Abstract
The exact immunology pathogenesis of hepatitis B virus (HBV) infection remains unclear currently. The dendritic cells (DCs) dysfunction is evident in adolescents with chronic HBV infection in the immune tolerant phase. DCs, as the most efficient professional antigen-presenting cells (APCs), possess the strongest antigen presenting the effect in the body and can stimulate the initial T cell activation and proliferation, depending on their stage of maturation. The recently classified type III interferon group, interferon-λ1 (IL-29), interferon-λ2 (IL-28A), and interferon-λ3 (IL-28B) displays immunomodulatory and antiviral activity. In the current study, we describe a way to stimulate the DCs maturation. As a result, IFN-λ1 combined with recombinant human granulocyte-macrophage colony stimulating factor (rhGM-CSF) and recombinant human interleukin-4 (rhIL-4) can induce the DCs maturation and promote the costimulatory molecules such as CD80, CD83, CD86 and human leucocyte antigen DR (HLA-DR) expression in the immune tolerance and the clearance phases. This study demonstrates that the DCs function is remarkably impaired both in the immune tolerant phase and the immune clearance phase in adolescents with chronic HBV infection compared with healthy youth control. At the same time, this study has developed a theoretical basis for the application of IFN-λ1 breaking immune tolerance and improving the body’s immune system to clear HBV.
Keywords: Dendritic cells, interferon-λ1, immune tolerance, adolescents, hepatitis B
Introduction
Despite an effective vaccine, chronic infection with hepatitis B virus (HBV) affects 350 million persons worldwide [1-3]. Long-term sequelae of CHB infection, which include cirrhosis, hepatic decompensation, and hepatocellular carcinoma, affect approximately one million persons annually [4]. HBV infection is a significant threat to public health and an enormous burden on society. To understand the exact immunology pathogenesis of HBV infection is very important because it can guide the clinician in deciding on the need and optimal timing for initiating antiviral therapy. In recent years, dendritic cells (DCs) in the pathogenesis of chronic hepatitis B cause more and more attention. During the past decade, multiple research groups have focused on DCs, in hopes of unraveling an HBV-specific DC signature or DC-dependent mechanisms of antiviral immunity that would lead to a successful HBV elimination strategy. Antigen presenting cells (APCs) are the initiators of the immune response. DCs, as the most efficient professional APCs [5,6], possess the strongest antigen presenting effect in the body and can stimulate the initial T cell activation and proliferation, which is different from other APCs [7]. In humans, the existence of phenotypically and functionally distinct DCs subsets has been reported: myeloid DC (mDC) and plasmacytoid DC (pDC) [8]. The functional status of DCs directly influences the cellular and humoral immune response. The differentiation process of DCs results in two main types of DCs: one is immature DCs (imDCs), which have large phagocytic capacity. The other is mature DCs, which can stimulate the initial T cell activation and produce co-stimulatory molecules and molecules of MHC class I and II. The function of DCs depends on their stage of maturation. Most DCs show the immaturity in most tissues where they are capable of capturing antigens [9]. These immature DCs lack of costimulatory molecules such as CD80, CD83, and CD86, which can stimulate the initial T cell activation. DCs reside in an immature form, being on alert for microbial pathogens that they can efficiently bind and internalize bacteria. In the process of antigen capture, antigen processing, and the formation of MHC-antigen peptide compounds, the expression of costimulatory molecules such as CD80, CD83, and CD86 increase. And with the increase of costimulatory molecules, DCs begin to mature and thus the functions of DCs begin to switch from an antigen-capturing mode to an antigen-presenting and T cell-stimulating mode [10]. So the functional defects in DCs could be an important mechanism of the virus to evade host immune response. When infected with chronic HBV, the amount and function of DCs in infection patients decrease in varying degrees. The costimulatory molecules expression on the surface of DCs is reduced and the secretion of interleukin-12 (IL-12) that inducing T cells to differentiate into Thl is declined as well as the ability to stimulate T cell proliferation is lower. Recently, a novel interferon-λ1 (IFN-λ1) has been discovered which can induce the DCs maturation and enhance immune function [11]. This study aimed to observe the effect of IFN-λ1 on the function of peripheral blood mononuclear cell (PBMC) DCs in adolescents with chronic HBV infection both in the immune tolerant and the immune clearance phases.
Materials and methods
Patients
44 adolescents with chronic HBV infection who were inpatients or outpatients at the Third Hospital of Hebei Medical University were chosen from March 2011 to August 2012. There were 30 males and 14 females aged 12 to 28 years among the 44 adolescent patients. The mean age was 18.9 ± 4.7 years. All the diagnoses were consistent with EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection [3]. The HBsAg, HBeAg and anti-HBc were positive, and HBV DNA > 1 × 105 IU/mL in all the patients. Liver puncture biopsy was performed in all of the 44 patients, and the pathological reports were made by professional pathologist. All of the 44 patients, there were 23 cases in the immune tolerant phase: Serum ALT and AST ≤ 2 × upper limit of normal (ULN), and the hepatic histology showed mild or no liver necroinflammation (G ≤ 1). There were 21 cases in the immune clearance phase: Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) sustainedly or repeatedly increased and > 2 × ULN, inflammatory necrosis lesions were seen in hepatic histology (G ≥ 2). The serological markers of hepatitis virus A, C, D and E were negative in all the selected cases. Patients with liver injury caused by other factors, combined with other acute or chronic diseases, treated with immunomodulators or antiviral agents within the past six months were excluded. Another ten healthy adolescents were chosen as a control group. The study was approved by the Ethics Committee, Third Hospital of Hebei Medical University, and informed consent was signed by all the participants.
Reagents and materials
The serum-free RPMI 1640 media were purchased from HyClone company in the US, interleukin-4 (rhIL-4), recombinant human granulocyte macrophage colony stimulating factor (rhGM-CSF), recombinant human IFN-λ1 were purchased from PeproTech company in the US. Phycoerythrin (PE) mouse anti-human CD80 and mouse IgG1 (PE) isotype control, PE mouse anti-human CD86 and mouse IgG2b (PE) isotype control, PE mouse anti-human HLA-DR and PE mouse IgG2a isotype control were purchased from BioLegend Company in the US. IFN-γ and interleukin-12 (IL-12), enzyme-linked immunoassay (ELISA) kits were purchased from Banner Company in the US.
Serum biochemical and virological indicators
Liver function was estimated by using Japanese Olympus AU 2700 automatic biochemical analyzer. HBsAg, anti-HBs, HBeAg, anti-HBe and anti-HBc were measured by ELISA. HBV-DNA quantification was examined using real-time PCR assay, and the lowest detection limit was 15 IU/ml.
Cell culture and isolation
The PBMCs were separated from the participants mentioned above using density gradient centrifugation. The separated PBMCs were suspended, precipitated, resuspended and cultured in 5% CO2 atmosphere at 37°C for 2~4 h, then the adherent cells were harvested. The following stimuli were added as indicated: IFN-λ1 group: cultured with IFN-λ1 100 ng/mL only; routine group: cultured with IL-4 100 ng/mL and GM-CSF 100 ng/mL; combination group: adding IFN-λ1 100 ng/mL, IL-4 100 ng/mL and GM-CSF 100 ng/mL. Cells were then cultivated in serum free medium and were observed under an inverted microscope on the 0th, 2nd, 5th and 7th day of cultivation. Culture supernatant was collected after seven days of cultivation and stored at -80°C for examination.
DC surface molecule detection
The cultured mature DC suspension was collected and centrifuged at 2500 r/min for 20 minutes with a 16.5 cm centrifugal radius. The supernatant was abandoned. Precipitation cells were rinsed twice in PBS, centrifuged at 1500 r/min for 5 minutes with a 16.5 cm centrifugal radius. Precipitation cells were suspended into > 5 × 105/tube with PBS and 20 μL of PE-CD80, PE-CD83, PE-CD86 and PE-HLA-DR were added respectively, and isotype control was made. They were then centrifuged at 1500 r/min for 5 minutes with a 16.5 cm centrifugal radius and washed once with PBS. Cells were resuspended in 500 μL PBS and placed on. Gating DC and counting 10,000 cells to analyze by flow cytometry. IFN-γ and IL-12 in cell culture supernatant were detected by ELISA conducting strictly according to the instructions.
Statistical analysis
The database was established using SPSS 13.0 statistical software for statistical analysis. The results were as means ± standard deviation (X̅ ± s). One way ANOVA was used to compare means of multiple samples. Independent samples t-test was used in the comparisons between two groups. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
Of all the 44 adolescents with chronic HBV infection, there were 23 cases including 15 males and 8 females in the immune tolerant phase aged 15-26 years with a mean age of (17.9 ± 4.8) years. The remaining cases including 15 males and 6 females were in the immune clearance phase aged 14-28 years with a mean age of (18.2 ± 7.3) years. The ALT and AST were (238.38 ± 97.55) U/L and (132.02 ± 48.07) U/L respectively for patients in the immune clearance phase, while patients in the immune tolerant phase were (70.36 ± 40.28) U/L and (65.99 ± 27.98) U/L respectively (t = 7.59 and 5.63 respectively, P < 0.01 all two pairings). The HBV DNA of patients in the immune clearance phase and the immune tolerant phase were (6.93 ± 1.89) lg IU/mL and (6.78 ± 1.34) lg IU/mL respectively (t = 0.31, P > 0.05).
DC morphology changes in each group
No morphological changes of the adherent cells were observed in IFN-λ1 group on the 2nd, 5th and 7th day of culture, neither did 11 days later. These cells from IFN-λ1 group were mostly suspended, but there was no change in cell volume (Figure 1A). Conversely, in the routine and combination group, the shape of the cells changed significantly. The amount of adherent cells decreased and the adherent cells gradually became suspended and increased in volume after an overnight culturing. Adherent cell stretching was seen after two days of induction and cultivation. A notable feature of activated DCs was the occurrence of cell clusters in the routine group after five days of culture (Figure 1B). After maturation was inducted with IFN-λ1, rhGM-CSF and rhIL-4, a large of cell clusters occurred and dendrite crossed on the surface of cells that formed the typical DC morphology on the 7th day as shown in Figure 1C.
Figure 1.
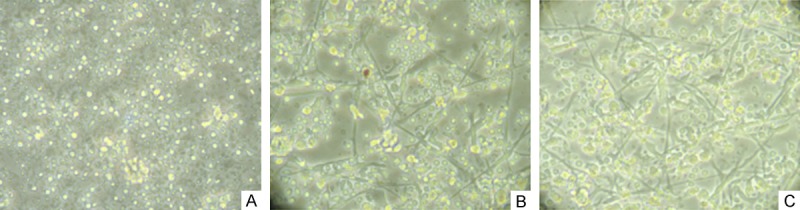
The morphology of DCs in different groups on the 7th day (× 400). A. IFN-λ1 group: No morphological changes of the adherent cells were observed. These cells from IFN-λ1 group were mostly suspended, but there was no change in cell volume. B. Routine group: The shape of the cells changed: the adherent cells gradually became suspended and increased in volume, then adherent cell stretching was seen and cell clusters formed in the surface of the cells after five days of culture. C. Combination group: dendrite crossed on the surface of cells that formed the typical DC morphology.
The detection results of cell surface molecules by flow cytometry
Because terminal DC maturation determines the outcome of immune responses, the surface markers of DCs were evaluated in each group to determine the maturation state of DCs. As shown in Figure 2, the expression of CD80, CD83, CD86 and HLA-DR in healthy control group was higher than not only the routine group but also the combination group both in the immune tolerant phase and the immune clearance phase after 7 days culture (P < 0.01). With co-cultivation, the expression of DC surface molecules both in the immune tolerant phase and the immune clearance phase showed a significant increase compared with the routine group (P < 0.01). In the routine group, the expression of CD80, CD83, CD86 and HLA-DR in the immune clearance phase showed a significant increase compared with the immune tolerant phase (P < 0.01). In the combination group, the expression of CD80 and CD83 in the immune clearance phase were significantly increased compared with the immune tolerant phase (P < 0.01), while the levels of CD86 and HLA-DR were increased in the immune clearance phase, but did not reach statistical significance (P > 0.05). These results demonstrate that IFN-λ1 combined with rhGM-CSF and IL-4 can induce DC maturation and increase the the expression of costimulatory molecules to improve the body’s immune ability.
Figure 2.
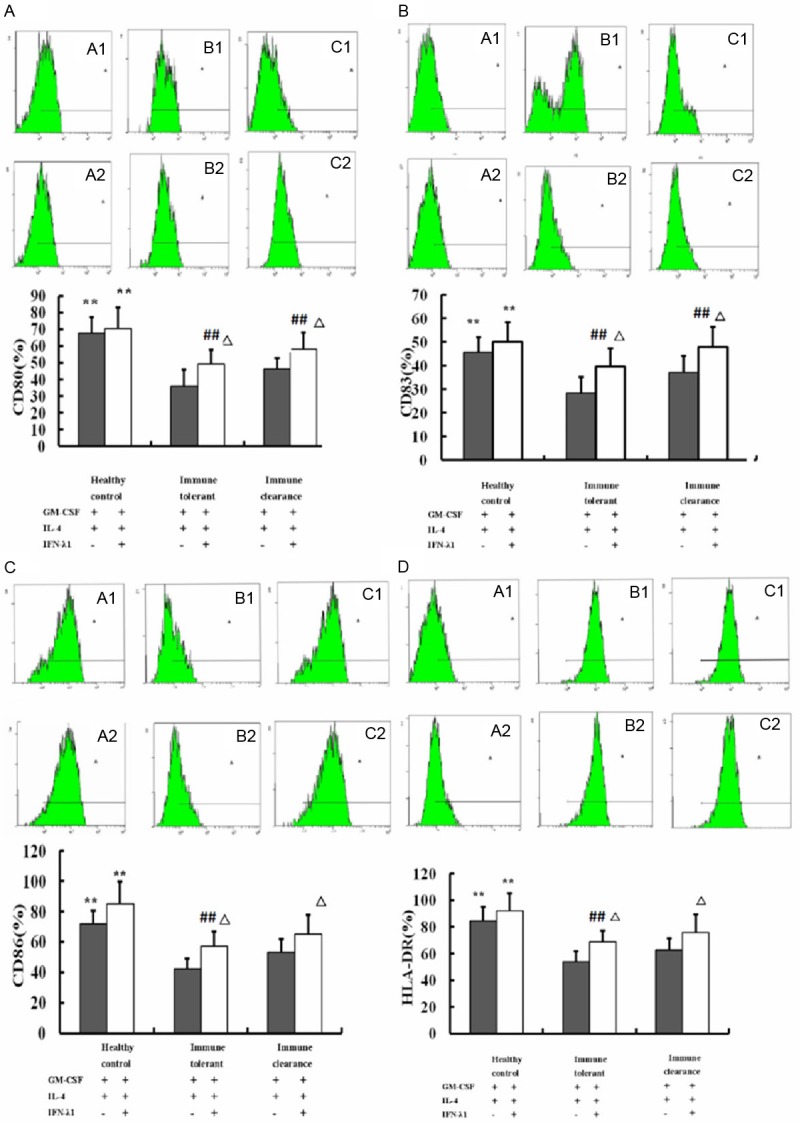
Effect of IFN-λ1 on DCs costimulatory molecules expression. Data were exhibited as a percentage. The expression of CD80, CD83, CD86 and HLA-DR in healthy control group was higher than not only the routine group but also the combination group both in the immune tolerant phase and the immune clearance phase. **P < 0.01 compared with the immune tolerant and the immune clearance phases. In the routine group, the expression of CD80, CD83, CD86 and HLA-DR in the immune clearance phase showed a significant increase compared with the immune tolerant phase (##P < 0.01). In the combination group, the expression of CD80 and CD83 in the immune clearance phase were significantly increased compared with the immune tolerant phase (##P < 0.01), while the levels of CD86 and HLA-DR were increased in the immune clearance phase, but did not reach statistical significance (P > 0.05). The expression of CD80, CD83, CD86 and HLA-DR in the combination group was higher than the routine group both in the immune tolerant phase and the immune clearance phase. ΔP < 0.01 in comparison with routine group.
IFN-λ1 alters the expression of IL-12 and IFN-γ on human DCs
The secretion of IL-12 and IFN-γ of DCs in healthy control group was higher than not only the routine group but also the combination group both in the immune tolerant phase and the immune clearance phase (P < 0.01). The secretion of IL-12 and IFN-γ of DCs in a combination group was higher than routine group both in the immune tolerant phase and the immune clearance phase (P < 0.01). In the current study, we can get the result that after adding of IFN-λ1, the secretion of IL-12 and IFN-γ of DCs showed a significant increase in the combination group. This result is consistent with the concept that IFN-λ1 combined with rhGM-CSF and IL-4 can induce DC maturation and increase the secretion of cytokines as shown in Figure 3.
Figure 3.
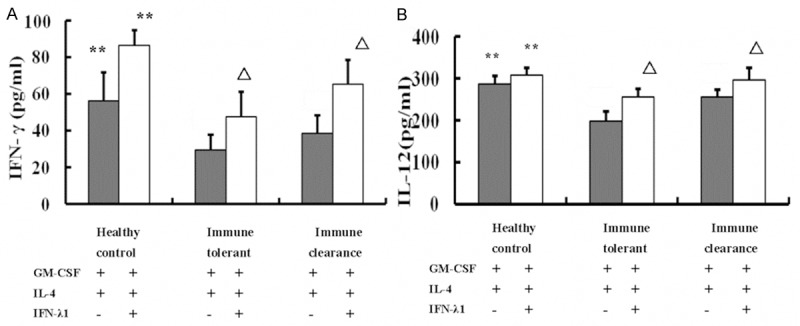
Effects of IFN-λ1 on DCs cytokine release. The secretion of IL-12 and IFN-γ of DCs in healthy control group was higher than not only the routine group but also the combination group both in the immune tolerant phase and the immune clearance phase. **P < 0.01 compared with the immune tolerant and immune clearance phases. The secretion of IL-12 and IFN-γ of DCs in combination group showed a significant increase compared with the routine group both in the immune tolerant phase and the immune clearance phase. ΔP < 0.01 in comparison with the routine group.
Discussion
DCs are the most powerful APCs, the only cells to activate resting T lymphocytes discovered so far, they defense against viral infections through the secretion of IL-12, IFN-γ and other Thl cytokines as well as by presenting antigen to induce T cells to clear the virus. DCs responds to microbial infections by undergoing phenotypic maturation and producing multiple cytokines [12], such as human mDCs and pDCs can produce high levels of IL-28 and IL-29. DCs are located in nonlymphoid and peripheral lymphoid tissues, where they act as of environment cues and orchestrate the interplay between the innate immune system and adaptive immune system to provoke a successful response [13]. IL-12 can induce differentiation of Th0 cells into Th1 cells and enhance the cytotoxicity of cytotoxic T lymphocytes (CTLs) [13]. The body clears HBV mainly through cellular immune restricted by HLA-I molecules and mediated by response CD8+ CTL. Massive replication of the persistent virus not only outpaces T-cell differentiation but also may induce the T-cell exhaustion that prevents long-term virus clearance [14]. As DCs were the starters and undertakers of immune response, thus the abnormality in number, phenotype and function of DC may affect the outcome of HBV infection, and DCs are closely related with the persistent HBV infection [15].
Natural history of HBV infection in infants and young children is very complicated. Four phases are included: an immune tolerant phase, an immune clearance phase, low or non-replicative phase, and reactivation phase [16,17]. The immune tolerant phase is thought to occur most frequently in persons who are infected from HBeAg-positive mothers via perinatal transmission. HBeAg may act as an immune tolerant protein that aids the virus in avoiding detection by the immune system. In the clearance phase, the host’s immune system recognizes HBV as being foreign and initiates an immune response that results in hepatocyte damage [18]. But the exact immunology pathogenesis of HBV infection remains unclear currently.
In this study, among the 44 adolescents with chronic HBV infection, the mothers of 37 cases had chronic HBV infection, indicating that the perinatal periods or infancy may be the infection time for adolescents with chronic HBV infection. The immune tolerant phase is more common in patients infected in infants and young children period and childhood which characterized by HBeAg-positive, a high HBV DNA loads and mild pathological changes of liver tissue [19]. Zhang et al. found that there were abnormalities in DC function, quantity and phenotype of children with HBV infection [20]. Vander Molen et al. suggested that the expression of DC surface molecules was inhibited in varying degrees after HBV infection and resulted to the obstacles in the process of immature DCs transferring into mature DCs [21]. The functional defect of DCs in chronic HBV infection patients can affect the differentiation of Thl/Th2 and cause insufficient CTL response. Another study showed that the expression frequency of HBcAg18-27-specific CD8+ T cells in peripheral blood of patients in the immune tolerant phase was significantly lower than those in the immune clearance phase [22].
IFNs can regulate the expression of many innate immune receptors, stimulate cytokine and chemokine production, regulate cell differentiation and polarization, inhibit cell proliferation and regulate apoptosis [23], besides having direct antiviral effects. The recently classified type III interferon group, IFN-λ, consists of IFN-λ1 (IL-29), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B) discovered by American scientists Kotenko and Sheppard et al. in 2003 [24,25]. A number of different cell types have been reported to produce IFN-λ in response to viral infection and/or maturation stimuli including monocytes, monocyte-derived dendritic cells (MDDC) and pDC [26,27]. IFN-λ and IFN-α activate the same signaling pathway through different receptors, IFN-λ like the type I IFNs, trigger signal transduction via the JAK/STAT pathway, including the activation of JAK1 and TYK2 kinases, the phosphorylation of STAT proteins, and the activation of the transcription complex of IFN-stimulated gene factor 3 [28,29]. A major difference between type III and type I IFNs is the distribution of their respective receptor complexes. Moreover, IFN-λ seems to have immunomodulatory functions because IFN-λ stimulated human monocyte-derived DCs induce proliferation of FOXP3-expressing suppressor T cells with contact-dependent suppressive activity on T cell proliferation [30]. IFN-λ plays an important role in regulating the maturation and function of DC, enhancing the cytotoxicity of NK cells and T cells, enhancing the function of Thl cells, inhibiting the production of Th2 cytokine and upregulating the expression of major histocompatibility complex (MHC) class I molecules [31]. IFN-λ1 (IFN-k1/IL-29), the prototype member of the human interferon lambda family, inhibits the development of human Th2 [32].
In the past study, Megjugorac et al. found that IFN-λ1 could significantly increase the expression of CD80, CD83 and IL-28Rα in DCs by cultivated normal human PBMC-derived DCs, and the herpes simplex virus type 1 (HSV-1), imiquimod (5 μg/mL) and IFN-λl (100 ng/mL) were added to stimulate their maturation [33]. Caux et al. reported that GM-CSF combined with TNF-α successfully induced the differentiation from CD34+ stem cells into DCs in 1992 [34]. It was reported that both GM-CSF combined with IFN-α and GM-CSF combined with IL-4/TNF-α resulted in CD11c+CD86+ HLA-DR+ cells with a typical DC morphology that could efficiently stimulate T cells by culturing PBMCs [35]. The other report showed that a combination of GM-CSF and IL-4 provided the best conditions for the generation of cells from PBMCs with the characteristic phenotype and functional properties of DCs (high expression of CD1, class II and B7, and high stimulatory capacity in allogeneic and autologous mixed leukocyte reaction) [36]. Another study showed that by culturing cord blood monocytes with GM-CSF (100 ng/ml) and IL-4 (10 ng/ml), cord blood-adherent cells became nonadherent, acquired DC morphology, and showed increased expression of CD1a, CD80, CD86 and HLA-DR. At the same time, they lost membrane CD14 and some cells with the expression of CD83 and CMRF-44 were generated. This study demonstrated that GM-CSF combined with IL-4 can induce the generation of DC [37]. Recently, GM-CSF combined with IL-4 has been a classic culture combination to DC. However, very little research on the ability of IFN-λl to regulate DC function has been done. In this study, we provide morphological, phenotypical, and functional evidence that IFN-λ1 combined with rhGM-CSF and IL-4 cultivation can modulate the differentiation of monocyte-derived DCs in vitro. The distinctive morphology of mDCs is dendrite formation. In this study, we observed that the DCs induced by IFN-λ1 combined with rhGM-CSF and IL-4 exhibited a different morphology compared to routine-induced DCs. IFN-λ1 induced dendritic morphology formation, including the formation of clusters suggesting that IFN-λ1 could induce the differentiation and maturation process of DCs.When stimulated with IFN-λ1 combined with rhGM-CSF and IL-4, the imDCs would convert into the mature phenotype, characterized by increased the expression of costimulatory molecules and MHC molecules. We observed that IFN-λ1 up-regulated MHC molecules, DC specific maturation molecules (CD80, CD83, CD86 and HLA-DR). These molecules are characteristic markers that are highly expressed on mature DCs. The higher expression of CD80, CD83, CD86 and HLA-DR on IFN-λ1-induced cells indicates an improvement of the DC maturation process. We observed an increase in the expression of various surface molecules known to play important roles in the process of T cell activation by mediating intercellular contact and delivering essential costimulatory activity. Importantly, IFN-λ1 modulated cytokine production by DCs. The major Th1-skewing factors IL-12 and IFN-γ were substantially increased when stimulated with IFN-λ1 combined with rhGM-CSF and IL-4, which might critically influence the development of a subsequent T cell response. These data suggest that IFN-λ1 plays an important role in the early stage of DC differentiation by promoting the process of maturation from monocytic precursors into mature DCs, and thus potentially repairing the function of DCs. Currently, IFN-λ1 combined with rhGM-CSF and IL-4, has been a classic culture combination to DC. In this study, the IFN-λ1 combined with rhGM-CSF and IL-4 cultivation can significantly increase the expression of DC surface molecules and the secretion of IL-12 and IFN-γ in the immune tolerant and the immune clearance phase compared with the routine cultivation, further suggesting that IFN-λ1 is involved in regulating the maturation and function of DCs.
In conclusion, the DC dysfunction is evident in adolescents with chronic HBV infection in the immune tolerant phase. IFN-λ1 combined with rhGM-CSF and IL-4 can induce DC maturation, increase the expression of the costimulatory molecules and stimulate cytokine and chemokine production in the immune tolerant phase and the immune clearance phase. This study has developed a theoretical basis for the application of IFN-λ1 breaking immune tolerance and improved the body’s immune ability to clear HBV.
Acknowledgements
This study was supported by HeBei province science funds (142777762D).
Disclosure of conflict of interest
None.
References
- 1.Popalis C, Yeung LT, Ling SC, Ng V, Roberts EA. Chronic hepatitis B virus (HBV) infection in children: 25 years’ experience. J Viral Hepat. 2013;20:e20–26. doi: 10.1111/jvh.12019. [DOI] [PubMed] [Google Scholar]
- 2.Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK Hong Kong Liver Fibrosis Study Group. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395–401. doi: 10.1002/hep.21724. [DOI] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Byrne DD, Newcomb CW, Carbonari DM, Nezamzadeh MS, Leidl KB, Herlim M, Yang YX, Hennessy S, Kostman JR, Leonard MB, Localio AR, Lo Re V 3rd. Risk of hip fracture associated with untreated and treated chronic hepatitis B virus infection. J Hepatol. 2014;61:210–218. doi: 10.1016/j.jhep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 6.Dolganiuc A, Szabo G. Dendritic cells in hepatitis C infection: can they (help) win the battle? J Gastroenterol. 2011;46:432–447. doi: 10.1007/s00535-011-0377-y. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Chang CH, Rossi EA, Cardillo TM, Goldenberg DM. Interferon-lambda1 linked to a stabilized dimer of Fab potently enhances both antitumor and antiviral activities in targeted cells. PLoS One. 2013;8:e63940. doi: 10.1371/journal.pone.0063940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshio S, Kanto T, Kuroda S, Matsubara T, Higashitani K, Kakita N, Ishida H, Hiramatsu N, Nagano H, Sugiyama M, Murata K, Fukuhara T, Matsuura Y, Hayashi N, Mizokami M, Takehara T. Human blood dendritic cell antigen 3 (BDCA3)(+) dendritic cells are a potent producer of interferon-lambda in response to hepatitis C virus. Hepatology. 2013;57:1705–1715. doi: 10.1002/hep.26182. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Li L, Min J, Wang J, Wu H, Zeng Y, Chen S, Chu Z. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 2012;277:66–73. doi: 10.1016/j.cellimm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Gottenberg JE, Chiocchia G. Dendritic cells and interferon-mediated autoimmunity. Biochimie. 2007;89:856–871. doi: 10.1016/j.biochi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Liu X, Zhou Y, Su SB. Interferon-lambdas: the modulators of antivirus, antitumor, and immune responses. J Leukoc Biol. 2009;86:23–32. doi: 10.1189/jlb.1208761. [DOI] [PubMed] [Google Scholar]
- 12.Osterlund P, Veckman V, Siren J, Klucher KM, Hiscott J, Matikainen S, Julkunen I. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol. 2005;79:9608–9617. doi: 10.1128/JVI.79.15.9608-9617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kis-Toth K, Szanto A, Thai TH, Tsokos GC. Cytosolic DNA-activated human dendritic cells are potent activators of the adaptive immune response. J Immunol. 2011;187:1222–1234. doi: 10.4049/jimmunol.1100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng G, Luo B, Li J, Zhao D, Wu W, Chen F, Chen Z. Hepatitis B e-antigen Persistency is Associated with the Properties of HBV-Specific CD8 T Cells in CHB Patients. J Clin Immunol. 2010;31:195–204. doi: 10.1007/s10875-010-9483-5. [DOI] [PubMed] [Google Scholar]
- 16.Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, Amarapurkar D, Cooksley G, Jafri W, Mohamed R, Hou JL, Chuang WL, Lesmana LA, Sollano JD, Suh DJ, Omataet M. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 17.Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29(Suppl 1):100–107. doi: 10.1111/j.1478-3231.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- 18.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49(Suppl 5):S45–55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy PT, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan AT, Naik S, Foster GR, Bertoletti A. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology. 2012;143:637–645. doi: 10.1053/j.gastro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Chen D, Yao J, Zhang H, Jin L, Shi M, Zhang H, Wang FS. Increased infiltration of intrahepatic DC subsets closely correlate with viral control and liver injury in immune active pediatric patients with chronic hepatitis B. Clin Immunol. 2007;122:173–180. doi: 10.1016/j.clim.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 21.van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG, Kwekkeboom J, Janssen HL. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738–746. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenegger FS, Mueller K, Otte B, Beck B, Hiddemann W, Schendel DJ, Subklewe M. CD86 and IL-12p70 are key players for T helper 1 polarization and natural killer cell activation by Toll-like receptor-induced dendritic cells. PLoS One. 2012;7:e44266. doi: 10.1371/journal.pone.0044266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaslavsky E, Hershberg U, Seto J, Pham AM, Marquez S, Duke JL, Wetmur JG, Tenoever BR, Sealfon SC, Kleinstein SH. Antiviral response dictated by choreographed cascade of transcription factors. J Immunol. 2010;184:2908–2917. doi: 10.4049/jimmunol.0903453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 25.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nature Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 26.Yin Z, Dai J, Deng J, Sheikh F, Natalia M, Shih T, Lewis-Antes A, Amrute SB, Garrigues U, Doyle S, Donnelly RP, Kotenko SV, Fitzgerald-Bocarsly P. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J Immunol. 2012;189:2735–2745. doi: 10.4049/jimmunol.1102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi B, Dickensheets HL, Spann KM, Alston MA, Luongo C, Dumoutier L, Huang J, Renauld JC, Kotenko SV, Roederer M, Beeler JA, Donnelly RP, Collins PL, Rabin RL. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J Virol. 2006;80:5032–5040. doi: 10.1128/JVI.80.10.5032-5040.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witte K, Witte E, Sabat R, Wolk K. IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties. Cytokine Growth Factor Rev. 2010;21:237–251. doi: 10.1016/j.cytogfr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mennechet FJ, Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 2006;107:4417–4423. doi: 10.1182/blood-2005-10-4129. [DOI] [PubMed] [Google Scholar]
- 31.Hillyer P, Mane VP, Schramm LM, Puig M, Verthelyi D, Chen A, Zhao Z, Navarro MB, Kirschman KD, Bykadi S, Jubin RG, Rabin RL. Expression profiles of human interferon-alpha and interferon-lambda subtypes are ligand- and cell-dependent. Immunol Cell Biol. 2012;90:774–783. doi: 10.1038/icb.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Megjugorac N, Gallagher G, Gallagher G. 270 Production of, and response to, IFN-λ1 by plasmacytoid dendritic cells. Cytokine. 2008;43:308–308. [Google Scholar]
- 33.Megjugorac NJ, Gallagher GE, Gallagher G. Modulation of human plasmacytoid DC function by IFN- 1 (IL-29) J Leukoc Biol. 2009;86:1359–1363. doi: 10.1189/jlb.0509347. [DOI] [PubMed] [Google Scholar]
- 34.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 35.Korthals M, Safaian N, Kronenwett R, Maihofer D, Schott M, Papewalis C, Diaz Blanco E, Winter M, Czibere A, Haas R, Kobbe G, Fenk R. Monocyte derived dendritic cells generated by IFN-alpha acquire mature dendritic and natural killer cell properties as shown by gene expression analysis. J Transl Med. 2007;5:46. doi: 10.1186/1479-5876-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sallusto F, Lanzavecchia A. Human Dendritic Cells Is Maintained by Granulocyte/Macrophage Colony-stimulating Factor Plus Interleukin 4 and Downregulated by Tumor Necrosis Factor α. J Exp Mede. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Z, Takahashi M, Narita M, Toba K, Liu A, Furukawa T, Koike T, Aizawa Y. Generation of dendritic cells from adherent cells of cord blood by culture with granulocyte-macrophage colony-stimulating factor, interleukin-4, and tumor necrosis factor-alpha. J Hematotherapy Stem Cell Res. 2000;9:453–64. doi: 10.1089/152581600419116. [DOI] [PubMed] [Google Scholar]


