Abstract
ACE2-Ang (1-7) axis is a key regulator in cardiac hypertrophy, myocardial remodeling and development of heart failure. To investigate how ACE2-Ang (1-7) axis function in pressure-overload-induced heart failure, male SD rats (weighing about 250 g) were used to establish the model of pressure-overload-induced heart failure using aortic stenosis surgery. The level of plasma ACE2, ACE and Ang (1-7) from heart failure group were significantly up-regulated compared with the sham group by ELISA test. The mRNA and protein expression of ACE2 in myocardial tissue from heart failure group also showed remarkably increased. Importantly, we found that the expression of ACE2 and Ang (1-7) were reversed in heart failure group after treatment with AT1 receptor antagonist telmisartan. Compared with heart failure group, the level of plasma ACE2, ACE and Ang (1-7) were significantly decreased in telmisartan treated group. The mRNA and protein expression of ACE2 in cardiac tissue from telmisartan group was also significantly decreased, while Mas mRNA and protein level was increased. Taken together, these studies demonstrated that the expression of ACE2-Ang (1-7) axis was induced in pressure-overload-induced heart failure model, suggesting that ACE2-Ang (1-7) axis may have a protective role in the development of heart failure and may provide a new target for drug development of heart failure.
Keywords: ACE2, Ang (1-7), rat, pressure-overload-induced heart failure
Introduction
Cardiovascular diseases are predicted to be the most common cause of death worldwide by 2020. Renin-angiotensin system (RAS) is an important mediators of myocardial fibrosis, pathological hypertrophy, and heart failure [8,18]. Angiotensin Converting Enzyme (ACE), Angiotensin II (Ang II), and Angiotensin II type I Receptor (AT1), namely AngII-ACE-AT1 axis is thought to be classic RAS pathway [13,16]. Drugs that target Ang II and the Ang II type 1 receptor (AT1) are widely used for the treatment of cardiovascular diseases such as hypertension, myocardial infarction, and heart failure [17,19]. Recently, it has found that there are still new members in RAS system, that is Angiotensin 1-7 (Ang (1-7)), Angiotensin-converting enzyme 2 (ACE2), and the specific receptor Mas. ACE2-Ang (1-7)-Mas axis is known as the second metabolic axis of RAS. Actually, ACE2-Ang (1-7)-Mas axis plays a pivotal role in regulating the occurrence and development of cardiovascular disease [3,11,15]. Therefore, study on ACE2-Ang (1-7)-Mas has become a hot spot in biological medicine research.
Angiotensin-converting enzyme 2 (ACE2) is a pleiotropic monocarboxypeptidase capable of metabolizing several peptide substrates, including Ang I, Ang II, des-Arg9-bradykinin, apelin-13, and opioids [3]. Ang (1-7), one of the major enzymatic products of ACE2, is a cardiovascular protective peptide in RAS [4,9,14]. It has been shown that Ang (1-7) can reduce Ang II-induced cardiac hypertrophy and remodeling and pressure-overload-induced heart failure [14]. ACE2 knockout mice can develop impaired cardiac function with adverse ventricular remodeling, enhanced oxidative stress, and inflammatory cytokine expression [2,12]. Ang II infusion induces hypertension, myocardial hypertrophy, fibrosis, and diastolic dysfunction, which are exacerbated in ACE2 deficient mice, whereas rhACE2 attenuated Ang II- and pressure-overload-induced adverse myocardial remodeling [21].
To delineate the expression of ACE2-Ang (1-7)-Mas axis in animals of heart failure, we established a pressure-overloaded rat model. The expression of ACE2 and Ang (1-7) remarkably increased following development of heart failure, while Mas expression decreased in heart failure model. Telmisartan intervention, an AT1 receptor antagonist, reversed the expression of each component of ACE2-Ang (1-7)-Mas axis. Thus, ACE2-Ang (1-7)-Mas axis plays an important role in development of heart failure, which may provide a new target for heart failure drug design and therapeutic strategy.
Materials and methods
Animals
SD rats were provided by Experimental Animal Center, Xi’an Jiaotong University School of Medicine. Rats used in this study were housed under 12 hours light- 12 hours dark cycle, in a pathogen free animal facility accredited by the Association for the Advancement and Accreditation of Laboratory Animal Care International. Rats were maintained on a standard rodent chow (Teklad #7904; Harlan-Teklad, Indianapolis, IN) and water ad libitum. All animal procedures used in this study were reviewed and preapproved by the Xi’an Jiaotong University Institutional Animal Care and Use Committee.
Aortic stenosis-induced pressure overload
Male SD rats (n=90) weighing 250 g ± 20 g were randomly divided into three groups: sham group, heart failure group and telmisartan intervention group. Sham-operated rats were served as controls. Under ether inhalation anesthesia, along rat ventral midline, laparotomy was performed, the abdominal aorta was carefully separated from above the right renal artery, and a silver clip with an inner diameter of 0.8 mm was placed, so that the annular aortic stenosis formed. The incision was sutured, with an intramuscular injection of 40,000 units of penicillin only to prevent infection. For sham group, the operation was same as those in experimental group except for the silver clip was not placed around the separated abdominal aorta. For telmisartan intervention group, telmisartan (30 mg/100 ml) was administered in drinking water after operation for 3 days. Rats were killed after surgery for 14 and 35 days. Blood collected from the inferior vena cava was used for assaying plasma content of ACE2 using ELISA kits. Myocardial tissue was collected and froze in liquid nitrogen for mRNA analysis.
Enzyme-linked immunosorbent assay (ELISA)
ELISA double-antibody sandwich assay kits for ACE2, Ang (1-7), ACE, and Ang II were purchased from Australia ProSearch International Australia Pty Company. According to the Kit instructions, the reaction system and standard curve were established. The absorbance of each sample was measured by enzyme-linked immunosorbent analyzer. The content of ACE2, Ang (1-7), ACE, and Ang II were calculated in accordance with the standard curve.
RNA preparation and real-time PCR
Total RNA was prepared from rat cardiac tissue using TRIzol reagent (Invitrogen). Reverse transcription was performed with 1 μg of total RNA by PrimeScriptTM RT Reagent Kit with gDNA Eraser (TaKaRa). Q-PCR was carried out in triplicates for the amplification of specific genes and normalized by comparison with GAPDH. Each PCR reaction was composed of 0.8 μl (10 μmol) of forward and reverse primers and 10 μl of 2×SYBR Green PCR Master Mix to make a final volume of 20 μl and performed by using Takara TP800 (Thermal Cycler Dice Real Time System). The generation of specific PCR products was confirmed by melting curve analysis and relative gene expression changes were measured using the comparative Ct method, X=2-ΔΔCt. The primer sequences are listed in Table 1.
Table 1.
Specific Primers for Q-PCR
| Genes name | Primers forward | Primers reverse |
|---|---|---|
| ACE2 | CATTGGAGCA AGTGTTGGAT | GAGCTAATGCATGCCATTCA |
| AT-1a | CCTACATTCTAACACACCCT | GCATATTGTGCCAGTACAAT |
| MAS | AGACACGAATTCACCAGG | AGACTCGAGTTAGACGAG |
| GAPDH | CGGATTTGGTCGTATTGGG | TCTCGCTCCTGGAAGATGG |
Western blot analysis
40 μg heart protein samples were subjected to 10% SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted using ACE2, Mas, and AT-1α antibodies. GAPDH (American, California, epitomics) was used as loading control to determine the relative expression levels of the target protein in the sample.
Statistical analysis
Experimental data are shown as Mean ± SD. Main and interactive effects were analyzed by One-Way ANOVA using SPSS11.5 software. When justified by One-Way ANOVA, differences between individual group means were analyzed by Fisher’s LSD test. Differences were considered statistically significant at P < 0.05, while P < 0.01 represents more significant change.
Results
Plasma levels of ACE2, Ang (1-7), ACE and Ang II
To generate the pressure overloaded animal model, rat aortic stenosis surgery was operated. Surgical schematic diagram was shown in Figure 1. As we expected, compared with sham group, heart weight and left ventricle mass index were significantly increased in aortic stenosis group after aortic stenosis surgery for 14 days (P < 0.05), and more obviously for 35 days (P < 0.01) (Figure 2A and 2B). However, heart weight and left ventricle mass index seemed no significantly changed between 14 days and 35 days operation (Figure 2A and 2B). Interestingly, intervention of telmisartan, an AT1 receptor antagonist, attenuated myocardial hypertrophy caused by aortic stenosis surgery (Figure 2A and 2B).
Figure 1.

Model to illustrate the operation of aortic stenosis in SD rat.
Figure 2.
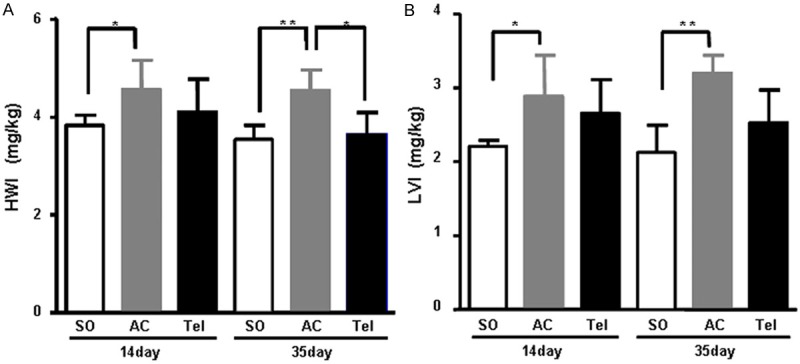
Heart-body weight ratio in aortic stenosis group or telmisartan intervention group (n=6) (*P < 0.05, **P < 0.01). A. Heart-body weight index. B. Left heart-body weight index. SO represents sham operation group. AC represents aortic stenosis group. Tel represents telmisartan intervention group.
Plasma level of ACE2, Ang (1-7), ACE and Ang II were determined after aortic stenosis surgery for 14 and 35 days. Compared with sham group, plasma level of ACE2 and Ang (1-7) was significantly increased in aortic stenosis group (P < 0.05) (Figure 3A and 3B). The content of plasma ACE also remarkably increased in aortic stenosis group (P < 0.01) (Figure 3C). However, The level of plasma Ang II in aortic stenosis group obviously increased only after surgery for 35 days, while slightly increased after surgery for 35 days (Figure 3D). After intervention with telmisartan, plasma level of ACE2, Ang (1-7), ACE and Ang II were reversed. Compared with aortic stenosis group, the content of plasma ACE2, Ang (1-7) and ACE significantly decreased in telmisartan treated group after surgery for 14 and 35 days (Figure 3A-C). Plasma level of Ang II also showed remarkably decreased in telmisartan treated group after surgery for 35 days (P < 0.05) (Figure 3D).
Figure 3.
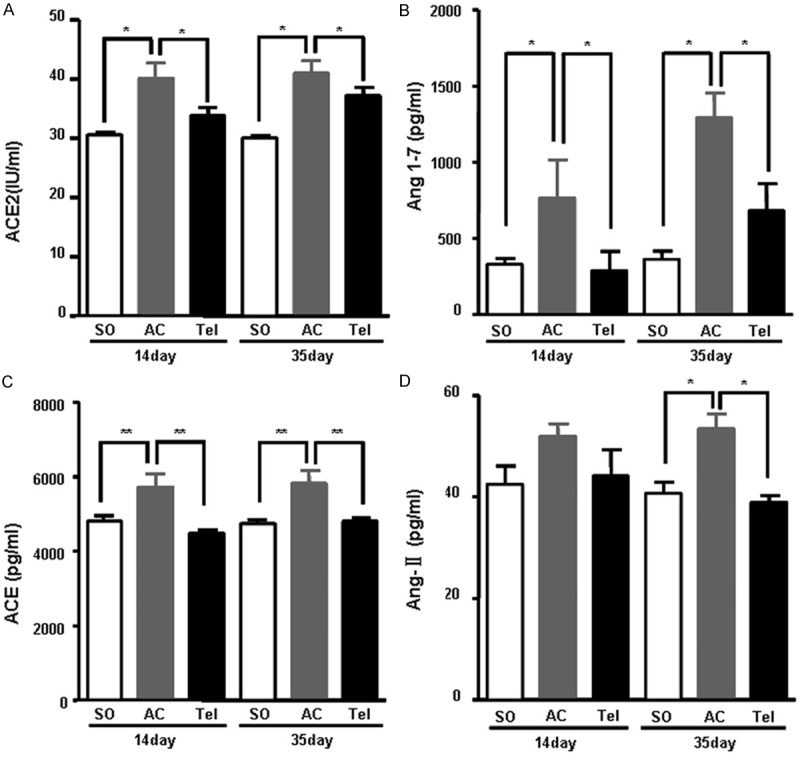
Plasma level of ACE2 (A), Ang (1-7) (B) ACE (C) and Ang II (D). SO represents sham operation group. AC represents aortic stenosis group. Tel represents telmisartan intervention group.
The mRNA expression of ACE2, Mas and AT1α in myocardial tissue
To test mRNA level of ACE2-Ang (1-7)-Mas, we extracted RNA from myocardial tissue and did RT Real time PCR. Real time PCR results showed that ACE2 mRNA expression was significantly increased in aortic stenosis group, especially after surgery for 14 days (P < 0.01) (Figure 4A). After treatment with telmisartan, an AT1 receptor antagonist, ACE2 mRNA expression was decreased (P < 0.05) (Figure 4A). In contrast, the mRNA expression of Mas in aortic stenosis group significantly decreased compared with sham group, which was increased after telmisartan intervention (Figure 4B). Compared with sham group, AT1α mRNA level significantly increased in aortic stenosis group (P < 0.05), while its expression decreased after telmisartan intervention (Figure 4C).
Figure 4.
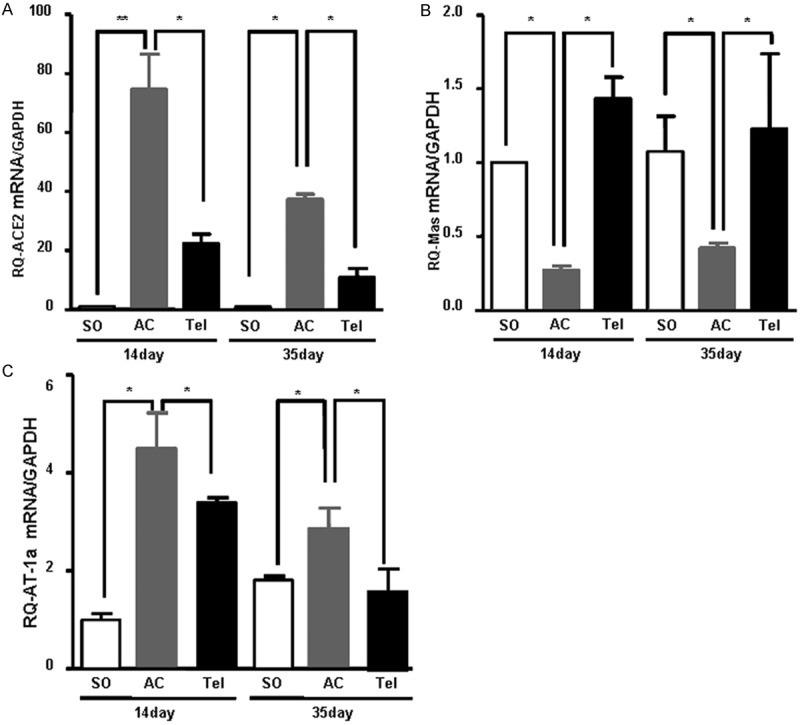
The mRNA expression of ACE2 (A), Mas (B) and AT1α (C) in myocardial tissue. Total RNA of myocardial tissue isolated from SD rats after surgery of aortic stenosis or telmisartan treatment was used for Real time PCR analysis. The specific amplification of target genes was normalized with GAPDH and the fold-change values are shown. SO represents sham operation group. AC represents aortic stenosis group. Tel represents telmisartan intervention group. Data from 3 independent Real time PCR determinations are expressed as mean ± SD.
Protein expression of ACE2, Mas and AT1α in myocardial tissue
To investigate the protein expression of ACE2, Mas and AT1α, we extracted the total protein from myocardial tissue. Consistent with mRNA level, ACE2 protein expression significantly increased in aortic stenosis group compared with sham group after surgery for 14 and 35 days (P < 0.01) (Figure 5A and 5B). After treatment with telmisartan, ACE2 protein expression decreased (P < 0.05) (Figure 5A and 5B). The protein expression of Mas significantly decreased in aortic stenosis group, which recovered in telmisartan treated group after surgery for 35 days (P < 0.05). However, Mas level did not change obviously after surgery for 14 days either in aortic stenosis group or telmisartan treated group (Figure 5A and 5C). The protein expression of AT1α also showed no changes either in aortic stenosis group or telmisartan treated group after surgery for 14 days (Figure 5A and 5D). After surgery for 35 days, AT1α protein expression significantly increased in aortic stenosis group, which decreased in telmisartan group (P < 0.05) (Figure 5A and 5D).
Figure 5.
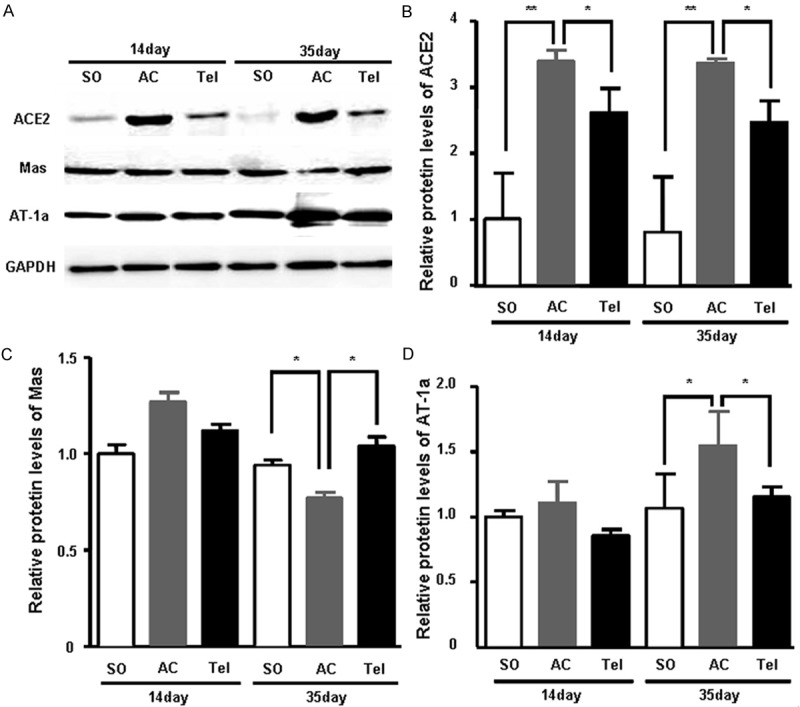
The protein expression of ACE2, Mas and AT1α in myocardial tissue. A. Myocardial tissue homogenates from SD rats after surgery of aortic stenosis or telmisartan treatment were subjected to SDS-PAGE. The non-responsive gene GAPDH is shown as loading control. B. Quantitive analysis of ACE2 protein expression. C. Quantitive analysis of Mas protein expression. D. Quantitive analysis of AT1α protein expression. SO represents sham operation group. AC represents aortic stenosis group. Tel represents telmisartan intervention group.
Discussion
ACE2 is the first known homolog of human ACE and functions as a pleiotropic monocarboxypeptidase responsible for the degradation of a range of peptides with a high catalytic efficiency. The discovery of ACE2 has dramatically changed the direction of cardiovascular research in view of the pivotal role of this enzyme in the regulation of the renin-angiotensin system (RAS). Many studies have shown that ACE2 is involved in maintaining cardiac function. High expression of ACE2 is observed in the myocardial tissue from patients, who suffered with ischemic heart failure, idiopathic dilated cardiomyopathy, or primary pulmonary hypertension [1,22]. Also, ACE2 gene expression is strongly up-regulated in cross zone between infarct and marginal, and survival myocardial tissue from mice with myocardial infarction. Consistently, in the present study, we found that the plasma ACE2 level, mRNA and protein expression of ACE2 in myocardial tissue significantly increased in aortic stenosis-induced pressure overloaded rats, suggesting that ACE2 may play a protective role in development of heart failure.
In animal models of cardiac hypertrophy and heart failure, the formation of Ang II can be blocked by ACEI or ARB, which leads to the increased transcription and translation of ACE2 gene, following up-regulation of Ang (1-7) in myocardial tissue. Reversely, infusion of human recombinant ACE2 can reduce Ang II-induced myocardial fibrosis, cardiac hypertrophy and cardiac insufficiency in rats [21]. Overexpression of ACE2 by intramyocardial injection of Ad-ACE2 can attenuated left ventricular fibrosis and improved left ventricular remodeling and systolic function in a rat model of myocardial infarction [20]. Additionally, overexpression of ACE2 by lenti-mACE2 in rats results in protective effects on angiotensin II-induced cardiac hypertrophy and fibrosis [5]. Similarly, in ACE2 gene null old rats, heart systolic function significantly decreased, accompanied by mild ventricular dilation, which can cause ventricular wall thinning [2]. Clinical studies have also shown that expression of ACE2 gene had a positive correlation with left ventricular end-diastolic diameter, but was not significantly correlated with left ventricular ejection fraction (EF) or brain natriuretic peptide (BNP) in plasma, indicating that upregulation of ACE2 gene in myocardium of patients with severe heart failure depends on the degree of left ventricular dilation, which is the body’s important compensatory mechanism for delaying left ventricular remodeling [10].
Ang (1-7) is a cardiovascular protective peptide in RAS against cardiovascular toxic effects of Ang II [7]. Many studies show that Ang (1-7) has a protective effect on the heart function. Acutely perfusion of Ang (1-7) leads to the increase of CO and SV in heart of normal rat. Chronically perfusion of Ang (1-7) can improve the endothelial function of aorta, and coronary perfusion has a protective effect on the left coronary artery ligation-induced cardiac function failure. Moreover, Ang (1-7) also has anti-angiogenic effect and can reduce stent-induced neointimal proliferation [7]. Ang (1-7) binds to specific receptors on cardiac fibroblasts to play a role in anti-myocardial fibrosis and anti-myocardial hypertrophy [6]. The present study also found that plasma Ang (1-7) level increased in overload induced heart failure rats following with high content of ACE2, while its content decreased after given telmisartan. These studies suggest that high level of ACE2 and Ang (1-7) may be a compensatory response to pressure overload induced heart failure.
Like other peptides, Ang (1-7) has physiological role when it binds to specific receptor [23]. Studies have found that Ang (1-7) is an intrinsic ligand of Mas receptor. Mas, a functional antagonist of Ang II 1 receptor (AT1), can cause AT1 receptor isomers to block Ang II function. Studies have confirmed that Ang (1-7) can inhibit Ang II induced proliferation of myocardial cells by activating Mas receptor [4]. Mas gene knockout mouse shows cardiac dysfunction, with decrease of contractile force, dropping of left ventricular filling pressure, and the reduction of dp/dt and cardiac output either in vitro or the whole state. Consistently, our study found that the expression of Mas significantly reduced in pressure overload-induced heart failure of rats while recovered after telmisartan intervention, suggesting a protective role of Mas in development of heart failure.
In summary, this study presents that ACE2-Ang (1-7)-Mas axis is closely related with the occurrence and development of pressure overload-induced heart failure of rats. At different stages of heart failure, the activities of components of ACE2-Ang (1-7)-Mas axis in plasma are different. The level of ACE2 and Ang (1-7) increased both in plasma and myocardial tissue from heart failure group, while Mas level decreased, and their level reversed when treatment with telmisartan, suggesting a potential protective role of these factors in heart failure, which will provide a new target for drug design, and gene therapy for heart failure.
Acknowledgements
This work was partly supported by 2011 funds of cross-disciplinary research projects of Fundamental Research of Xi’an Jiaotong University (YL) and National Natural Science Foundation of China (No. 81200207) and the Fundamental Research Funds for the Central Universities (LB).
Disclosure of conflict of interest
None.
References
- 1.Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26:369–75. doi: 10.1093/eurheartj/ehi114. discussion 22-4. [DOI] [PubMed] [Google Scholar]
- 2.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–8. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 4.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7) Am J Physiol Heart Circ Physiol. 2007;292:H736–42. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 5.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–90. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 6.Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, Greenberg BH. Angiotensin-(1-7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol. 2005;289:H2356–63. doi: 10.1152/ajpheart.00317.2005. [DOI] [PubMed] [Google Scholar]
- 7.Langeveld B, van Gilst WH, Tio RA, Zijlstra F, Roks AJ. Angiotensin-(1-7) attenuates neointimal formation after stent implantation in the rat. Hypertension. 2005;45:138–41. doi: 10.1161/01.HYP.0000149382.83973.c2. [DOI] [PubMed] [Google Scholar]
- 8.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 9.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–26. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsuki M, Morimoto S, Izawa H, Ismail TF, Ishibashi-Ueda H, Kato Y, Horii T, Isomura T, Suma H, Nomura M, Hishida H, Kurahashi H, Ozaki Y. Angiotensin converting enzyme 2 gene expression increased compensatory for left ventricular remodeling in patients with end-stage heart failure. Int J Cardiol. 145:333–4. doi: 10.1016/j.ijcard.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 11.Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 12.Oudit GY, Kassiri Z, Patel MP, Chappell M, Butany J, Backx PH, Tsushima RG, Scholey JW, Khokha R, Penninger JM. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75:29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 14.Santos RA, Ferreira AJ. Angiotensin-(1-7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122–8. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- 15.Santos RA, Ferreira AJ, Simões E Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin (1-7)-Mas axis. Exp Physiol. 2008;93:519–27. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 16.Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002;89:3A–9A. doi: 10.1016/s0002-9149(01)02321-9. discussion 10A. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Nagueh SF. Current perspectives on cardiac function in patients with diastolic heart failure. Circulation. 2009;119:1146–57. doi: 10.1161/CIRCULATIONAHA.108.822676. [DOI] [PubMed] [Google Scholar]
- 18.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and reninangiotensin-aldosterone system. Circulation. 1991;83:1849–65. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 19.Zaman MA, Oparil S, Calhoun DA. Drugs targeting the renin-angiotensin-aldosterone system. Nat Rev Drug Discov. 2002;1:621–36. doi: 10.1038/nrd873. [DOI] [PubMed] [Google Scholar]
- 20.Zhao YX, Yin HQ, Yu QT, Qiao Y, Dai HY, Zhang MX, Zhang L, Liu YF, Wang LC, Liu de S, Deng BP, Zhang YH, Pan CM, Song HD, Qu X, Jiang H, Liu CX, Lu XT, Liu B, Gao F, Dong B. ACE2 overexpression ameliorates left ventricular remodeling and dysfunction in a rat model of myocardial infarction. Hum Gene Ther. 21:1545–54. doi: 10.1089/hum.2009.160. [DOI] [PubMed] [Google Scholar]
- 21.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–28. doi: 10.1161/CIRCULATIONAHA.110.955369. 18 p following 28. [DOI] [PubMed] [Google Scholar]
- 22.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–12. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 23.Zucker IH, Xiao L, Haack KK. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 2014;126:695–706. doi: 10.1042/CS20130294. [DOI] [PMC free article] [PubMed] [Google Scholar]


