the progression of the disease [33]. Recently, it has been demonstrated that changes in clinical and physiologic variables of survival predictionin BLM induced pulmonary fibrosis may not correlate with biochemical and histological endpoints [34]. Pulmonary function tests are important predictors of survivals in patients with pulmonary fibrosis. These tests more directly correlate with survival than the severity of biochemical and histopathological patterns in humans.
In summary, the present study has shown that treatment with dihydroartemisinin attenuates bleomycin-induced pulmonary fibrosis. The beneficial effect of dihydroartemisinin was possibly related to the inhibition of inflammatory cell recruitment and decrease of TGF-β1, TNF-α, α-SMA and NF-κB expression and collagen synthesis. Our findings suggest that dihydroartemisinin may be a potential therapeutic candidate in the treatment of pulmonary fibrosis.
Acknowledgements
This work was supported by the Science Plan Projects of University in Shandong (J14LK52), Nature Science Foundation from Shandong Province (ZR2011HL064, ZR2011HQ006), Science plan projects of medicine and health development in Shandong (2014WS0184) and National Nature Science Foundation of China (NSFC81370730, NSFC31470415 and NSFC81273957).
Disclosure of conflict of interest
None.
References
- 1.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 2.Perri D, Cole DE, Friedman O, Piliotis E, Mintz S, Adhikari NK. Azathioprine and diffuse alveolar hemorrhage: the pharmacogenetics of thiopurine methyltransferase. Eur Respir J. 2007;30:1014–7. doi: 10.1183/09031936.00026107. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y, Yang X, Lian F, Sun L. Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology (Oxford) 2007;46:920–926. doi: 10.1093/rheumatology/kem014. [DOI] [PubMed] [Google Scholar]
- 5.Li WD, Dong YJ, Tu YY, Li ZB. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int Immunophamlacol. 2006;6:1243–50. doi: 10.1016/j.intimp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Wang CM, Li HX, Zhang XF. RoIe of Fas, FasL and Caspase-3 in artesunate-induced apoptosis of human embryonic lung fibroblasts. J C1inRehabil Tissue Eng Res. 2011;15:3785–3788. [Google Scholar]
- 7.Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med. 2011;17:355–361. doi: 10.1097/MCP.0b013e328349ac2b. [DOI] [PubMed] [Google Scholar]
- 8.Thrall RS, McCormick JR, Jack RM, McReynolds RA, Ward PA. Bleomycin-induced pulmonary fibrosis in the rat. AM J Pathol. 1979;95:117–127. [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Q, Tao Y, Piao H, Du MR, Li DJ. Trophoblasts and Decidual Stromal Cells Regulate Decidual NK Cell Functions Via Interaction between Collagen and LAIR-1. Am J Reprod Immunol. 2014;71:368–378. doi: 10.1111/aji.12211. [DOI] [PubMed] [Google Scholar]
- 10.Yuan WD, Yang DX, Sun XH, Liu W, Wang L, Li XY, Man XJ, Fu Q. Effects of hydroxysafflor yellow A on proliferation and collagen synthesis of rat vascular adventitial fibroblasts induced by angiotensin II. Int J Clin Exp Pathol. 2014;7:5772–5781. [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu Y, Dobashi K, Iizuka K, Horie T, Suzuki K, Tukagoshi H, Nakazawa T, Nakazato Y, Mori M. Contribution of small GTPase Rho and its target protein ROCK in amurine model of lung fibrosis. Am J Respir Crit Care Med. 2001;163:210–217. doi: 10.1164/ajrccm.163.1.2001089. [DOI] [PubMed] [Google Scholar]
- 12.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 13.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein RH, Fine A. Potential therapeutic initiatives for fibrogenic lung diseases. Chest. 1995;108:848–855. doi: 10.1378/chest.108.3.848. [DOI] [PubMed] [Google Scholar]
- 15.Bringardner BD, Baran CP, Eubank TD. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10:287–301. doi: 10.1089/ars.2007.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghu G, Brown KK, Costabel U, Cottin V, Bois RM, Lasky JA. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178:948–55. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 17.Thavarajah K, Wu P, Rhew EJ, Yeldandi AK, Kamp DV. Pulmonary complications of tumor necrosis factor-targeted therapy. Respir Med. 2009;103:661–669. doi: 10.1016/j.rmed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, Piguet PF, Vassalli P. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250–9. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa M, Fujimoto M, Kikuchi K. Elevated serum tumor necrosis factor-alpha levels in patients with systemic sclerosis: association with pulmonary fibrosis. J Rheumatol. 1997;24:663–5. [PubMed] [Google Scholar]
- 20.Piguet PF, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J. 1994;7:515–518. doi: 10.1183/09031936.94.07030515. [DOI] [PubMed] [Google Scholar]
- 21.Singh R, Wang B, Shirvaikar A, Khan S, Kamat S, Schelling JR, Konieczkowski M, Sedor JR. The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J Clin Invest. 1999;103:1561–1570. doi: 10.1172/JCI5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CG, Kang HR, Homer RJ, Chupp G, Elias JA. Transgenic modeling of transforming growth factor-beta (1): Role of apoptosis in fibrosis and alveolar remodeling. Proc Am Thorac Soc. 2006;3:418–423. doi: 10.1513/pats.200602-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strieter RM. What differentiates normal lung repair and fibrosis inflammation, resolution of repair and fibrosis. Proc Am Thorac Soc. 2008;5:305–310. doi: 10.1513/pats.200710-160DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: Down-regulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- 25.Bergeron A, Soler P, Kambouchner M, Loiseau P, Milleron B, Valeyre D, Hance AJ, Tazi A. Cytokine profiles in idiopathic pulmonary fibrosis suggest an important role for TGF-beta and il-10. Eur Respir J. 2003;22:69–76. doi: 10.1183/09031936.03.00014703. [DOI] [PubMed] [Google Scholar]
- 26.Kenyon NJ, Ward RW, McGrew G, Last JA. TGF-β1 causes airway fibrosis and increased collagen I and III mRNA in mice. Thorax. 2003;8:772–777. doi: 10.1136/thorax.58.9.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marimoto D, Kim H, Oyabu T. Effect of long-term inhalation of toner on exttacellular matrix in the lung of rat invivo. Inhal Toxical. 2005;17:153–159. doi: 10.1080/08958370590904517. [DOI] [PubMed] [Google Scholar]
- 28.Lee CG, Cho S, Homer RJ, Elias JA. Genetic control of transforming growth factor-beta1-induced emphysema and fibrosis in the murine lung. Proc Am Thorac Soc. 2006;3:476–477. doi: 10.1513/pats.200603-040MS. [DOI] [PubMed] [Google Scholar]
- 29.Cutroneo KR, White SL, Phan SH, Ehrlich HP. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol. 2007;211:585–589. doi: 10.1002/jcp.20972. [DOI] [PubMed] [Google Scholar]
- 30.Montaner S, Perona R, Saniger L, Lacal JC. Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. doi: 10.1074/jbc.273.21.12779. [DOI] [PubMed] [Google Scholar]
- 31.Gurujeyalakshmi G, Wang Y, Gift SN. Taurine and niacin block lung injury and fibrosis by down-regulating bleomycin-induced activation of transcription nuclear factor-kappaB in mice. J Pharmacol Exp Ther. 2000;293:82–90. [PubMed] [Google Scholar]
- 32.Ghosh S. Regulation of inducible gene expression by the transcription factor NF-kappaB. Immunol Res. 1999;19:183–189. doi: 10.1007/BF02786486. [DOI] [PubMed] [Google Scholar]
- 33.Voltz JW, Card JW, Carey MA, Degraff LM, Ferguson CD, Flake GP. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:45–52. doi: 10.1165/rcmb.2007-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collard HR, King TE Jr, Bartelson BB. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–42. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]


