Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disorder that affects approximately 1% of the world’s population. The pathogenesis of RA is not understood fully. It is assumed that endothelial function is associated with the proinflammatory state of RA. Endothelial dysfunction/activation reflects the increased level of von Willebrand factor (vWF) and a shift toward prothrombotic activity of the endothelium. The present study was performed to investigate the possible relationships between vWF and claudin-5 and the level of disease activity in patients with RA. The study population was divided into four groups according to the disease activity score in 28 joints (DAS28): remission group (RG), 18 patients (DAS28 < 2.6); low disease activity group (LDAG), 23 patients (DAS28 > 2.6-3.2); moderate disease activity (MDAG), 23 patients (DAS28 > 3.2-5.1); high disease activity group (HDAG), 14 patients (DAS28 > 5.1); and control group (CG), 10 healthy subjects. Claudin-5 and vWF assessment were derived from serum samples gathered from the patients known to have RF and anti-CCP titers in the normal ranges. A high positive association of claudin-5 and vWF with the MDAG was observed (P < 0.001). The results of our study indicated that the relationship between vWF and claudin-5, which are indicators of endothelial cell dysfunction and tight junction activity, may be a predictor of disease activity. Further studies are required to investigate these pathways to shed light on the roles of claudin-5 and vWF in the progression of inflammation and other vascular conditions.
Keywords: Claudin-5, von Willebrand factor (vWF), rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disorder that affects approximately 1% of the world’s population [1]. Unfortunately, its management has a significant impact on quality of life unfortunatelly, its management has a significant impact on quality of life [2]. C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) are routinely used in evaluation of disease activity in RA [3]. However, these parameters are not disease-specific. Investigation of effective new treatments and prevention strategies to improve the progression of disease activity in RA are needed. However, the pathogenesis of RA is not understood fully. It is assumed that endothelial function is associated with the proinflammatory state of RA [4]. Endothelial cells are recognized as gatekeepers that control the infiltration of leukocytes and plasma proteins into the walls of blood vessels. Endothelial cell-cell junctions can also function as signaling structures directly or indirectly [5]. Tight junction (TJ) proteins are located at endothelial cell-cell contacts. TJ protein complexes are composed of transmembrane, cytoplasmic, and cytoskeletal proteins [6]. The roles of several TJ proteins, including zonula occludens (ZO), occludin, and junctional adhesion molecules (JAMs), have been demonstrated in RA [7,8]. The organization of endothelial junctional complexes is affected by cytokines in many diseases. Endothelial dysfunction/activation reflects the increased level of von Willebrand factor (vWF) and a shift toward prothrombotic activity of the endothelium. The present study was performed to investigate the possible relationships between vWF and claudin-5 and the level of disease activity in patients with RA. The roles of this mechanism and possible association with disease activity have not been studied extensively.
Materials and methods
A total of 78 patients who were followed up at the Rheumatology Policlinics in the Medicine Faculty Hospital, Sakarya University between April 2012 and September 2013 and fulfilled the ACR/EULAR 2010 RA classification criteria were enrolled in this study. Ten healthy control subjects who were followed up at the checkup polyclinic were also included in the study. Patients were randomly selected, according to the criteria outlined below, from seronegative patients negative for rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibody. The study population was divided into four groups according to the disease activity score in 28 joints (DAS28): remission group (RG), 18 patients (DAS28 < 2.6); low disease activity group (LDAG), 23 patients (DAS28 > 2.6-3.2); moderate disease activity (MDAG), 23 patients (DAS28 > 3.2-5.1); high disease activity group (HDAG), 14 patients (DAS28 > 5.1); and control group (CG), 10 healthy subjects. The patients’ age, gender, body mass index (BMI), and smoking habits were registered. Clinically, the patients’ duration of symptoms, duration of disease, delay in diagnosis, clinical remission, levels of relevant disease-modifying antirheumatic drugs (DMARD), and family history were noted. To evaluate the patients’ quality of life, the Health Assessment Questionnaire (HAQ) disability index consisting of 20 questions was used. To determine whether the patients were in remission, the ACR/EULAR 2011 remission criteria published in 2011, involving the CRP, swollen and tender joint numbers and the patient’s global evaluation were used. Claudin-5 and vWF assessment were derived from serum samples gathered from the patients known to have RF and anti-CCP titers in the normal ranges. RF was measured by nephelometry; a level of 20 U/mL was considered positive as suggested by Beckman Coulter IMMAGE® Immunochemistry Systems (Beckman Coulter, Brea, CA). Anti-CCP antibodies were measured by Enzyme Linked Immunosorbent Assay (ELISA; Abbott Laboratories, Green Oaks, IL) and considered positive above a cut-off value of 5 arbitrary units, as suggested by Abbott ARCHITECT i1000SR. Claudin-5 and vWF were assessed by ELISA (Eastbiopharm Co., Ltd., Wuhan, China).
Ethics approval
The study protocol was approved by the Ethics Committee of Sakarya University. This work was performed as part of a laboratory-based study, with no direct involvement with the affected patients. All clinical data, including specimen source and relevant patient information, were carefully recorded from laboratory request forms. The assays were calibrated and quality control materials were analyzed according to the respective manufacturer’s instructions.
Statistical analysis
SPSS version 20.0 statistical software was used for statistical analysis (IBM Corp., Armonk, NY, USA). Quantitative variables (clinical, laboratory) are presented as means, SDs, and ranges. Correlations among clinical, laboratory parameters and autoantibodies were analyzed by Pearson’s correlation and one-way ANOVA. In all analyses, P < 0.05 was taken to indicate statistical significance.
Results
Age, sex, BMI, and smoking habits were similar between RA patients and controls. Both claudin-5 and vWF value distributions showed homogeneous characteristics in terms of demographic characteristics. With regard to claudin-5 and vWF, there were no statistically significant differences among RA patients in the duration of symptoms, disease duration, or delay in diagnosis. Clinically, the remission rate was 58.8% in RA patients. The demographic and baseline clinical characteristics of the study groups are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the study groups
| Characteristics | RA patients | Controls | P |
|---|---|---|---|
| Age, mean ± SD years | 47.75 ± 14.91 | 45.31 ± 14.42 | > 0.05 |
| Sex, % women | 74.42 | 60.00% | |
| BMI, kg/cm2 | 27.51 ± 3.05 | 25.84 ± 2.65 | |
| Cigarette smoking, % | 29.83 | 40.0 | |
| Duration of symptoms, mean ± SD months | 44.93 ± 24.47 | NA | |
| Disease duration, mean ± SD months | 39.13 ± 23.8 | NA | |
| Delay in diagnosis, mean ± SD months | 4.98 ± 4.05 | NA | |
| HAQ total scores, mean ± SD | 2.94 ± 2.26 | NA |
BMI: Body Mass Index; HAQ: Health Assessment Questionnaire; NA: Not applicable.
All patients and controls were negative for RF and anti-CCP antibody. ESR and CRP values were significantly correlated (r = 0.725, P < 0.001). Within groups, the most significant changes were observed in the ESR (~6-fold in level) followed by CRP. The mean ESR and mean serum CRP concentration were significantly higher in the tested patient groups than the controls (P < 0.001 and P = 0.032, respectively). Table 2 presents the ESR and CRP in RA patients and the controls.
Table 2.
Laboratory characteristics of the study groups
| Groups | n (%) | ESRa | CRPb | Claudin-5c | vWFc |
|---|---|---|---|---|---|
| Control | 10 (11.4) | 8.4 ± 2.1 [1.2] | 2.4 ± 0.9 [0.8] | 12.9 ± 5.9 [9.5] | 12.3 ± 5.8 [7.4] |
| RG | 18 (20.5) | 27.2 ± 8.9 [10.1] | 7.8 ± 4.1 [6.3] | 17.3 ± 10.1 [5.6] | 14.6 ± 12.1 [9.6] |
| LDAG | 23 (26.1) | 28.2 ± 9.8 [25.7] | 7.8 ± 4.6 [8.1] | 33.4 ± 26.1 [32.8] | 28.8 ± 25.7 [32.2] |
| MDAG | 23 (26.1) | 31.7 ± 13.5 [30.6] | 8.6 ± 5.9 [9.2] | 56.9 ± 35.8 [63.9] | 55.5 ± 35.9 [69.2] |
| HDAG | 14 (15.9) | 52.5 ± 15.7 [32.4] | 14.1 ± 5.9 [13.2] | 25.2 ± 14.9 [9.1] | 24.5 ± 17.1 [12.2] |
| P d | < 0.001 | 0.032 | < 0.001 | < 0.001 |
Presented data were sorted according to the mean ± standard deviation [interquartile rage].
Range: 0-200 mm/h;
Range: 1-960 mg/L;
ng/mL;
One-way ANOVA (within groups).
With the exception of a slight difference between the controls and RG (~5 ng/mL, P > 0.05), claudin-5 concentrations were significantly different with respect to the stage of disease activity in other groups. A high positive association of claudin-5 with the MDAG was observed (P < 0.001). Figure 1 shows the association of claudin-5 with regard to the stage of disease activity. Similarly, with the exception of a slight difference between the controls and RG (~2 ng/mL, P > 0.05), vWF concentrations were significantly different with regard to the stage of disease activity in the other groups. A high positive association of vWF with the MDAG was observed (P < 0.001). Figure 2 shows the association of vWF with respect to the stage of disease activity.
Figure 1.
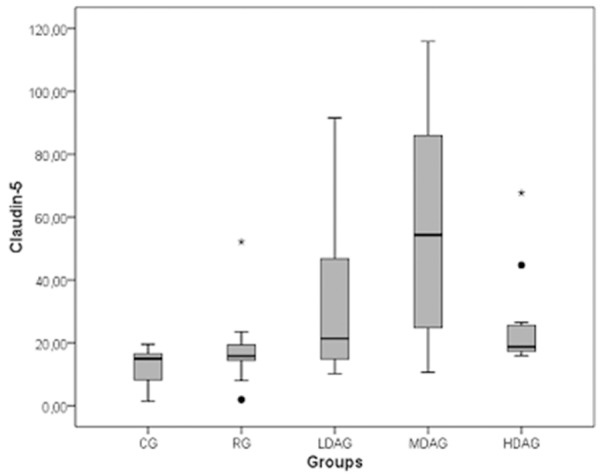
Association of claudin-5 with disease activity.
Figure 2.
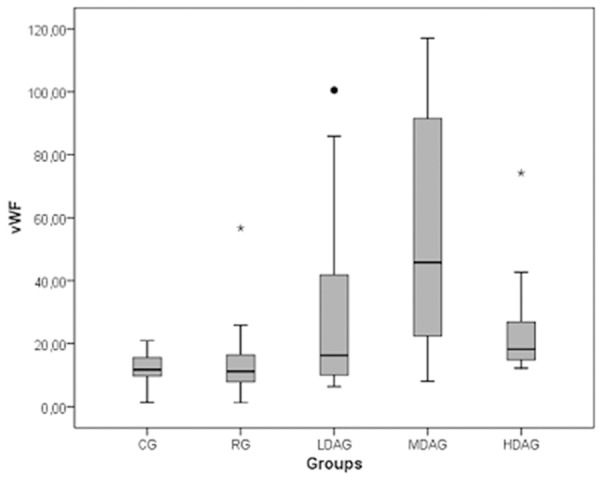
Association of vWF with disease activity.
Both claudin-5 and vWF were significantly correlated with the ESR, CRP, and DAS28 score (P < 0.05), but not with RF or anti-CCP antibody. In addition, among all patients involved in this study, the average HAQ score was 2.9 ± 2.3. The means ± SD of the HAQ scores of the RG, LDAG, MDAG, and HDAG were 3.7 ± 2.1, 3.1 ± 1.9, 2.4 ± 2.6, and 1.2 ± 1.7, respectively. Within groups, there were negative correlations among claudin-5 (r = -0.129), vWF (r = -0.156), and HAQ scores, but these were not statistically significant (P > 0.05).
Discussion
Recent studies have indicated that immunity leads to vascular injury via the effects of inflammation. In this context, many molecules involved in different signaling pathways have been investigated in endothelial cells. In addition, the mechanisms underlying the pathogenesis of various diseases associated with endothelial dysfunction mediated by the cytokine pathways are currently under investigation. Endothelial dysfunction has been shown to be related to many events, such as oxidative stress, metabolic abnormalities, genetic predisposition, polymorphisms, and cardiovascular disorders. Changes in inflammatory activity in RA patients may be one of the main reasons for endothelial dysfunction [9]. Increased vascular inflammation in diseases such as RA and atherosclerosis is the result of increased endothelial cell permeability. This increase in permeability of the endothelial cells leads to an increase in paracellular leakage of plasma fluid and proteins. Claudin-5, which is the integral membrane protein involved in endothelial tight junctions, and its transcriptional and posttranslational regulation are involved in many physiological and pathological processes [10]. Claudins control pericellular permeability as well as barrier function [11,12]. Moreover, Claudin-5 knockout mice died within 1 day after birth and were reported to show blood–brain barrier defects. It is suggested that endothelial dysfunction/activation is reflected by high levels of leukocyte adhesion molecules (e.g., vascular cell adhesion molecule-1 [VCAM-1], intercellular adhesion molecule-1 [ICAM-1], and E-selectin), increased levels of vWF, and a shift toward prothrombotic activity in the endothelium [9,12]. vWF in RA patients has been investigated in many studies as a marker of endothelial activity as well as for its role in platelet adhesion. vWF is released from both endothelial cells and megakaryocytes, but the source of vWF in the circulation originates only from endothelial cells. A previous study evaluating the relationship between endothelial activity markers and inflammatory parameters in patients with RA indicated that inflammatory markers, such as IL-8, CRP, and RF, and vWF, are correlated positively. Thus, increased RA activity indicated endothelial injury. Foster et al [13] compared the levels of endothelial activation markers (plasma vWF and soluble E-selectin) between early-onset inflammatory arthritis (EA) and healthy controls. Interestingly, the level of vWF was significantly higher in EA patients, indicating endothelial dysfunction and damage. Furthermore, classical inflammation markers, such as CRP, ESR, and IL-6 levels, were also evaluated and were not associated with endothelial damage in these patients. Consequently, endothelial damage/dysfunction has been reported in cases of early-onset inflammatory arthritis but not has not been directly associated with inflammation markers. However, we found a positive correlation between vWF, a marker of endothelial dysfunction, and disease activity in patients with RA. Many other clinical studies indicated that high levels of vWF can also be used as a marker to estimate cardiovascular risk in RA patients [14-21]. Veselinovic et al [22] suggested that RA patients have significantly elevated serum vWF level compared with controls associated with the development of accelerated atherosclerosis, and that it can be used for the estimation of cardiovascular risk. de Groot et al [23] reported that significantly elevated levels of vWF as well as advanced glycation end products (AGEs) are associated with increased endothelial activation and dysfunction in RA patients. A variety of therapeutic agents that act as AGE crosslink breakers were shown to not only induce the degradation of AGEs but also to facilitate the recovery of endothelial dysfunction in experimental models [24]. There have been no previous reports regarding the relationship between RA and claudin-5. However, a recent study indicated the importance of epithelial cell-cell and cell-extracellular matrix interactions as well as the disruption of tight junctions in Sjögren’s syndrome [25]. In an experimental mouse model of RA, occludin and ZO-1 levels were evaluated in the rat brain by immunoblot and immunofluorescence analyses. The results indicated decreased expression of occludin but no changes in ZO-1 levels. Moreover, a pathway and network-oriented genome-wide association study (GWAS) analysis (PANOGA) by Bakir-Gungor and Sezerman [26] identified the genes related to tight junctions and RA. However, many studies have shown no association between the vWF levels and effects of claudins, which play key roles in the regulation of paracellular permeability, on endothelial cells. Jin et al [27] investigated the changes of claudin-3, -4, -5, -18 expression in epithelial and endothelial cells in Pseudomonas aeruginosa (PA)-induced lung injury. The results indicated that claudin-4, -18, and -5 mRNA levels were increased 24 h after application of PA in alveolar epithelial and endothelial cells, but Western blot analysis indicated no significant increases in protein levels. Furthermore, imaging of endothelial damage by electron microscopy did not show any significant changes in the expression of vWF. We propose that the differences in expression of claudin-5 and vWF between the MDAG and HDAG may be related to disease activity and/or homeostatic mechanisms that interfere with the various proteins/immune mediators in the clinical progression of RA. These results may be due to increased disease activity and may restrict endothelial dysfunction in the HDAG. Further studies are required to investigate these differences.
The results of our study indicated that the relationship between vWF and claudin-5, which are indicators of endothelial cell dysfunction and tight junction activity, may be a predictor of disease activity. Further studies are required to investigate these pathways to shed light on the roles of claudin-5 and vWF in the progression of inflammation and other vascular conditions.
Disclosure of conflict of interest
None.
References
- 1.Nguyen NT, Nakahama T, Kishimoto T. Aryl hydrocarbon receptor and experimental autoimmune arthritis. Semin Immunopathol. 2013;35:637–44. doi: 10.1007/s00281-013-0392-6. [DOI] [PubMed] [Google Scholar]
- 2.Ibn Yacoub Y, Amine B, Laatiris A, Hajjaj-Hassouni N. Health-related quality of life in Moroccan patients with rheumatoid arthritis. Clin Rheumatol. 2012;31:1471–7. doi: 10.1007/s10067-012-2037-x. [DOI] [PubMed] [Google Scholar]
- 3.Gary SF, Ralph CB, Sherine EG, Iain BM, James RO, editors. Kelley’s Textbook of Rheumatology. 9th edition. Elsevier; 2013. pp. 476–492.pp. e6 [Google Scholar]
- 4.He M, Liang X, He L, Wen W, Zhao S, Wen L, Liu Y, Shyy JY, Yuan Z. Endothelial dysfunction in rheumatoid arthritis: the role of monocyte chemotactic protein-1-induced protein. Arterioscler Thromb Vasc Biol. 2013;33:1384–91. doi: 10.1161/ATVBAHA.113.301490. [DOI] [PubMed] [Google Scholar]
- 5.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 6.Förster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishioku T, Yamauchi A, Takata F, Watanabe T, Furusho K, Shuto H, Dohgu S, Kataoka Y. Disruption of the blood-brain barrier in collagen-induced arthritic mice. Neurosci Lett. 2010;482:208–11. doi: 10.1016/j.neulet.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Szekanecz Z, Besenyei T, Szentpétery A, Koch AE. Angiogenesis and vasculogenesis in rheumatoid arthritis. Curr Opin Rheumatol. 2010;22:299–306. doi: 10.1097/BOR.0b013e328337c95a. [DOI] [PubMed] [Google Scholar]
- 9.Klimek E, Skalska A, Kwaśny-Krochin B, Surdacki A, Sulicka J, Korkosz M, Fedak D, Kierzkowska I, Wizner B, Grodzicki TK. Differential associations of inflammatory and endothelial biomarkers with disease activity in rheumatoid arthritis of short duration. Mediators Inflamm. 2014;2014:681635. doi: 10.1155/2014/681635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burek M, Steinberg K, Förster CY. Mechanisms of transcriptional activation of the mouse claudin-5 promoter by estrogen receptor alpha and beta. Mol Cell Endocrinol. 2014;392:144–51. doi: 10.1016/j.mce.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Jang AS, Concel VJ, Bein K, Brant KA, Liu S, Pope-Varsalona H, Dopico RA Jr, Di YP, Knoell DL, Barchowsky A, Leikauf GD. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:483–90. doi: 10.1165/rcmb.2009-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Ceunynck K, De Meyer SF, Vanhoorelbeke K. Unwinding the von Willebrand factor strings puzzle. Blood. 2013;121:270–7. doi: 10.1182/blood-2012-07-442285. [DOI] [PubMed] [Google Scholar]
- 13.Foster W, Lip GY, Raza K, Carruthers D, Blann AD. An observational study of endothelial function in early arthritis. Eur J Clin Invest. 2012;42:510–6. doi: 10.1111/j.1365-2362.2011.02607.x. [DOI] [PubMed] [Google Scholar]
- 14.Alekperov RT, Baranov AA, Abaĭtova NE. Clinical associations of C-reactive protein in systemic sclerosis. Ter Arkh. 2006;78:30–5. [PubMed] [Google Scholar]
- 15.McEntegart A, Capell HA, Creran D, Rumley A, Woodward M, Lowe GD. Cardiovascular risk factors, including thrombotic variables, in a population with rheumatoid arthritis. Rheumatology (Oxford) 2001;40:640–4. doi: 10.1093/rheumatology/40.6.640. [DOI] [PubMed] [Google Scholar]
- 16.Artemenko NA, Sizyakina LP. Clinico-Immunologic Peculiarities of Different Forms of Rheumatoid Arthritis. Russ J Immunol. 1998;3:167–172. [PubMed] [Google Scholar]
- 17.Baranov AA, Nasonov EL, Shilkina NP, Samoriadova OG, Balabanova RM, Bimbazhapov RS. The von Willebrand factor antigen in patients with rheumatoid arthritis: a method for its determination and the clinical significance. Ter Arkh. 1993;65:69–72. [PubMed] [Google Scholar]
- 18.Duţu A, Rus V, Boloşiu HD, Parasca I, Cristea A, Malide D. von Willebrand factor in the plasma of patients with rheumatic diseases. Med Interne. 1989;27:273–8. [PubMed] [Google Scholar]
- 19.Miller JJ 3rd, Olds LC, Silverman ED, Milgrom H, Curd JG. Different patterns of C3 and C4 activation in the varied types of juvenile arthritis. Pediatr Res. 1986;20:1332–7. doi: 10.1203/00006450-198612000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Nusinow SR, Federici AB, Zimmerman TS, Curd JG. Increased von Willebrand factor antigen in the plasma of patients with vasculitis. Arthritis Rheum. 1984;27:1405–10. doi: 10.1002/art.1780271211. [DOI] [PubMed] [Google Scholar]
- 21.Federici AB, Fox RI, Espinoza LR, Zimmerman TS. Elevation of von Willebrand factor is independent of erythrocyte sedimentation rate and persists after glucocorticoid treatment in giant cell arteritis. Arthritis Rheum. 1984;27:1046–9. doi: 10.1002/art.1780270912. [DOI] [PubMed] [Google Scholar]
- 22.Veselinovic M, Jakovljevic V, Jurisic-Skevin A, Toncev S, Djuric DM. Carotid enlargement and serum levels of von Willebrand factor in rheumatoid arthritis: a follow-up study. Clin Rheumatol. 2012;31:1727–32. doi: 10.1007/s10067-012-2079-0. [DOI] [PubMed] [Google Scholar]
- 23.de Groot L, Hinkema H, Westra J, Smit AJ, Kallenberg CG, Bijl M, Posthumus MD. Advanced glycation endproducts are increased in rheumatoid arthritis patients with controlled disease. Arthritis Res Ther. 2011;13:R205. doi: 10.1186/ar3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soro-Paavonen A, Zhang WZ, Venardos K, Coughlan MT, Harris E, Tong DC, Brasacchio D, Paavonen K, Chin-Dusting J, Cooper ME, Kaye D, Thomas MC, Forbes JM. Advanced glycation end-products induce vascular dysfunction via resistance to nitric oxide and suppression of endothelial nitric oxide synthase. J Hypertens. 2010;28:780–8. doi: 10.1097/HJH.0b013e328335043e. [DOI] [PubMed] [Google Scholar]
- 25.Barrera MJ, Bahamondes V, Sepúlveda D, Quest AF, Castro I, Cortés J, Aguilera S, Urzúa U, Molina C, Pérez P, Ewert P, Alliende C, Hermoso MA, González S, Leyton C, González MJ. Sjögren’s syndrome and the epithelial target: a comprehensive review. J Autoimmun. 2013;42:7–18. doi: 10.1016/j.jaut.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Bakir-Gungor B, Sezerman OU. A new methodology to associate SNPs with human diseases according to their pathway related context. PLoS One. 2011;6:e26277. doi: 10.1371/journal.pone.0026277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin W, Rong L, Liu Y, Song Y, Li Y, Pan J. Increased claudin-3, -4 and -18 levels in bronchoalveolar lavage fluid reflect severity of acute lung injury. Respirology. 2013;18:643–51. doi: 10.1111/resp.12034. [DOI] [PubMed] [Google Scholar]


