Abstract
Soft tissue tumors are rare tumors that show a heterogeneous structure; thus far, their molecular behavior has not been elucidated. The aim of our study was to define the relationship between microvessel density (MVD), evaluated with CD31, and other immunohistochemical markers, such as vascular endothelial growth factor (VEGF), cyclooxygenase-2 (COX-2), CD34, maspin, DOG-1, and c-KIT. Immunostains were done in 55 cases consisting of benign and malignant tumors, such as liposarcomas, dermatofibrosarcomas, and tumors with histiocytic differentiation. Renal tubes were used as external control for VEGF, maspin, and DOG-1. Although DOG-1 is considered a specific marker for gastrointestinal tumors (GISTs), its positivity, correlated with c-KIT and VEGF immunoexpression, was also shown by dermatofibrosarcomas and tumors with histiocytic and lipomatous differentiation, suggesting its possible pro-angiogenic role. Maspin expression was observed in adipose tissue tumors only. Regarding angiogenesis, 31 of the 55 cases were VEGF-positive, such positivity being directly correlated with COX-2 and CD34 positivity as evaluated in the tumor cells and also with MVD. Although no significant differences in angiogenic activity were found between benign and malignant non-lipomatous tumors, the MVD was directly correlated with the histological type/grade of liposarcomas. Based on these aspects, we conclude that VEGF/COX-2-induced angiogenesis is specific for non-lipomatous tumors, whereas liposarcomas are dependent on the VEGF/maspin angiogenic pathway. The DOG-1/c-KIT/VEGF target may be used for further personalized therapy of soft tissue sarcomas. No data about DOG-1 and maspin positivity in liposarcomas have been published to date.
Keywords: Soft tissue, angiogenesis, maspin, c-KIT, DOG-1, COX-2
Introduction
Soft tissue tumors (STTs) are relatively rare tumors of mesenchymal lineage that can develop in any part of the human body and present a highly heterogeneous microscopic aspect [1,2]. Although rarely diagnosed, in the case of sarcomas, local recurrences and distant metastases are common, and the mortality rate is higher than 50% [1,3,4]. The prognosis and therapy depend on the primary site, size, resection margins, histological subtype, malignancy grade, stage, and molecular signature of the tumor [1,4,5]. Moreover, the specific cell origin in each microscopic type of STTs is still unclear [4]; new systems of subclassification have been proposed, the latest introduced by the World Health Organization in 2013 [6]. The biological behavior of STTs is influenced by some processes, such as angiogenesis, which could inhibit the tumor cell apoptosis [2]. However, other molecular pathways, such as chromosomal translocations, gene fusion [5], and other genomic alterations, have been described. An understanding of these mechanisms would be very useful not only for prognostic assessment but also for targeted therapy of soft tissue sarcomas.
In this study, we aimed to explore the characteristics of some immunohistochemical (IHC) markers whose expressions have not been intensively explored in the literature. Based on previous studies that reported an important prognostic and predictive role of VEGF (vascular endothelial growth factor) -dependent angiogenesis in STTs [1], we tried to define the particularities by using two markers that have been evaluated in the tumor cells, namely, VEGF-A and cyclooxygenase-2 (COX-2), as well as the endothelial marker CD31.
As original elements, on the one hand, the immunoexpression of maspin and its significance in STTs were analyzed and correlated with other markers. Maspin or serpin B5, a serine protease, is considered to play antiproliferative, proapoptotic, and antiangiogenic roles in carcinomas; however, its role in STTs is not yet known. On the other hand, c-KIT and DOG-1 reactivity within tumor cells was examined for its possible diagnostic and predictive relevance. These markers are known to be relatively specific for gastrointestinal tumors (GIST) [7,8]. Whereas c-KIT immunoexpression has been observed in several other STTs, such as Kaposi sarcoma [9], tumors with myofibroblastic differentiation, and even melanomas, no extensive studies about DOG-1 specificity have been published thus far. Knowing the predictive value of c-KIT positivity, correlated with the c-KIT mutations in exons 9 and 11 [8], this study aimed to identify those STTs that could respond to receptor tyrosine kinase inhibitors. To the best of our knowledge, no studies about DOG-1 immunoexpression in adipose tissue tumors, or even about the relationship between VEGF, CD31, and maspin in STTs, have been published to date.
Material and methods
Patients and tumor samples
The clinicopathological features of tumors located in dermis and subcutaneous tissues were investigated in 55 consecutive cases that underwent radical tumor resection from 2010 to 2013. The study was conducted at the Department of Pathology of the University of Medicine and Pharmacy of Tirgu-Mures, Romania. Signed written informed consent was obtained from the patient in each case. All cases were primary tumors; no recurrences or metastases were included. In each of the cases, the age and gender of the patients were considered, and the localization and microscopic type of the tumor were analyzed by using the newest classification of soft tissue tumors [6]. According to this system of classification, liposarcomas were grouped as follows [6]: atypical lipomatous tumors (formerly reffered to as well-differentiated liposarcomas), dedifferentiated liposarcomas (liposarcomas with varied non-lipomatous components), myxoid liposarcomas (including the round cell variant), and pleomorphic liposarcomas.
Immunohistochemistry
In each of the 55 cases, immunohistochemical (IHC) stains were done on 5-μm thick formalin-fixed paraffin-embedded tissues by using a panel of seven antibodies. Table 1 presents the characteristics of these antibodies. The cases were processed with the Novolink Polymer detection system (Novocastra, Newcastle-upon-Tyne, UK) and developed with the DAB (diaminobenzidine) solution (Novocastra). Counterstaining was done with Mayer’s hematoxylin (Novocastra). For negative controls, incubation was done with omission of specific antibodies.
Table 1.
Main characteristics of antibodies used for the immunohistochemical (IHC) stains (GIST, gastrointestinal stromal tumor)
| Antibody (company) | Clone | Dilution | Antigen retrieval | Positive control |
|---|---|---|---|---|
| Maspin (Novocastra, Newcastle-upon-Tyne, UK) | EAW24 | 1:25 | Incubation with citrate buffer (pH 6.0) - 60 minutes at 100°C | • external - renal tubes |
| • internal - NS | ||||
| CD31/PECAM-1 (LabVision, Fremont, CA, USA) | JC/70A | 1:25 | Incubation with high pH-solution (pH 10.0) - 30 minutes at 100°C | • external - placenta |
| • internal - blood vessels - endothelial cells | ||||
| VEGF-A (LabVision) | VG1 | 1:50 | Incubation with high pH-solution (pH 10.0) - 30 minutes at 100°C | • external - renal tubes |
| • internal - normal mature vessels - endothelial cells | ||||
| COX-2 (Novocastra) | monoclonal | 1:100 | Incubation with citrate buffer (pH 6.0) - 60 minutes at 100°C | • external - brain |
| • internal - lymphocytes | ||||
| CD34 (Dako Glostrup, Denmark) | Qbend/10 | 1:100 | Incubation with high pH-solution (pH 10.0) - 30 minutes at 100°C | • external - placenta |
| • internal - blood vessels - endothelial cells | ||||
| DOG-1 (Novocastra) | K9 | 1:50 | Incubation with citrate buffer (pH 6.0) - 60 minutes at 100°C | • external - renal tubes |
| • internal - NS | ||||
| CD117 (Dako) | polyclonal rabbit | 1:500 | Incubation with high pH-solution (pH 10.0) - 30 minutes at 100°C | • external - intestine |
| • internal - NS |
The IHC assessment was done with the use of a Nikon 800E optical microscope with digital camera. The cutoff point for CD34, VEGF-A, COX-2, maspin, c-KIT (CD117), and DOG-1 cytoplasmic positivity was set at 10%. The renal tubes were used as external control for VEGF positivity; incidental DOG-1 and maspin positivity was also observed in the renal tubes (Figure 1).
Figure 1.

Maspin (A) and DOG-1 positivity (B) in renal tubes that can be used as external positive control.
The microvessel density (MVD) was evaluated through CD31 staining by digital pictures and the image analysis software ImageJ (NIH). The MVD was determined by counting the positive vessels in the highly vascularized areas (hot spots) at 200x high-power fields. The percentage of positive endothelial area versus the total area of the microscopic field was batch-measured. The ulcerated and inflammatory areas were not taken into account in the assessment of angiogenesis. Although CD34 marked the endothelial cells, it was counted only in the tumor cells and was not used to quantify the MVD.
Statistical analysis
The statistical data were analyzed by using the GraphPad InStat 3 statistical software and descriptive analysis. A P-value < 0.05 with 95% confidence interval was considered statistically significant. The frequencies and percentages specific for the analyzed parameters, as well as the means and standard deviations, were used for continuous variables. The t test and ANOVA were used for univariate analysis.
Results
Clinicopathological features
The median age of the 55 patients was 57.23±14.88 years (range, 29-87 years), with a male/female ratio of 1:1.1 (26 males and 29 females). Most of the cases (34 of 55) were liposarcomas. Of the remaining 21 cases, 8 were benign tumors (lipomas and fibrohystiocytic tumors) and 13 were malignant non-lipomatous STTs (Table 2). Most of the tumors with lipomatous differentiation were diagnosed in the subcutaneous tissue of the abdominal wall, whereas the other tumor types predominantly developed in the lower extremities.
Table 2.
Immunohistochemical profile of soft tissue tumors
| Tumor histological type (n = 55) | CD31 median value | VEGF | COX-2 | CD34 | c-KIT | DOG-1 | maspin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| + | - | + | - | + | - | + | - | + | - | + | - | ||
| Liposarcoma (n = 34) | 8.35±3.45 | 27 | 7 | 5 | 29 | 7 | 27 | 5 | 29 | 5 | 29 | 9 | 25 |
| Atypical lipomatous tumor (n = 10) | 5.98±2.41 | 8 | 2 | 1 | 9 | - | 10 | - | 10 | - | 10 | 1 | 9 |
| Myxoid-type (n = 14) | 8.30±4.21 | 11 | 3 | 2 | 12 | - | 14 | 3 | 11 | 3 | 11 | 3 | 11 |
| Dedifferentiated type (n = 3) | 8.88±2.43 | 1 | 2 | 1 | 2 | - | 3 | - | 3 | - | 3 | - | 3 |
| Pleomorphic type (n = 7) | 9.44±2.02 | 7 | - | 1 | 6 | 7 | - | 2 | 5 | 2 | 5 | 5 | 2 |
| Dermatofibrosarcoma (n = 6) | 8.98±2.56 | 1 | 5 | 6 | - | 6 | - | 1 | 5 | 1 | 5 | - | 6 |
| Malignant fibrous histiocytoma (n = 4) | 9.33±3.68 | 2 | 2 | 4 | - | - | 4 | 1 | 3 | 1 | 3 | - | 4 |
| Fibrosarcoma (n = 3) | 8.27±2.31 | 1 | 2 | 3 | - | - | 3 | - | 3 | - | 3 | - | 3 |
| Benign tumors (n = 8) | 9.88±5.11 | - | 8 | 8 | - | 2 | 6 | 2 | 6 | 2 | 6 | 1 | 7 |
The endothelial area
By using the CD31 antibody, the median endothelial area (EA) was determined to be 9.08±4.05 (range, 3.17-18.71), without significant differences between benign and malignant tumors (P = 0.61). In the case of the benign tumors, the median EA was 9.88±5.11 (range, 3.17-15.78), whereas for the malignant tumors, independent of histological type, the median EA was 8.96±3.93, with a range of 3.95-18.91 (Table 2).
Regarding the liposarcomas, the median EA was 8.35±3.45 (range, 3.95-17.48). This was correlated with the tumor type, which was significantly smaller (P = 0.02) in atypical lipomas compared with the other histological groups (Table 2). The EA was also bigger in liposarcomas compared with lipomas, the latter having a median EA of 3.33±1.21 (P = 0.01).
Correlation of the endothelial area with other markers
Independent of the tumor type, the EA was directly correlated with the VEGF (P < 0.0001), COX-2 (P = 0.03), and CD34 expression in the tumor cells (P < 0.0001). No correlations were observed between the EA and the other IHC markers, such as maspin (P = 0.11), c-KIT (P = 0.09), and DOG-1 (P = 0.44).
Immunoexpression of VEGF, COX-2, and CD34 in the tumor cells
Of the 55 cases, 31 showed VEGF positivity in the tumor cells; all of these 31 cases were malignant STTs (Figures 2, 3). COX-2 marked the tumor cells of the 13 malignant non-lipomatous STTs, all benign tumors being COX-2 negative. Of the 34 liposarcomas, only 5 cases showed COX-2 positivity, without any relation to the histological type/grade of differentiation (Table 2).
Figure 2.
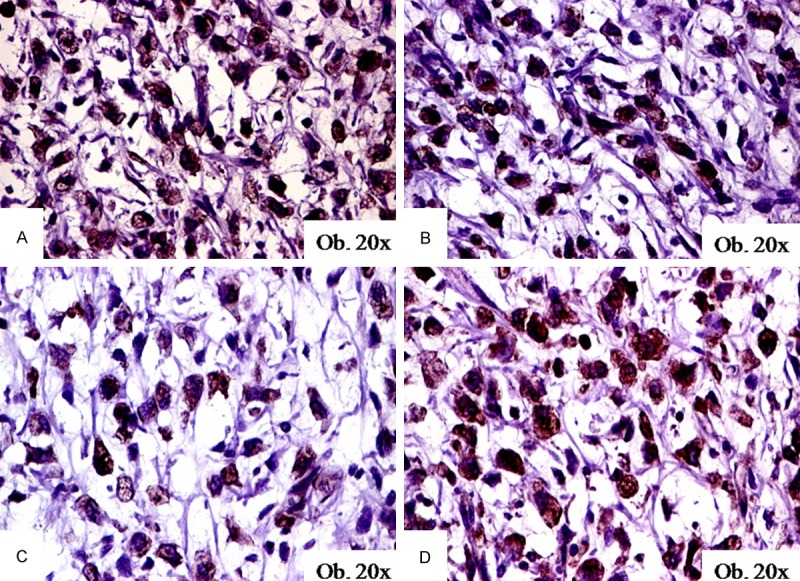
Immunohistochemical expression of DOG-1 (A), maspin (B), c-KIT (C), and VEGF (D) in myxoid liposarcomas.
Figure 3.
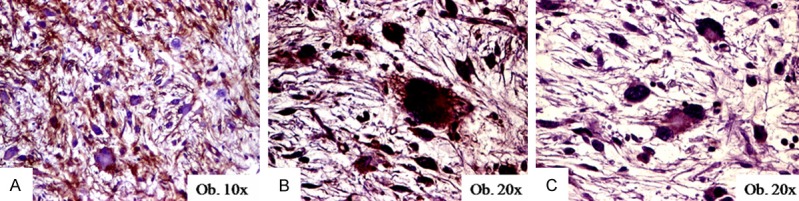
Immunohistochemical expression of CD34 (A), VEGF (B), and DOG-1 (C) in pleomorphic liposarcomas.
The CD34 positivity of the tumor cells was observed in 15 of the 55 cases. The two benign CD34-positive cases were lipomas (Table 2). All of the seven pleomorphic liposarcomas were diffusely marked by both CD34 and VEGF (Figure 3).
Immunoexpression of c-KIT and DOG-1 in the tumor cells
DOG-1 positivity was observed in 9 of the 55 cases, both in benign (one lipoma and one benign fibro-histiocytic tumor) and malignant tumors (three myxoid liposarcomas, two pleomorphic liposarcomas, one dermatofibrosarcoma, and one malignant fibrous histiocytoma). All of the nine DOG-1-positive cases also showed c-KIT and VEGF positivity (Figure 2). Regarding the DOG-1-maspin correlation, the three non-lipomatous tumors were maspin-negative cases, while the six tumors with lipomatous differentiation also showed maspin positivity (Table 2). DOG-1 positivity was also observed in the mature adipocytes.
Particularities of maspin immunoexpression in the tumor cells
There was marked maspin cytoplasmic positivity in 10 of the 55 cases, all of which presented lipomatous differentiation: one lipoma, one atypical lipomatous tumor, three myxoid liposarcomas (Figure 2) and five pleomorphic liposarcomas were maspin-positive (Table 2).
Discussion
Although the angiogenesis of STTs has been relatively extensively studied in the literature, most reports are focused on sarcomas. Similar to other solid tumors, VEGF is known to be secreted by mesenchymal tumor cells and peritumoral mast cells and to induce angiogenesis [10]. At the same time, VEGF and eight other hypoxia-related genes (MIF, SCD1, P4HA1, ENO1/MBP1, FAM162A/HGTD-P, SLC16A1/MCT1, FN1, and STAT1) seem to inhibit the proapoptotic activity of the bax gene, assuming that anti-VEGF therapy could increase not only the rate of apoptosis of endothelial cells but also the rate of induced death of tumor cells [2,5]. In line with these data, a direct correlation between endothelial area, VEGF, and COX-2 was observed in our study. Because anti-VEGF therapy does not achieve the best results in soft tissue sarcomas, a combined anti-COX-2/anti-VEGF therapy may improve the therapeutic results.
Experimentally, the inhibition of maspin in carcinoma cell lines has been proven to have antiproliferative and antiangiogenic effects, suggesting that anti-maspin drugs could be synthesized for antiangiogenic purposes. However, the confinement of maspin positivity to adipose tissue tumors suggests that it is not involved in the biological behavior of dermatofibrosarcomas or tumors with histiocytic differentiation. In non-lipomatous tumors, angiogenesis seems to be rather dependent on the VEGF and COX-2 activity, whereas a maspin-dependent pathway could be involved in the histogenesis of liposarcomas. COX-2 overexpression induces angiogenesis, enhancing tumor proliferation, and promotes suppression of apoptosis, which is a negative prognostic factor in soft tissue sarcomas [11].
Regarding DOG-1 positivity, although this biomarker is considered to have higher sensitivity and 100% specificity in distinguishing GISTs from other tumors, including leiomyomas and leiomyosarcomas [7,8,12], this study proved for the first time that DOG-1 can also mark other tumors, such as dermatofibrosarcomas, histiocytomas, and tumors with lipomatous differentiation. Its previously described positivity in salivary gland tumors [13], pseudopapillary neoplasm and acinic cell carcinomas of the pancreas [14,15], melanomas [15], carcinomas of the endometrium and colorectal segments [15], schwannomas [15], and uterine leiomyosarcomas [16], confirms our supposition. Referring to normal cells, DOG-1 has been previously reported to mark pancreatic centroacinar cells [15], its concomitant expression with chromogranin and synaptophysin suggesting a potential DOG-1-induced neuroendocrine activity [17]. Its expression in mature adipose cells and renal collecting tubules, observed in our study, has not yet been reported in the literature.
The DOG-1/VEGF concomitant expression in the tumor cells of soft tissue sarcomas and in the renal tubes could indicate a possible pro-angiogenic role of DOG-1. This unreported property of DOG-1 could explain the response of both metastatic renal cell carcinomas and metastatic soft tissue sarcomas to pazopanib, one of the most recently approved angiogenesis inhibitors targeting VEGF-, PDGF-, and c-KIT receptors, which is used for second-line therapy of renal cell carcinoma and soft tissue sarcomas [18].
Conclusions
In STTs with histiocytic differentiation and dermatofibrosarcomas the angiogenic activity is dependent on the VEGF-A/COX-2 pathway, whereas a VEGF-A/maspin relationship seems to be more characteristic of liposarcomas. Combined anti-COX-2/anti-VEGF drugs could be effective for non-lipomatous malignant STTs, whereas liposarcomas could be responsive to anti-maspin/anti-VEGF therapy. Moreover, concomitant DOG-1/c-KIT/VEGF-A positivity might be a potential useful target for further personalized antiangiogenic/anti-tyrosine kinase therapy of STTs; this suggests a potential role of DOG-1 in tumor angiogenesis. Renal tubes can be used as external control for both DOG-1 and maspin.
Acknowledgements
The English-language manuscript was polished by SPI Global Professional Editing Service.
Disclosure of conflict of interest
None.
References
- 1.Kilvaer TK, Smeland E, Valkov A, Sorbye SW, Bremnes RM, Busund LT, Donnem T. The VEGF and PDGF-family of angiogenic markers have prognostic impact in soft tissue sarcomas arising in the extremities and trunk. BMC Clin Pathol. 2014;14:5. doi: 10.1186/1472-6890-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Win TT, Jaafar H, Yusuf Y. Relationship of angiogenic and apoptotic activities in soft-tissue sarcoma. South Asian J Cancer. 2014;3:171–174. doi: 10.4103/2278-330X.136799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Pobirici DD, Bogdan F, Pobirici O, Petcu CA, Rosca E. Study of malignant fibrous histiocytoma: clinical, statisitic and histopathological interrelation. Rom J Morphol Embryol. 2011;52:385–388. [PubMed] [Google Scholar]
- 5.Takahashi A, Nakayama R, Ishibashi N, Doi A, Ichinohe R, Ikuyo Y, Takahashi T, Marui S, Yasuhara K, Nakamura T, Sugita S, Sakamoto H, Yoshida T, Hasegawa T, Takahashi H. Analysis of gene expression profiles of soft tissue sarcoma using a combination of knowledge-based filtering with integration of multiple statistics. PLoS One. 2014;9:e106801. doi: 10.1371/journal.pone.0106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher CDM, Bridge JA, Hogendoorn PCW, editors. IARC. 2013. WHO classification of tumours of soft tissue and bone. [Google Scholar]
- 7.Hwang DG, Qian X, Hornick JL. DOG1 antibody is a highly sensitive and specific marker for gastrointestinal stromal tumors in cytology cell blocks. Am J Clin Pathol. 2011;135:448–453. doi: 10.1309/AJCP0PPKOBNDT9LB. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Jin MS, Zou YB, Gao JN, Li XB, Peng F, Wang HY, Wu ZD, Wang YP, Duan XM. Diagnostic significance of DOG-1 and PKC-θ expression and c-Kit/PDGFRA mutations in gastrointestinal stromal tumours. Scand J Gastroenterol. 2013;48:1055–1065. doi: 10.3109/00365521.2013.816770. [DOI] [PubMed] [Google Scholar]
- 9.Gurzu S, Ciortea D, Munteanu T, Kezdi-Zaharia I, Jung I. Mesenchymal to endothelial transition in Kaposi sarcoma. PLoS One. 2013;8:e71530. doi: 10.1371/journal.pone.0071530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baneth V, Raica M, Cimpean AM. Assessment of angiogenesis in soft-tissue tumors. Rom J Morphol Embryol. 2005;46:323–327. [PubMed] [Google Scholar]
- 11.Hakozaki M, Tajino T, Konno S, Kikuchi S, Yamada H, Yanagisawa M, Nishida J, Nagasawa H, Tsuchiya T, Ogose A, Abe M, Hojo H. Overexpression of cyclooxygenase-2 in malignant peripheral nerve sheath tumor and selective cyclooxygenase-2 inhibitor-induced apoptosis by activating caspases in human malignant peripheral nerve sheath tumor cells. PLoS One. 2014;9:e88035. doi: 10.1371/journal.pone.0088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fei BY, Yang JM, Zao ZS. Differential clinical and pathological characteristics of esophageal stromal tumors and leiomyomata. Dis Esophagus. 2014;27:30–35. doi: 10.1111/dote.12032. [DOI] [PubMed] [Google Scholar]
- 13.Chenevert J, Duvvuri U, Chiosea S, Dacic S, Cieply K, Kim J, Shiwarski D, Seethala RR. DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol. 2012;25:919–929. doi: 10.1038/modpathol.2012.57. [DOI] [PubMed] [Google Scholar]
- 14.Bergmann F, Andrulis M, Hartwig W, Penzel R, Gaida MM, Herpel E, Schirmacher P, Mechtersheimer G. Discovered on gastointestinal stromal tumor 1 (DOG1) is expressed in pancreatic centroacinar cells and in solid-pseudopapillary neoplasm-novel evidence for a histogenetic relationship. Hum Pathol. 2011;42:817–823. doi: 10.1016/j.humpath.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Hemminger J, Iwenofu OH. Discovered on gastrointestinal stromal tumours 1 (DOG1) expression in non-gastrointestinal stromal tumour (GIST) neoplasms. Histopathology. 2012;61:170–177. doi: 10.1111/j.1365-2559.2011.04150.x. [DOI] [PubMed] [Google Scholar]
- 16.Sah SP, McCluggage WG. DOG1 immunoreactivity in uterine leiomyosarcomas. J Clin Pathol. 2013;66:40–43. doi: 10.1136/jclinpath-2012-201150. [DOI] [PubMed] [Google Scholar]
- 17.Ardeleanu C, Arsene D, Hinescu M, Andrei F, Gutu D, Luca L, Popescu LM. Pancreatic expression of DOG1: a novel gastrointestinal stromal tumor (GIST) biomarker. Appl Immunohistochem Mol Morphol. 2009;17:413–418. doi: 10.1097/PAI.0b013e31819e4dc5. [DOI] [PubMed] [Google Scholar]
- 18.Brotelle T, Bay JO. Pazopanib for treatment of renal cell carcinoma and soft tissue sarcomas. Bull Cancer. 2014;101:641–646. doi: 10.1684/bdc.2014.1981. [DOI] [PubMed] [Google Scholar]


