Abstract
Continuing advances in ‘omics methodologies and instrumentation is enhancing the understanding of how plants cope with the dynamic nature of their growing environment. ‘Omics platforms have been only recently extended to cover horticultural crop species. Many of the most widely cultivated vegetable crops belong to the genus Brassica: these include plants grown for their root (turnip, rutabaga/swede), their swollen stem base (kohlrabi), their leaves (cabbage, kale, pak choi) and their inflorescence (cauliflower, broccoli). Characterization at the genome, transcript, protein and metabolite levels has illustrated the complexity of the cellular response to a whole series of environmental stresses, including nutrient deficiency, pathogen attack, heavy metal toxicity, cold acclimation, and excessive and sub-optimal irradiation. This review covers recent applications of ‘omics technologies to the brassicaceous vegetables, and discusses future scenarios in achieving improvements in crop end-use quality.
Keywords: genomics, transcriptomics, metabolomics, proteomics, crop, microbiomics
Introduction
Brassicaceous vegetables, which are cultivated worldwide, belong to the taxa Brassica oleracea (cabbage, broccoli, cauliflower, kale, Brussels sprouts, collard greens, savoy, kohlrabi, and Chinese kale) and B. rapa (turnip, mizuna, napa cabbage, cime di rapa, and turnip rape). Just as for crops generally, maintaining their productivity in the light of incipient climate change and the dynamically changing pest and pathogen community represents a major challenge for the biotechnologist and the plant breeder (Augustine et al., 2014). The importance of this class of vegetables lies not only in their contribution to the vitamin and mineral components of the human diet, but also in their beneficial effect on human health, which reflects the action of the glucosinolates, a group of secondary plant metabolites almost exclusively associated with this plant family (Higdon et al., 2007; Jeffery and Araya, 2009; Wu et al., 2013). The aliphatic glucosinolates (and their break down products) have attracted scientific attention (Reichelt et al., 2002; Halkier and Gershenzon, 2006; Sonderby et al., 2010; Wittstock and Burow, 2010); of particular note in this context is the anti-carcinogen isothiocyanate sulforaphane, the major break down product of glucoraphanin in broccoli (Fahey et al., 1997; Shapiro et al., 2001, 2006).
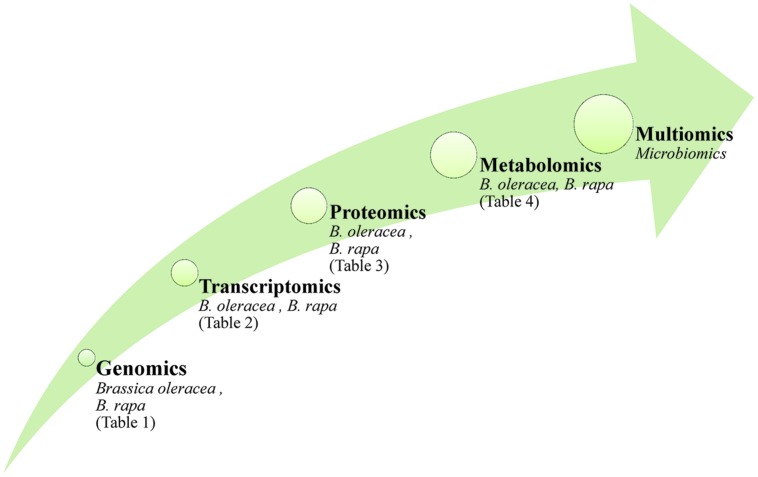
The use of ‘omics technologies, which current gather information either at the DNA, RNA, protein or metabolite levels, can potentially provide a comprehensive picture of cellular physiology. DNA sequence (although not the epigenome) is largely independent of the growing environment, while the transcriptome, proteome and metabolome are all highly responsive (Figure 1). Tailoring a crop cultivar to a specific environment is the central challenge for the plant breeder, and reflects the reality that genotype on its own will not generally be sufficient to support a biotechnology-driven crop improvement program. Rather, a combination of one or more of the ‘omics platforms is required to deliver reliable information. Such multiple ‘omics data sets tend to be very large, as they represent a series of time point and/or treatment samplings; their analysis can only be addressed computationally (Bieda, 2012; Kohl et al., 2014; Schumacher et al., 2014). The demand for better data processing led to the release of new software packages freely available or provided by vendors (examples are given in Katajamaa and Oresic, 2007), however, the visualization of multi-omic data sets remains an important task for bioinformatics. Practical tools should create clear and meaningful visualizations without being overwhelmed by the complexity of the data sets (recent approaches are discussed by Gehlenborg et al., 2010).
FIGURE 1.
The application of ‘omics technologies in brassicaceous vegetables. Arrow size reflect the complexity of the molecular data and the impact on phenotype.
The prior acquisition of a full genome sequence aids materially in the interpretation of such data. The genome sequence of the model plant Arabidopsis thaliana, a member of the Brassicaceae family, has been known for nearly 15 years (Kaul et al., 2000), and the full genome sequences of both B. rapa (Wang et al., 2011) and B. oleracea (Ayele et al., 2005; Liu et al., 2014b) have been published more recently. Substantial amounts of transcript-based data have been acquired for B. oleracea (Gao et al., 2014; Izzah et al., 2014; Kim et al., 2014).
Genomics
Genomic research has a great capability in speeding up breeding processes and several applications for crop improvement, through, e.g., marker-assisted selection and gene pyramiding. In case of brassicaceous vegetables, several populations have been generated to establish linkage maps using simple sequence repeat (SSR), amplified fragment length polymorphism (AFLP), nucleotide binding site or expressed sequence tag (EST) markers with the aim to genetically localize favorable traits by quantitative trait locus (QTL) analysis (summarized in Table 1). A survey of available online tools, covering mapping populations, linkage maps, gene sequences, and QTL, is presented by Li et al. (2013). Linkage maps were generated by crossing different genotypes of B. oleracea (Camargo et al., 1997; Iniguez-Luy et al., 2008) and B. oleracea var. botrytis (Gu et al., 2008). In the previous examples, a segregating offspring population was used to establish marker order and spacing. The same was shown for doubled haploid lines where offspring are homozygous (see Pink et al., 2008 for review). A doubled haploid population derived from crosses of two broccoli cultivars was genotyped by SSR and AFLP markers and QTL analysis identified loci for horticulturally important characteristics (Walley et al., 2012). Plant genetic resources have been characterized using AFLP markers to assess the huge genetic diversity present in gene banks, including Dutch and Italian B. oleracea (van Hintum et al., 2007; Maggioni et al., 2014) and Czech B. oleracea var. capitata accessions (Faltusova et al., 2011). A B. rapa collection consisting of 239 accessions was genotyped using SSR markers and subsequent association mapping identified two markers associated with flowering time (Zhao et al., 2010). Genetic basis of flowering time was also investigated in B. rapa by association mapping using natural variation and recombinant inbred lines (Lou et al., 2011). Phylogenetic relationships were established in B. rapa subspecies by AFLP markers (Takuno et al., 2007). The development of genetic maps from Brassica vegetables paved the way for QTL analysis and, together with accurate phenotyping, morphological, qualitative and yield traits were evaluated in different species. QTL mapping was conducted for eleven yield- and heading-related traits in doubled haploid Chinese cabbage lines and identified 46 main QTL (Liu et al., 2013b). In addition, 27 QTL were found for leaf and heading-related traits in a segregating population of Chinese cabbage (Ge et al., 2011). A segregating population of a cross between a Chinese cabbage with a turnip was used to identify loci related to morphological characteristics of the tap root (Lu et al., 2008). To investigate the genetic background of premature bolting under cold stress, a Chinese cabbage population was constructed based on crossing early and late bolting genotypes and QTL analysis identified 26 QTL (Wang et al., 2014c). Seven morphological traits were screened in a population derived from crossing a Chinese cabbage with a vegetable turnip and resulted in the detection of eight QTL (Kubo et al., 2010). A doubled haploid population of B. oleracea var. capitata served for detecting 13 QTL for heading-related traits (Lv et al., 2014). QTL analysis of a B. oleracea var. italica population, derived from a cross of a heat-sensitive and a heat-tolerant cultivar, identified AFLP markers correlated to floral development under heat stress (Lin et al., 2013). A high-density linkage map was established for a B. oleracea population segregating for carotenoid concentration in florets and three carotenoid QTL were found (Brown et al., 2014). Authors applied the B. napus SNP array and presented a 96% coverage of the B. oleracea genome. Localizing underlying factors affecting another major pigment, chlorophyll, in a Chinese cabbage population revealed five QTL for chlorophyll a and five QTL for chlorophyll b content (Ge et al., 2012). Vegetables with high nutrient use efficiency are also developed in order to reduce fertilizer application. Association mapping of B. oleracea accessions identified some QTL related to potassium concentration in shoot and those were tested using substitution lines (White et al., 2010). The gain of knowledge on the genetic localization of favorable traits is transferred to breeding new lines through marker-assisted selection. Accumulating three major loci for clubroot (Plasmodiophora brassicae) resistance genes resulted in the development of Chinese cabbage lines with strengthened resistance (Matsumoto et al., 2012).
Table 1.
Published genomics analyses in the brassicaceous vegetables.
| Brassica species | Population | Plant material | Reference |
|---|---|---|---|
| B. oleracea | Segregating population | Cross of contrasting geno-types | Camargo et al. (1997) |
| B. oleracea | Segregating population | Cross of contrasting genotypes | Sebastian et al. (2000) |
| B. oleracea | Segregating population | Cross of contrasting genotypes | Gao et al. (2007) |
| B. oleracea | Gene bank accessions | – | van Hintum et al. (2007) |
| B. oleracea | Doubled-haploid | Cross of contrasting genotypes | Iniguez-Luy et al. (2008) |
| B. oleracea | Gene bank accessions | DNA methylation | Salmon et al. (2008) |
| B. oleracea | TILLING | EMS mutagenesis | Himelblau et al. (2009) |
| B. oleracea | Gene bank accessions | – | White et al. (2010) |
| B. oleracea | Segregating population | Cross of contrasting genotypes | Brown et al. (2014) |
| B. oleracea | DNA methylation | Parkin et al. (2014) | |
| B. oleracea var. botrytis | Segregating population | Cross of contrasting genotypes | Gu et al. (2008) |
| B. oleracea var. capitata | Gene bank accessions | – | Faltusova et al. (2011) |
| B. oleracea var. capitata | Doubled-haploid | Cross of contrasting genotypes | Wang et al. (2012) |
| B. oleracea var. capitata | Doubled-haploid | Cross of contrasting genotypes | Lv et al. (2014) |
| B. oleracea var. italica | Doubled-haploid | Cross of contrasting genotypes | Walley et al. (2012) |
| B. oleracea var. italica | Segregating population | Cross of contrasting genotypes | Lin et al. (2013) |
| B. oleracea, B. rupestris | Wild and cultivated populations, hybrid population | Maggioni et al. (2014) | |
| B. rapa | Wild and cultivated populations | – | Takuno et al. (2007) |
| B. rapa | Segregating population | Cross of contrasting genotypes | Lu et al. (2008) |
| B. rapa | Segregating population | Cross of contrasting genotypes | Kubo et al. (2010) |
| B. rapa | TILLING | EMS mutagenesis | Stephenson et al. (2010) |
| B. rapa | Gene bank accessions | – | Zhao et al. (2010) |
| B. rapa | Segregating population, gene bank accessions | Cross of contrasting genotypes | Lou et al. (2011) |
| B. rapa | Segregating population | Cross of contrasting genotypes | Bagheri et al. (2012) |
| B. rapa | Hypermethylated population | DNA methylation | Amoah et al. (2012) |
| B. rapa | Segregating population | Cross of contrasting genotypes | Wang et al. (2014c) |
| B. rapa ssp. pekinensis | Segregating population | Cross of contrasting genotypes | Ge et al. (2011) |
| B. rapa ssp. pekinensis | Segregating population | Cross of contrasting genotypes | Ge et al. (2012) |
| B. rapa ssp. pekinensis | Marker-assisted selection | Gene pyramiding | Matsumoto et al. (2012) |
| B. rapa ssp. pekinensis | Doubled-haploid | Cross of contrasting genotypes | Liu et al. (2013b) |
Advances in next generation sequencing technologies enabled surveying genotype-phenotype-relationships with the highest resolution to date (Wei et al., 2013; Varshney et al., 2014). A high-density linkage map was derived from the whole genome shotgun sequence of B. oleracea var. capitata, based on 1,227 genetic markers (Wang et al., 2012). The availability of B. rapa genome sequence aided in the generation of a linkage map for a recombinant inbred line descending from a vegetable leafy and an yellow sarson oilseed genotype of B. rapa (Bagheri et al., 2012). Recently, genomes of all three sequences Brassicas were compared to develop SSR markers and a total of 115,869, 185,662, and 356,522 primer pairs were designed from B. rapa, B. oleracea, and B. napus, respectively (Shi et al., 2014). EST markers were developed based on the sequenced transcriptomes of two cabbage lines susceptible or tolerant to black rot disease, demonstrating the feasibility especially for species without a reference genome sequence (Izzah et al., 2014).
One of the main strategies in reverse genetics is Targeting Induced Local Lesions IN Genomes (TILLING) and numerous applications are shown in functional genomics of model plants and crops (Kurowska et al., 2011; Chen et al., 2014). Random point mutations are generated by chemical mutagenesis and high-throughput screening for SNPs in the target gene isolates mutants with loss-of-function or gain-of-function phenotypes. Currently, two TILLING populations are established for brassicaceous vegetables, B. oleracea1 (Himelblau et al., 2009) and B. rapa2 (Stephenson et al., 2010) and both are freely accessible.
Next generation sequencing and AFLP mapping of methylation polymorphism allowed determining the DNA cytosine methylation status and, hence, enabled tackling the epigenome to understand gene expression variation (Niederhuth and Schmitz, 2014). The level of genome methylation was assessed in 30 B. oleracea populations and lines, and then related to phenotypic variability (Salmon et al., 2008). The B. oleracea genome was correlated to the leaf transcriptome and methylome and provided insights into polyplody events (Parkin et al., 2014). A chemically induced hypermethylated B. rapa population was developed recently and can serve for epiallele discovery (Amoah et al., 2012).
Transcriptomics
Transcriptomics is based on the characterization and quantification of RNAs present in a given plant, organ, tissue, or cell. A particular attraction of assaying transcription is that it presages gene expression and so has the potential to provide a link between genotype and phenotype. Rapid developments in the relevant technologies now allow for the design of very large-scaled experiments which can in principle capture and enumerate every transcript present in a given biological sample (summarized in Table 2). The early transcriptomic platforms were built around the concept of immobilizing transcripts in an array, which was used to capture the mRNA content of a sample on the basis of nucleotide sequence complementarity. A more sophisticated form of microarray replaced transcripts with 20–70 nt long oligonucleotides designed to target known gene sequences (Knudsen, 2004). In both cases, the protocol requires bathing the array in a solution of labeled RNA extracted from the test sample. Hybridization of the probe at a given site on the array is detected by the fluorescence emitted by the labeled probe. Typically, two parallel experiments need to be run, in which one sample is derived from a plant exposed to a certain treatment while the other is derived from a plant which has not been exposed (control). Alternatively, genetic contrasts (such as wild type vs. a mutant, wild type vs. a transgenic) can replace the treatment contrast. Many of such experiments have been reported in a range of plant species (for example, Rabbani et al. (2003) in rice, Yu and Setter (2003) in maize, Lee and Yun (2006) in pepper). The quantity of such data collected from the brassicaceous vegetable species is relatively limited, partly perhaps because their full genome sequences have only very recently been acquired. The close phylogenetic relationship between Brassica spp. and A. thaliana has encouraged a number of attempts to use an A. thaliana microarray to analyze Brassica spp. transcriptomes. With the recent acquisition of Brassica spp. genome sequences, a number of Brassica-specific microarrays are finally becoming available – these include the B. rapa 24 K oligo microarray and the Agilent Brassica microarray, which is able to detect the transcription of >27,000 unigenes in a range of Brassica spp. The genetic closeness of Brassica spp. and A. thaliana has allowed large numbers of Brassica unigenes to be assigned a function, but nevertheless, substantial numbers appear to encode either Brassica-specific proteins or represent non-coding RNA (Trick et al., 2009).
Table 2.
Published transcriptomic analyses in the brassicaceous vegetables.
| Brassica species | Plant organ/developmental stage | Study objective | Methodology | Reference |
|---|---|---|---|---|
| B. oleracea | Flowers | Male sterility | Microarray | Kang et al. (2008) |
| B. oleracea var. italica | Seeds and sprouts | Glucosinolate metabolism | RNA-seq analysis | Gao et al. (2014) |
| B. rapa | Flowers | Self-incompatibility | Microarray | Kwun et al. (2004) |
| B. rapa | plants | Abiotic stress | Microarray | Lee et al. (2008) |
| B. rapa | Seedlings, roots, petioles, leaves, flowers | Comparative analysis | RNA-seq | Kim et al. (2012) |
| B. rapa | Seedlings, roots, leaves, petiole | Abiotic stress | RNA-Seq | Devisetty et al. (2014) |
| B. rapa ssp. pekinensis | Seedlings, root tips | Cold stress | Microarray | Yang et al. (2005) |
| B. rapa ssp. pekinensis | Leaves | Heat shock transcription factor | Comparative genomic analysis | Song et al. (2014) |
| B. rapa ssp. pekinensis | Seedlings | Plant-microbe interactions | RNA-seq | Sun (2014) |
| B. rapa ssp. rapa | Seedlings | Etiolation | RNA-seq | Zhou et al. (2014) |
A cDNA microarray constructed from a Chinese cabbage (B. rapa ssp. pekinensis) pistil-specific cDNA library approach was used by Kwun et al. (2004) to investigate the effect of CO2 on self-incompatibility, and a similar strategy was followed by Yang et al. (2005) to identify genes up-regulated by low temperature stress. Meanwhile Lee et al. (2008) synthesized an oligo-based microarray from the sequences of 24,000 B. rapa unigenes to characterize the response to low temperature, salinity and drought. Based on the identification of candidate genes using a 2 × 104 k Brassica microarray to compare the gene expression of untreated and methyl jasmonate treated pak choi sprouts, Wiesner et al. (2013a, 2014) were able to identify the homologues genes involved in the synthesis of 1-methoxyindole-3-ylmethyl glucosinolate.
The major alternative to microarray technology relies on amplification rather than on hybridization. The most commonly used method, termed RNA-seq, is designed to generate a de novo assembly of the transcriptome. This has largely replaced the earlier approach called “serial analysis of gene expression” (SAGE). In general, a population of RNA (total or fractionated) is converted to a library of cDNA fragments with adaptors attached to one or both ends. Each molecule, with or without amplification, is then sequenced in a high-throughput manner to obtain short sequences from one end (single-end sequencing) or both ends (pair-end sequencing). The reads are typically 300–400 bp, depending on the DNA-sequencing technology used (Wang et al., 2009). In principle, any high-throughput sequencing technology can be used for RNA-Seq. The data generated from RNA-Seq experiments are in the form of an absolute frequency of each transcript identified, which obviates the need for using a reference sequence. For this reason, it can provide a greater level of insight and accuracy than microarray analysis can (Marioni et al., 2008; T’Hoen et al., 2008). In broccoli, the RNA-Seq approach has been taken to describe the transcription of genes involved in glucosinolate metabolism (Gao et al., 2014). Of the nearly 20 k unigenes recovered, more than 2,500 appeared to be differentially transcribed in a contrast between the seed and the seedling, and a large proportion of these were putative transcription factors. Curiously, the up-regulation of candidate glucosinolate synthesis genes was negatively correlated with the glucosinolate content of the germinating seedling; a possible explanation offered by the authors was that the high transcript abundance of an TGG2 orthologue (encoding a myrosinase) had the effect of degrading aliphatic glucosinolates (Gao et al., 2014).
Small RNAs, which range in length from 19 to 25 nt (most are either 21 nt or 24 nt long), are ubiquitous in eucaryotic cells. Extensive transcriptome sequencing has revealed the presence of a large number of such RNAs, which cannot be translated into a protein, but rather act as an important class of regulators, especially in the context of plant development, signal transduction, metabolism and the response to biotic and abiotic stress (Ellendorff et al., 2009; Sun, 2012). The abundance of a particular species of a class of small RNAs, referred to as microRNAs (miRNAs), varies from plant species to plant species, from developmental stage to developmental stage and from tissue to tissue (Carrington and Ambros, 2003; Bartel, 2004; Kenan-Eichler et al., 2011). The transcriptional response of Chinese cabbage to infection by Erwinia carotovora ssp. carotovora included a marked alteration in the abundance of certain small RNAs (Sun, 2014). In turnip (B. rapa ssp. rapa), it appeared that various miRNAs are involved in the regulation of plant growth, development and differentiation in the absence of light (Zhou et al., 2014). A number of Brassica species are polyploids, the genomes of which have been markedly altered by the polyploidization event (Kenan-Eichler et al., 2011). Some of these changes have been associated with the activity of small RNAs. Kim et al. (2012) identified 412 distinct miRNAs in B. rapa, of which 216 were novel. The same study identified 29 novel miRNAs which were only found in the flower.
Proteomics
The acquisition of the complete genome sequence of a growing number of plant species3 along with their transcriptomes continues apace. However, much of the physiology of the cell is determined by gene products (particularly, but not exclusively, proteins) rather than by nucleic acid. A proteomic analysis seeks to characterize the full protein complement present in a particular organism, organ, tissue, or cell. Initial attempts to derive this were based on two dimensional gel electrophoresis (2-DE), as described by O’Farrell (1975). Besides 2-DE, current proteomics platforms exploit more versatile LC (liquid chromatography)-based methods (Rabilloud et al., 2010; Matros et al., 2011), coupled with mass spectrometry (MS). Complicating the analysis, and unlike the nucleic acids, protein molecules are subject to a range of functional modification, such as phosphorylation, glycosylation, and acetylation (Cox and Mann, 2011). Nevertheless, proteomic approaches have been successfully applied to a number of plant species to study various developmental processes and environmental adaptation (see reviews by Barkla et al., 2013; Vanderschuren et al., 2013; Zhuang et al., 2014). Kehr and Buhtz (2011) have provided a summary of the literature regarding proteomic analyses in Brassica spp., a list dominated by experiments based on oilseed rape. In the following, we give emphasis to reports on how proteomic techniques are applied to catalog and characterize special, developmental-driven or environmentally induced alterations in the proteome of brassicaceous vegetables (summarized in Table 3).
Table 3.
Published proteomic analyses in the brassicaceous vegetables.
| Brassica species | Plant organ/developmental stage | Study objective | Methodology | Reference |
|---|---|---|---|---|
| Members of Brassicaceae family | Etiolated leaves of seedlings | Genotypic variation | 2-DE | Marquès et al. (2001) |
| B. oleracea var. capitata, B. rapa var. pekinensis, synthesized B. napus | Seeds | Genotypic variation | 2-DE and MS/MS | Hossain et al. (2014) |
| B. oleracea | Xylem saps | Mapping | 1-DE and MS/MS | Buhtz et al. (2004) |
| B. oleracea | Vacuoles | Mapping | MS/MS | Schmidt et al. (2007) |
| B. oleracea | Xylem saps | Mapping, N-glycosylation | MS/MS | Ligat et al. (2011) |
| B. oleracea | Phloem tissues of stem | Mapping | MS/MS | Anstead et al. (2013) |
| B. oleracea var. alboglabra | Leaves, stems | Genotypic variation | 2-DE | Albertin et al. (2005) |
| B. oleracea var. alboglabra | Leaves | Genotypic variation | 2-DE and MS | Kong et al. (2010) |
| B. oleracea var. botrytis italica | Leaves, stems | Genotypic variation | 2-DE | Albertin et al. (2005) |
| B. oleracea var. botrytis | Mitochondria | Mapping | 2-DE and MS | Pawlowski et al. (2005) |
| B. oleracea var. capitata | Floral head | Effect of transgene | 2-DE and MS/MS | Liu et al. (2011) |
| B. oleracea L. var capitata | Leaves | Cropping systems | 2-DE and MS/MS | Nawrocki et al. (2011) |
| B. oleracea var. capitata | Stigma | Self-incompatibility | 2-DE | Zeng et al. (2012) |
| B. oleracea var. capitata | Floral head | Effect of transgene | 2-DE and MS/MS | Liu et al. (2013a) |
| B. oleracea var. capitata | Seeds | Genotypic variation | 2-DE and MS/MS | Hossain et al. (2014) |
| B. oleracea var. capitata | Floral heads | Effect of transgene | 2-DE and MS/MS | Liu et al. (2014a) |
| B. oleracea var. italica | Xylem sap | Salinity | 2-DE and MS/MS | Fernandez-Garcia et al. (2011) |
| B. oleracea var. italica | Floral heads | Sodium selenate nutrition | 2-DE and MS/MS | Sepúlveda et al. (2013) |
| B. rapa cvs. Natsumaki, Takamaru | Roots | Pathogen interaction | 2-DE | Kaido et al. (2007) |
| B. rapa | Leaves | Genotypic variation | 2-DE and MS | Kong et al. (2010) |
| B. rapa ssp. chinensis | Leaves | Effect of di-n-butyl phtalate | 2-DE and MS/MS | Liao et al. (2006) |
| B. rapa var. chinensis | Leaves | Effect of di-n-butyl phtalate | 2-DE and MS/MS | Liao et al. (2009) |
| B. rapa ssp. chinensis | Leaves | Mapping | 2-DE | Alam et al. (2013) |
| B. rapa ssp. chinensis | Leaves | Pathogen interaction | 2-DE and MS/MS | Sun et al. (2014) |
| B. rapa ssp. chinensis | Pistil | Self-incompatibility | 2-DE and MS/MS | Wang et al. (2014a) |
| B. rapa ssp. chinensis | Leaves and roots | Nitrogen nutrition | 2-DE and MS | Zhuang et al. (2014) |
| B. rapa ssp. pekinensis | Seedlings | Effect of atrazine | 2-DE and MS | Li et al. (2008) |
| B. rapa var. pekinensis | Seeds | Genotypic variation | 2-DE and MS/MS | Hossain et al. (2014) |
Using a conventional 2-DE approach, Alam et al. (2013) succeeded in detecting about 1,300 distinct low abundance leaf proteins in Chinese cabbage. A characterization of the proteomic content of the xylem sap of broccoli and oilseed rape (and some non- brassicaceous) undertaken by Buhtz et al. (2004) showed little evidence of any species specificity, while De Bernonville et al. (2014) were able to define the proteomic impact in the xylem sap induced by infection of B. oleracea with the pathogen Xanthomonas campestris pv. campestris. Ligat et al. (2011) expanded the B. oleracea data set by subjecting the xylem sap proteome to LC-MS/MS and the N-glycoproteome by prior enrichment with concanavalin A affinity chromatography and LC-MS/MS. Most of the ∼200 proteins identified proved to be involved in cell wall-related carbohydrate metabolism, although a number of oxido-reductases and proteases were also revealed. A proteomic analysis of broccoli tissue enriched with phloem allowed the identification of 379 proteins, some of which were structural and others associated with the biotic and/or abiotic stress response (Anstead et al., 2013). In cauliflower, an analysis of isolated tonoplast membranes revealed 102 tonoplast integral and 214 peripheral proteins (Schmidt et al., 2007). A further study in this species was able to show that its mitochondrial proteome was highly similar to that of A. thaliana, and included at least 51 proteins involved in the generation of ATP, protein folding and protein transport (Pawlowski et al., 2005).
The host-pathogen interaction is important in brassicaceous vegetables, as in all crops. A root proteomic comparison between a pair of turnip cultivars showing a contrasting reaction to the causal agent of clubroot disease was able to demonstrate the presence of certain proteins in the resistant cultivar which were absent from the profile of the sensitive one (Kaido et al., 2007). Sun et al. (2014) tracked the proteomic response of Chinese cabbage resulting from infection with downy mildew (Hyaloperonospora parasitica), and were able to identify some 91 proteins which altered in their abundance; some of these were involved in ethylene signaling. When the abundances of specific transcripts and their gene products were compared, a correlation could be established for only about half of the 33 genes investigated; a finding which was interpreted as implying that many of the early events in the host’s resistance response involved post-translational modification.
Abiotic stress is a further major production constraint. Analysis of the effect of the photosystem II inhibitor atrazine on the chloroplast proteome of Chinese cabbage has identified at least nine proteins as being differentially expressed; some of these were related to isoprene or protein synthesis (Li et al., 2008). Investigations of the impact of the industrial soil pollutant phthalate ester on either pak choi (Liao et al., 2006) or Chinese cabbage (Liao et al., 2009) have shown that the pollutant was responsible for a different set of up- or down-regulated proteins, even though the two species are so closely related to one another. Among the effects of salinity on broccoli are the down-regulation of several proteins involved in cell wall metabolism and the up-regulation of known plant defense-related proteins (Fernandez-Garcia et al., 2011). A third common source of abiotic stress is that imposed by nutrient supply. Wang et al. (2014b) have characterized the response of hydroponically grown pak choi to the supply of nitrogen in different forms. The plants responded to glycine supply by altering the expression of proteins involved in protein processing, amino acid metabolism and redox homeostasis. A similar experiment involving broccoli investigated the effect of supplying the plants with a non-limiting concentration of the essential trace element selenium (Sepúlveda et al., 2013), resulting in an enhanced accumulation in the inflorescence of a number of proteins associated with the general stress response, while that of pathogen defense-related proteins was reduced. The effect of an organic farming regime on the cabbage proteome has shown that the cropping system had a measurable effect on the accumulation of proteins involved in glycolysis, the Krebs cycle, amino acid metabolism and various detoxification processes (Nawrocki et al., 2011).
A plants protein composition is a key determinant of a species’ and a cultivar’s identity, and differences in specific plant features are better reflected by their proteome than by either their genome or transcriptome. Thus proteome variation has been suggested as providing a viable basis for the design of molecular markers able to accelerate breeding (Witzel et al., 2011). Marquès et al. (2001) made comparisons between the 2-DE profiles of various Brassica species in an attempt to establish the nature of the genetic relationship between them. PCR-based markers for the genomes A (B. rapa) and B (B. oleracea) were obtained by Kong et al. (2010) by aligning the proteomic profiles of young leaves. From some of those differentially expressed proteins, PCR-based markers were developed successfully. The effect of the polyploidization process on the proteome was assessed by Albertin et al. (2005), who made comparisons between the leaf and stem proteomes of haploid, diploid and tetraploid sprouting broccoli, and Chinese kale. The analysis concluded that the two different tissues were responsible for 40% of the proteomic variation observed, with only 10% ascribed to ploidy level differences. With the aim to identify proteins responsible for an improved vernalisation trait, the seed proteome of a somatic hybrid line was compared with those of its parent lines B. oleracea (cabbage) and B. rapa (Chinese cabbage) that showed low or high seed production during warm winter conditions, respectively (Hossain et al., 2014).
Understanding the basis of self-incompatibility has been a long-standing problem in the Brassica genus. A proteomic approach addressing this issue in Chinese cabbage was taken by Wang et al. (2014a), who analyzed the protein complement of the pistils from self-incompatible and compatible plants; in some cases, protein abundance was consistent with that of the relevant transcript, particularly with respect to those involved in energy metabolism, protein synthesis and the defense against stress. Recently, for a more cell-type specific proteomics approach related to self-incompatibility, a protocol for the analysis of cabbage stigma proteins was established (Zeng et al., 2012).
The market value of vegetables is greatly affected by their visual appearance and their post-harvest shelf life. As part of a transgenic-based attempt to prolong the marketability of broccoli, 2-DE was used to compare the proteomes of the inflorescences of wild type and transgenic lines produced to express delayed senescence (Liu et al., 2011). The conclusion was that a number of stress response related proteins and chaperones accumulated in the transgenic lines, while the build-up of 1-aminocyclopropane-1-carboxylate oxidase was only noted in the wild type. When the effect of exogenously supplying cytokinin (N6-benzylaminopurine) was studied at the proteomic level, Liu et al. (2013a) detected only a minor degree of overlap with the transgenic contrast, an observation which was taken to suggest that the molecular basis of the delayed post-harvest senescence induced by exogenous cytokinin differed from that induced by endogenous cytokinin. Of the up-regulated stress response related proteins detected in the transgenic material, 17 proved to be heat-stable following cooking and were therefore considered to represent potential allergens (Liu et al., 2014a).
Metabolomics
Metabolomics, defined as the systematic study of the by-products of cellular processes, is an ambitious field, as the number of chemically distinct molecules involved in a typical plant sample is estimated to be at least 100,000 (Wink, 1988), each varying with respect to their abundance (De Vos et al., 2007). A number of these compounds are of physiological importance and/or represent dietary sources of antioxidants and other health enhancing compounds; most importantly with respect to vegetable crops, they make a major contribution to product color and flavor. Often these latter quality aspects rely not on individual compounds, but rather on specific mixtures (Ferguson and Schlothauer, 2012). The state-of-the-art analytical platforms employed for metabolomic analyses are a sophisticated combination of nuclear magnetic resonance spectroscopy (NMR), MS, gas chromatography (GC), LC, and capillary electrophoresis (CE). Changes in the primary and secondary metabolite pool have been characterized using a GC-MS approach (Hummel et al., 2013), while CE-MS is more suitable for characterizing the products associated with the central metabolism (Yang et al., 2012). NMR is appropriate for the targeting of phenolic compounds, carbohydrates, organic acids and amino acids, and of particular interest in the context of brassicaceous vegetables, glucosinolates (Table 4). Electrospray ionization (ESI), conducted either in positive (resulting in protonated species) or in negative (deprotonated species) mode is used for the analysis of semi-polar metabolites. Together, these technologies can identify and quantify a wide range of primary and secondary metabolites. For LC-MS and NMR applications, samples are typically extracted in alcohol and not derivatized. In contrast, GC-MS separations require a derivatization step (Kopka et al., 2005). Particularly high mass resolution can be achieved by the use of time-of-flight (TOF)-MS devices, and additional information can be obtained from the MS/MS fragmentation patterns and UV-VIS spectra. Recent software developments have improved the capacity to recognize different metabolites. Profiles based on mass, retention time and signal amplitude provide the data required for filtering biomarkers. Typically a data processing pipeline can be divided into two steps: data processing (filtering, feature detection, alignment, and normalization) and data analysis (algorithm selection, training, evaluation, and model examination; further explanation see Boccard et al., 2010).
Table 4.
Published non-targeted metabolomic analyses in the brassicaceous vegetables.
| Brassica species | Plant organ/developmental stage | Study objective | Methodology | Compounds | Reference |
|---|---|---|---|---|---|
| B. oleracea collection | Leaves | Factor identification for thermal degradation of glucosinolates | HPLC-MS LC-MS | Semi-polar | Hennig et al. (2014) |
| B. oleracea var. botrytis collection | Inflorescences | Genotypic variation of colored cultivars | GC-TOF-MS | Primary and secondary compounds* | Park et al. (2013) |
| B. oleracea var. capitata | Leaves | Discrimination of conventional and organic farming | LC-MS | Semi-polar | Mie et al. (2014) |
| B. oleracea var. capitata | Leaves | Genotypic variation | LC-MS | Phenolic compounds | Park et al. (2014) |
| B. oleracea var. italic | Inflorescences | Genotypic variation, selenium treatment, crop management | LC-MS | Phenolic compounds | Luthria et al. (2008) |
| B. rapa collection | Leaves | Response to pre-harvest bacterial contamination | 1H NMR | Polar | Jahangir et al. (2008b) |
| B. rapa collection | Leaves | Genotypic variation | 1H NMR | Polar | Abdel-Farid et al. (2007) |
| B. rapa collection | Leaves | Effect of metal-ion treatment | 1H NMR | Polar | Jahangir et al. (2008a) |
| B. rapa collection | Leaves | Response to pre-harvest fungal infection | 1H NMR | Polar | Abdel-Farid et al. (2009) |
| B. rapa collection | Leaves | Genotypic variation | LC-MS | Semi-polar | Del Carpio et al. (2011) |
| B. rapa var. pekinensis | Leaves | Discrimination by geographical areas | 1H NMR | Polar | Kim et al. (2013a) |
| B. rapa var. rapa, Raphanus sativa | Leaves | Ontogenetic variation | NMR, HPLC | Primary and secondary compounds* | Muhammad et al. (2014) |
*Includes both a non-targeted and a targeted approach.
Nevertheless, metabolite identification in non-targeted approaches in the absence of authentic reference compounds remains difficult. Various platforms and spectral databases are available online4.
The metabolome of Brassica spp. as that of all plants, is influenced by both genotype (Luthria et al., 2008) and developmental stage (Abdel-Farid et al., 2007), as well as by the plant’s growing environment (Hall, 2006; Weimer and Slupsky, 2013). Metabolomic analyses in the brassicaceous vegetables have included both non-targeted (Table 4) and targeted (Table 5) approaches (Cevallos-Cevallos et al., 2009). The former have concern a range of both secondary (polyphenols and carotenoids) and primary metabolites (Hall, 2006), while the latter focused heavily on the identification and quantification of glucosinolates (Ramautar et al., 2006). The glucosinolates are a unique component of the brassicaceous vegetable metabolome (Verkerk et al., 2009). These compounds contain a sulfur-linked β-D-glucopyranose moiety and an amino acid-derived side chain, and fall into three definable classes: the aliphatic, indole and aromatic glucosinolates. They are structurally highly diverse, and within the Brassicaceae are mostly dominated by the aliphatic glucosinolates (Halkier and Gershenzon, 2006). Subsequently, aliphatic glucosinolates can be subdivided into straight- or branched-chain alk(en)yl glucosinolates with or without a hydroxy group and into a large group of those that contain an additional sulfur atom with various oxidation levels in the side chain (see for detailed structural overview, Hanschen et al., 2014). The glucosinolates are not known to be bioactive, but their hydrolysis products provide deterrence against feeding (Textor and Gershenzon, 2009), and their presence in the human diet has been associated with both health benefits (Vig et al., 2009) and taste (Beck et al., 2014).
Table 5.
Published targeted metabolomic analyses in the brassicaceous vegetables, featuring glucosinolates and their break-down products.
| Brassica species | Plant organ/Developmental stage | Study objective | Methodology | Reference |
|---|---|---|---|---|
| B. carinata | Leaves | (Onto)genetic variation, effect of water supply | HPLC-MS | Schreiner et al. (2009) |
| B. oleracea | Leaves | Genotypic variation | LC-MS | Rochfort et al. (2008) |
| B. oleracea var. italica | Inflorescences | Effect of nitrogen and sulfur supply | HPLC-MS | Schonhof et al. (2007a) |
| B. oleracea var. italica | Inflorescences | Effect of CO2 | HPLC-MS | Schonhof et al. (2007b) |
| B. oleracea var. italica | Sprouts | Effect of water supply and aphid treatment | HPLC-MS | Khan et al. (2011) |
| B. oleracea var. italica | Sprouts | Effect of UV-B | HPLC-MS | Mewis et al. (2012) |
| B. rapa | Roots | Effect of nitrogen and sulfur supply | HPLC-MS | Shumin et al. (2007) |
| B. rapa | Leaves | Effect of fungal infection | NMR | Abdel-Farid et al. (2010) |
| B. rapa | Leaves | Effect of fertilization | GC-MS | Okazaki et al. (2012) |
| B. rapa | Roots | Genotypic variation | LC-PDA-QTOF-MS | Lee etal. (2013) |
| B. rapa ssp. chinensis | Leaves, sprouts | Effect of methyl jasmonate | HPLC-MS | Wiesner et al. (2013a,b) |
| B. rapa ssp. pekinensis | Leaves, seeds | Organ differentiation | LC-ESI-MS, LC-UV | Hong et al. (2011) |
| B. rapa ssp. rapa | Leaves, roots, root exsudates | Effect of methyl jasmonate, salicylic acid | HPLC-MS | Schreiner et al. (2011) |
Gas chromatography/TOF-MS has been used to characterize the phytochemical diversity of the florets in cauliflower cultivars varying in inflorescence pigmentation (Park et al., 2013). A 1H-NMR platform proved informative for discriminating between a set of Chinese cabbage cultivars from China and Korea: differences with respect to both primary and secondary metabolites were uncovered, although insufficient attention has yet to be given to the effect of the growing environment (Kim et al., 2013a). A non-targeted strategy was chosen by Mie et al. (2014) to reveal the impact of organic farming on the metabolome of cabbage, and led to the conclusion that this approach has potential for the authentication of organic products. The exposure of B. carinata seedlings to lithium ions induced a marked effect on the plant’s lipid and phenolic content: sinapic acid esters and chloroplast lipids were replaced by benzoate derivatives, resveratrol and oxylipins (Li et al., 2009). When GC/TOF-MS was used to discriminate between cabbage cultivars varying with respect to their resistance to feeding by diamondback moth (Plutella xylostella) caterpillars, Kim et al. (2013b) were able to show that the levels of glycolic acid, quinic acid, inositol, fumaric acid, glyceric acid, trehalose, shikimic acid, and aspartic acid were all very different.
Non-targeted metabolic approaches have also been applied to the study of processing quality in brassicaceous vegetables. Changes in the content of certain flavonols have been associated with the thermal degradation of glucosinolates in B. oleracea (Hennig et al., 2014). Several analytical techniques have been used to assess the impact of industrial processing on the phytochemical composition of vegetable purées. In broccoli, most of the metabolites present in the purée were degraded by heating, including various health-related and flavor compounds, vitamins, carotenoids, flavonoids, glucosinolates, and oxylipins (Lopez-Sanchez et al., 2015). Post-harvest storage temperature also has a profound effect on the metabolome. For example, in radish (Raphanus sativus), the content of phenylpropanoids, flavonoids, and glucosinolates decreased with storage time (Jahangir, 2010).
Microbiomics
Microbiomics refers to the application of ‘omics technologies to the microbial community associated with the plant spermosphere, endosphere, rhizosphere, and phyllosphere. The plant microbiome has attracted the focus of several researchers in recent years (reviewed in Vorholt, 2012; Bakker et al., 2013; Bulgarelli et al., 2013), as it has an intimate interaction with the plant, affecting both its metabolism and physiology. Studies of the plant microbiome have relied to date either on the in vitro culture of the relevant micro-organisms or on the recognition of species identity from PCR amplicons derived from variable regions of their genome (such as the 16S rDNA locus) followed by sequencing, microarray analysis or various electrophoretic procedures (Rastogi et al., 2013; Turner et al., 2013; Muller and Ruppel, 2014). Metaproteomics approaches can complement such data sets to provide functional information relevant to the microbiome (Knief et al., 2012). As many vegetable are consumed as a fresh product, their associated microbiome will enter the human digestive system. Exemplarily, the lettuce (Hunter et al., 2010; Rastogi et al., 2012; Jackson et al., 2013; Williams and Marco, 2014) and spinach (Leff and Fierer, 2013; Lopez-Velasco et al., 2013) microbiomes are dependent on the host genotype, the cultivation conditions and the tissue sampled. The impact of commensal microbial diversity on crop disease has been investigated in a range of oilseed rape cultivars infected with the fungal pathogen Verticillium longisporum (Graner et al., 2003). Bacterial strains isolated from tolerant or susceptible plant cultivars showed biocontrol activity against the fungus and imply a possible application in crop protection. Meanwhile De Campos et al. (2013) used next generation sequencing to show how the components of the oilseed rape microbiome depended on the developmental stage of the plant. Links et al. (2014) demonstrated that the seeds of B. juncea, B. napus, and B. rapa, were associated with a core seed microbial population of geographically and ecologically different crops. The structure of the Brassica species -specific microbiome has been shown to be sensitive to the composition of the host’s secondary metabolites (Ruppel et al., 2008). Hunter et al. (2014) established a close relationship between the composition of the root exudate and the structure of the rhizosphere microbiome. Certain microbial strains had a significant impact on the capacity of the rhizosphere to solubilize phosphorus (Schilling et al., 1998; Deubel et al., 2000). Determining the functional interaction between the microbiome’s composition and plant metabolic activities is still at an early stage of development (Bakker et al., 2013).
Concluding Remarks
Many refinements in all the ‘omics technologies can be expected to come on-stream over the next few years, and these will provide exciting new avenues of research in the brassicaceous vegetables. Some of the topics likely to feature in the near future are flux analysis, the identification of informative breeding markers for the selection of beneficial bioactives and enhanced responses to both abiotic and biotic stress (Figure 2). The increasing volume of transcriptomic data acquired continues to provide a wealth of information relevant to obtaining a detailed picture of key regulatory mechanisms and pathways active in the plant. Improving the capacity to identify the components of the proteome is a fast moving area of technology development. So far, proteomic analysis in the brassicaceous vegetables has relied almost exclusively on 2-DE separation, even though the more sophisticated chromatography and MS platforms have been quite widely used in other (particularly model) plant species; these technologies are of particular value in illuminating post-translational modifications. The acquisition of full genome sequences for some (or all) of the brassicaceous vegetables can only aid in facilitating proteomic analysis in this groups of crop plants. Gaining some control over the composition of metabolites will surely provide a powerful means of varietal improvement and the optimization of post-harvest technology.
FIGURE 2.
Potential applications of ‘omics technologies for brassicaceous vegetables improvement.
The marriage of ‘omics technology and plant breeding should trigger a major efficiency breakthrough in crop improvement, and also generate novel opportunities in the fields of nutrigenomics (systems approach to understand the relationship between diet and health) and microbiomics. Although substantial progress has already been made both in the data acquisition and its interpretation, many technical and scientific challenges remain before the level of understanding of the workings of a complex biological system such as a plant rises above the current state. Global information of different hierarchies (genomics, transcriptomics, proteomics, and metabolomics) need to be integrated using mathematical and statistical methods to refine existing knowledge and make new discoveries.
There are successful studies were systems perspectives revealed correlations that had not been suggested by classical approaches in Arabidopsis, (Mochida and Shinozaki, 2011), tomato (Enfissi et al., 2010), maize (Casati et al., 2011), and others. Elucidating principles that govern relationships between biological instances will help to improve plant functions also in brassicaceous vegetables; i.e., stress-resistant plants and high- yield cultivars.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
- Abdel-Farid B. B., Kim H. K., Choi Y. H., Verpoorte R. (2007). Metabolic characterization of Brassica rapa leaves by NMR spectroscopy. J. Agric. Food Chem. 55 7936–7943 10.1021/jf071294b [DOI] [PubMed] [Google Scholar]
- Abdel-Farid I. B., Jahangir M., Mustafa N. R., Van Dam N. M., Van Den Hondel C., Kim H. K., et al. (2010). Glucosinolate profiling of Brassica rapa cultivars after infection by Leptosphaeria maculans and Fusarium oxysporum. Biochem. Syst. Ecol. 38 612–620 10.1016/j.bse.2010.07.008 [DOI] [Google Scholar]
- Abdel-Farid I. B., Jahangir M., Van Den Hondel C., Kim H. K., Choi Y. H., Verpoorte R. (2009). Fungal infection-induced metabolites in Brassica rapa. Plant Sci. 176 608–615 10.1016/j.plantsci.2009.01.017 [DOI] [Google Scholar]
- Alam I., Sharmin S. A., Kim K. H., Kim Y. G., Lee J. J., Lee B. H. (2013). An improved plant leaf protein extraction method for high resolution two-dimensional polyacrylamide gel electrophoresis and comparative proteomics. Biotech. Histochem. 88 61–75 10.3109/10520295.2012.729863 [DOI] [PubMed] [Google Scholar]
- Albertin W., Brabant P., Catrice O., Eber F., Jenczewski E., Chèvre A.-M., et al. (2005). Autopolyploidy in cabbage (Brassica oleracea L.) does not alter significantly the proteomes of green tissues. Proteomics 5 2131–2139 10.1002/pmic.200401092 [DOI] [PubMed] [Google Scholar]
- Amoah S., Kurup S., Lopez C. M. R., Welham S. J., Powers S. J., Hopkins C. J., et al. (2012). A hypomethylated population of Brassica rapa for forward and reverse epi-genetics. BMC Plant Biol. 12:193 10.1186/1471-2229-12-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstead J., Hartson S., Thompson G. (2013). The broccoli (Brassica oleracea) phloem tissue proteome. BMC Genomics 14:764 10.1186/1471-2164-14-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine R., Arya G. C., Nambiar D. M., Kumar R., Bisht N. C. (2014). Translational genomics in Brassica crops: challenges, progress, and future prospects. Plant Biotechnol. Rep. 8 65–81 10.1007/s11816-013-0298-8 [DOI] [Google Scholar]
- Ayele M., Haas B. J., Kumar N., Wu H., Xiao Y. L., Van Aken S., et al. (2005). Whole genome shotgun sequencing of Brassica oleracea and its application to gene discovery and annotation in Arabidopsis. Genome Res. 15 487–495 10.1101/gr.3176505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri H., El-Soda M., Van Oorschot I., Hanhart C., Bonnema G., Jansen-Van Den Bosch T., et al. (2012). Genetic analysis of morphological traits in a new, versatile, rapid-cycling Brassica rapa recombinant inbred line population. Front. Plant Sci. 3:183 10.3389/fpls.2012.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker P. A. H. M., Berendsen R. L., Doornbos R. F., Wintermans P. C. A., Pieterse C. M. J. (2013). The rhizosphere revisited: root microbiomics. Front. Plant Sci. 4:165 10.3389/fpls.2013.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkla B. J., Castellanos-Cervantes T., Diaz De León J. L., Matros A., Mock H.-P., Perez-Alfocea F., et al. (2013). Elucidation of salt stress defense and tolerance mechanisms of crop plants using proteomics - current achievements and perspectives. Proteomics 13 1885–1900 10.1002/pmic.201200399 [DOI] [PubMed] [Google Scholar]
- Bartel D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116 281–297 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- Beck T. K., Jensen S., Bjoern G. K., Kidmose U. (2014). The masking effect of sucrose on perception of bitter compounds in Brassica vegetables. J. Sens. Stud. 29 190–200 10.1111/joss.12094 [DOI] [Google Scholar]
- Bieda M. (2012). Kepler for ‘Omics Bioinformatics. Procedia Comput. Sci. 9 1635–1638 10.1016/j.procs.2012.04.180 [DOI] [Google Scholar]
- Boccard J., Veuthey J.-L., Rudaz S. (2010). Knowledge discovery in metabolomics: an overview of MS data handling. J. Sep. Sci. 33 290–304 10.1002/jssc.200900609 [DOI] [PubMed] [Google Scholar]
- Brown A. F., Yousef G. G., Chebrolu K. K., Byrd R. W., Everhart K. W., Thomas A., et al. (2014). High-density single nucleotide polymorphism (SNP) array mapping in Brassica oleracea: identification of QTL associated with carotenoid variation in broccoli florets. Theor. Appl. Genet. 127 2051–2064 10.1007/s00122-014-2360-5 [DOI] [PubMed] [Google Scholar]
- Buhtz A., Kolasa A., Arlt K., Walz C., Kehr J. (2004). Xylem sap protein composition is conserved among different plant species. Planta 219 610–618 10.1007/s00425-004-1259-9 [DOI] [PubMed] [Google Scholar]
- Bulgarelli D., Schlaeppi K., Spaepen S., Van Themaat E. V. L., Schulze-Lefert P. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64 807–838 10.1146/annurev-arplant-050312-120106 [DOI] [PubMed] [Google Scholar]
- Camargo L. E. A., Savides L., Jung G., Nienhuis J., Osborn T. C. (1997). Location of the self-incompatibility locus in an RFLP and RAPD map of Brassica oleracea. J. Hered. 88 57–60 10.1093/oxfordjournals.jhered.a023057 [DOI] [PubMed] [Google Scholar]
- Carrington J. C., Ambros V. (2003). Role of microRNAs in plant and animal development. Science 301 336–338 10.1126/science.1085242 [DOI] [PubMed] [Google Scholar]
- Casati P., Campi M., Morrow D. J., Fernandes J. F., Walbot V. (2011). Transcriptomic, proteomic and metabolomic analysis of UV-B signaling in maize. BMC Genomics 12:321 10.1186/1471-2164-12-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevallos-Cevallos J. M., Reyes-De-Corcuera J. I., Etxeberria E., Danyluk M. D., Rodrick G. E. (2009). Metabolomic analysis in food science: a review. Trends Food Sci. Technol. 20 557–566 10.1016/j.tifs.2009.07.002 [DOI] [Google Scholar]
- Chen L., Hao L. G., Parry M. A. J., Phillips A. L., Hu Y. G. (2014). Progress in TILLING as a tool for functional genomics and improvement of crops. J. Integr. Plant Biol. 56 425–443 10.1111/jipb.12192 [DOI] [PubMed] [Google Scholar]
- Cox J., Mann M. (2011). “Quantitative, high-resolution proteomics for data-driven systems biology,” in Annual Review of Biochemistry Vol. 80 eds Kornberg R. D., Raetz C. R. H., Rothman J. E., Thorner J. W. (Palo Alto, CA: Annual Reviews) 273–299. [DOI] [PubMed] [Google Scholar]
- De Bernonville T., Albenne C., Arlat M., Hoffmann L., Lauber E., Jamet E. (2014). “Xylem sap proteomics,” in Plant Proteomics eds Jorrin-Novo J. V., Komatsu S., Weckwerth W., Wienkoop S. (New York, NY: Humana Press) 391–405 10.1007/978-1-62703-631-3_28 [DOI] [PubMed] [Google Scholar]
- De Campos S. B., Youn J. W., Farina R., Jaenicke S., Junemann S., Szczepanowski R., et al. (2013). Changes in root bacterial communities associated to two different development stages of canola (Brassica napus L. var oleifera) evaluated through next-generation sequencing technology. Microb. Ecol. 65 593–601 10.1007/s00248-012-0132-9 [DOI] [PubMed] [Google Scholar]
- De Vos R. C. H., Moco S., Lommen A., Keurentjes J. J. B., Bino R. J., Hall R. D. (2007). Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2 778–791 10.1038/nprot.2007.95 [DOI] [PubMed] [Google Scholar]
- Del Carpio D. P., Basnet R. K., De Vos R. C. H., Maliepaard C., Visser R., Bonnema G. (2011). The patterns of population differentiation in a Brassica rapa core collection. Theor. Appl. Genet. 122 1105–1118 10.1007/s00122-010-1516-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel A., Gransee A., Merbach W. (2000). Transformation of organic rhizodepositions by rhizosphere bacteria and its influence on the availability of tertiary calcium phosphate. J. Plant Nutr. Soil Sci. 163 387–392 [DOI] [Google Scholar]
- Devisetty U. K., Covington M. F., Tat A. V., Lekkala S., Maloof J. N. (2014). Polymorphism identification and improved genome annotation of Brassica rapa through deep RNA sequencing. G3 (Bethesda) 4 2065–2078 10.1534/g3.114.012526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellendorff U., Fradin E. F., De Jonge R., Thomma B. P. (2009). RNA silencing is required for Arabidopsis defence against Verticillium wilt disease. J. Exp. Bot. 60 591–602 10.1093/jxb/ern306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enfissi E. M. A., Barneche F., Ahmed I., Lichtlé C., Gerrish C., Mcquinn R. P., et al. (2010). Integrative transcript and metabolite analysis of nutritionally enhanced DE-ETIOLATED1 downregulated tomato fruit. Plant Cell 22 1190–1215 10.1105/tpc.110.073866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. W., Zhang Y. S., Talalay P. (1997). Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. U.S.A. 94 10367–10372 10.1073/pnas.94.19.10367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faltusova Z., Kucera L., Ovesna J. (2011). Genetic diversity of Brassica oleracea var. capitata gene bank accessions assessed by AFLP. Electr. J. Biotechnol. 14 10 10.2225/vol14-issue3-fulltext-4 [DOI] [Google Scholar]
- Ferguson L. R., Schlothauer R. C. (2012). The potential role of nutritional genomics tools in validating high health foods for cancer control: broccoli as example. Mol. Nutr. Food Res. 56 126–146 10.1002/mnfr.201100507 [DOI] [PubMed] [Google Scholar]
- Fernandez-Garcia N., Hernandez M., Casado-Vela J., Bru R., Elortza F., Hedden P., et al. (2011). Changes to the proteome and targeted metabolites of xylem sap in Brassica oleracea in response to salt stress. Plant Cell Environ. 34 821–836 10.1111/j.1365-3040.2011.02285.x [DOI] [PubMed] [Google Scholar]
- Gao J. J., Yu X. X., Ma F. M., Li J. (2014). RNA-Seq analysis of transcriptome and glucosinolate metabolism in seeds and sprouts of broccoli (Brassica oleracea var. italic). PLoS ONE 9:e88804 10.1371/journal.pone.0088804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M. Q., Li G. Y., Yang B., Qiu D., Farnham M., Quiros C. (2007). High-density Brassica oleracea linkage map: identification of useful new linkages. Theor. Appl. Genet. 115 277–287 10.1007/s00122-007-0568-3 [DOI] [PubMed] [Google Scholar]
- Ge Y., Ramchiary N., Wang T., Liang C., Wang N., Wang Z., et al. (2011). Mapping quantitative trait loci for leaf and heading-related traits in Chinese cabbage (Brassica rapa L. ssp pekinesis). Hortic. Environ. Biotechnol. 52 494–501 10.1007/s13580-011-0031-x [DOI] [Google Scholar]
- Ge Y., Wang T., Wang N., Wang Z., Liang C., Ramchiary N., et al. (2012). Genetic mapping and localization of quantitative trait loci for chlorophyll content in Chinese cabbage (Brassica rapa ssp pekinensis). Sci. Hortic. 147 42–48 10.1016/j.scienta.2012.09.004 [DOI] [Google Scholar]
- Gehlenborg N., O’Donoghue S. I., Baliga N. S., Goesmann A., Hibbs M. A., Kitano H., et al. (2010). Visualization of omics data for systems biology. Nat. Methods 7 S56–S68 10.1038/nmeth.1436 [DOI] [PubMed] [Google Scholar]
- Graner G., Persson P., Meijer J., Alstrom S. (2003). A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol. Lett. 224 269–276 10.1016/S0378-1097(03)00449-X [DOI] [PubMed] [Google Scholar]
- Gu Y., Zhao Q. C., Sun D. L., Song W. Q. (2008). A genetic linkage map based on AFLP and NBS markers in cauliflower (Brassica oleracea var. botrytis). Bot. Stud. 49 93–99. [Google Scholar]
- Halkier B. A., Gershenzon J. (2006). Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57 303–333 10.1146/annurev.arplant.57.032905.105228 [DOI] [PubMed] [Google Scholar]
- Hall R. D. (2006). Plant metabolomics: from holistic hope, to hype, to hot topic. New Phytol. 169 453–468 10.1111/j.1469-8137.2005.01632.x [DOI] [PubMed] [Google Scholar]
- Hanschen F. S., Lamy E., Schreiner M., Rohn S. (2014). Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed. 53 11430–11450 10.1002/anie.201402639 [DOI] [PubMed] [Google Scholar]
- Hennig K., De Vos R. C. H., Maliepaard C., Dekker M., Verkerk R., Bonnema G. (2014). A metabolomics approach to identify factors influencing glucosinolate thermal degradation rates in Brassica vegetables. Food Chem. 155 287–297 10.1016/j.foodchem.2014.01.062 [DOI] [PubMed] [Google Scholar]
- Higdon J. V., Delage B., Williams D. E., Dashwood R. H. (2007). Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol. Res. 55 224–236 10.1016/j.phrs.2007.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himelblau E., Gilchrist E. J., Buono K., Bizzell C., Mentzer L., Vogelzang R., et al. (2009). Forward and reverse genetics of rapid-cycling Brassica oleracea. Theor. Appl. Genet. 118 953–961 10.1007/s00122-008-0952-7 [DOI] [PubMed] [Google Scholar]
- Hong E., Kim S. J., Kim G. H. (2011). Identification and quantitative determination of glucosinolates in seeds and edible parts of Korean Chinese cabbage. Food Chem. 128 1115–1120 10.1016/j.foodchem.2010.11.102 [DOI] [Google Scholar]
- Hossain M. M., Li X., Evans I. H., Rahman M. A. (2014). A proteomic analysis of seed proteins expressed in a Brassica somatic hybrid and its two parental species. J. Plant Tissue Cult. Biotechnol. 24 11–26 10.3329/ptcb.v24i1.19187 [DOI] [Google Scholar]
- Hummel J., Strehmel N., Bölling C., Schmidt S., Walther D., Kopka J. (2013). “Mass spectral search and analysis using the golm metabolome database,” in The Handbook of Plant Metabolomics eds Weckwerth W., Kahl G. (Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA) 321–343 10.1002/9783527669882.ch18 [DOI] [Google Scholar]
- Hunter P. J., Hand P., Pink D., Whipps J. M., Bending G. D. (2010). Both leaf properties and microbe-microbe interactions influence within-species variation in bacterial population diversity and structure in the lettuce (Lactuca species) Phyllosphere. Appl. Environ. Microbiol. 76 8117–8125 10.1128/AEM.01321-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P. J., Teakle G., Bending G. D. (2014). Root traits and microbial community interactions in relation to phosphorus availability and acquisition, with particular reference to Brassica. Front. Plant Sci. 5:27 10.3389/fpls.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez-Luy F. L., Voort A. V., Osborn T. C. (2008). Development of a set of public SSR markers derived from genomic sequence of a rapid cycling Brassica oleracea L. genotype. Theor. Appl. Genet. 117 977–985 10.1007/s00122-008-0837-9 [DOI] [PubMed] [Google Scholar]
- Izzah N. K., Lee J., Jayakodi M., Perumal S., Jin M., Park B. S., et al. (2014). Transcriptome sequencing of two parental lines of cabbage (Brassica oleracea L. var. capitata L.) and construction of an EST-based genetic map. BMC Genomics 15:149 10.1186/1471-2164-15-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. R., Randolph K. C., Osborn S. L., Tyler H. L. (2013). Culture dependent and independent analysis of bacterial communities associated with commercial salad leaf vegetables. BMC Microbiol. 13:274 10.1186/1471-2180-13-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangir M. (2010). Stress Response and Health Affecting Compounds in Brassicaceae. Ph.D. thesis, Department of Pharmacognosy and Metabolomics, Institute of Biology (IBL), Faculty of Science, Leiden University, Leiden. [Google Scholar]
- Jahangir M., Abdel-Farid I. B., Choi Y. H., Verpoorte R. (2008a). Metal ion-inducing metabolite accumulation in Brassica rapa. J. Plant Physiol. 165 1429–1437 10.1016/j.jplph.2008.04.011 [DOI] [PubMed] [Google Scholar]
- Jahangir M., Kim H. K., Choi Y. H., Verpoorte R. (2008b). Metabolomic response of Brassica rapa submitted to pre-harvest bacterial contamination. Food Chem. 107 362–368 10.1016/j.foodchem.2007.08.034 [DOI] [Google Scholar]
- Jeffery E. H., Araya M. (2009). Physiological effects of broccoli consumption. Phytochem. Rev. 8 283–298 10.1007/s11101-008-9106-4 [DOI] [Google Scholar]
- Kaido M., Ishikawa T., Hori H. (2007). Comparative proteomic analysis of the resistant response in Brassica rapa root culture to the clubroot disease agent Plasmodiophora brassicae. Bull. Facul. Agric. Niigata Univ. 60 67–71. [Google Scholar]
- Kang J., Zhang G., Bonnema G., Fang Z., Wang X. (2008). Global analysis of gene expression in flower buds of Ms-cd1 Brassica oleracea conferring male sterility by using an Arabidopsis microarray. Plant Mol. Biol. 66 177–192 10.1007/s11103-007-9261-9 [DOI] [PubMed] [Google Scholar]
- Katajamaa M., Oresic M. (2007). Data processing for mass spectrometry-based metabolism. J. Chromatogr. A 1158 318–328 10.1016/j.chroma.2007.04.021 [DOI] [PubMed] [Google Scholar]
- Kaul S., Koo H. L., Jenkins J., Rizzo M., Rooney T., Tallon L. J., et al. (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408 796–815 10.1038/35048692 [DOI] [PubMed] [Google Scholar]
- Kehr J., Buhtz A. (2011). “Brassica proteomics and metabolomics,” in Genetics, Genomics and Breeding of Oilseed Brassicas eds Edwards D., Batley J., Parkin I., Kole C. (Lebanon: Science Publishers, Inc) 174–193. [Google Scholar]
- Kenan-Eichler M., Leshkowitz D., Tal L., Noor E., Melamed-Bessudo C., Feldman M., et al. (2011). Wheat hybridization and polyploidization results in deregulation of small RNAs. Genetics 188 263–272 10.1534/genetics.111.128348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A. M., Ulrichs C., Mewis I. (2011). Water stress alters aphid-induced glucosinolate response in Brassica oleracea var. italica differently. Chemoecology 21 235–242 10.1007/s00049-011-0084-4 [DOI] [Google Scholar]
- Kim B., Yu H. J., Park S. G., Shin J. Y., Oh M., Kim N., et al. (2012). Identification and profiling of novel microRNAs in the Brassica rapa genome based on small RNA deep sequencing. BMC Plant Biol. 12:218 10.1186/1471-2229-12-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. A., Lim C. J., Kim S., Choe J. K., Jo S. H., Baek N., et al. (2014). High-throughput sequencing and de novo assembly of Brassica oleracea var. capitata L. for transcriptome analysis. PLoS ONE 9:e92087 10.1371/journal.pone.0092087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Jung Y., Song B., Bong Y. S., Ryu D. H., Lee K. S., et al. (2013a). Discrimination of cabbage (Brassica rapa ssp pekinensis) cultivars grown in different geographical areas using H-1 NMR-based metabolomics. Food Chem. 137 68–75 10.1016/j.foodchem.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Kim J. K., Choi S. R., Lee J., Park S.-Y., Song S. Y., Na J., et al. (2013b). Metabolic differentiation of diamondback moth (Plutella xylostella (L.)) resistance in cabbage (Brassica oleracea L. ssp. capitata). J. Agric. Food Chem. 61 11222–11230 10.1021/jf403441t [DOI] [PubMed] [Google Scholar]
- Knief C., Delmotte N., Chaffron S., Stark M., Innerebner G., Wassmann R., et al. (2012). Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. Isme J. 6 1378–1390 10.1038/ismej.2011.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen S. (2004). Guide to Analysis of DNA Microarray Data 2nd Edn. Hoboken, N.J: Wiley-Liss; 10.1002/0471670278 [DOI] [Google Scholar]
- Kohl M., Megger D. A., Trippler M., Meckel H., Ahrens M., Bracht T., et al. (2014). A practical data processing workflow for multi-OMICS projects. Biochim. Biophys. Acta 1844 52–62 10.1016/j.bbapap.2013.02.029 [DOI] [PubMed] [Google Scholar]
- Kong F., Ge C., Fang X., Snowdon R. J., Wang Y. (2010). Characterization of seedling proteomes and development of markers to distinguish the Brassica A and C genomes. J. Genet. Genomics 37 333–340 10.1016/S1673-8527(09)60051-5 [DOI] [PubMed] [Google Scholar]
- Kopka J., Schauer N., Krueger S., Birkemeyer C., Usadel B., Bergmüller E., et al. (2005). GMD@CSB.DB: the Golm Metabolome Database. Bioinformatics 21 1635–1638 10.1093/bioinformatics/bti236 [DOI] [PubMed] [Google Scholar]
- Kubo N., Saito M., Tsukazaki H., Kondo T., Matsumoto S., Hirai M. (2010). Detection of quantitative trait loci controlling morphological traits in Brassica rapa L. Breed. Sci. 60 164–171 10.1270/jsbbs.60.164 [DOI] [Google Scholar]
- Kurowska M., Daszkowska-Golec A., Gruszka D., Marzec M., Szurman M., Szarejko I., et al. (2011). TILLING - a shortcut in functional genomics. J. Appl. Genet. 52 371–390 10.1007/s13353-011-0061-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwun M., Choi Y., Yoon H., Park B. S., Kang B. J., Chung Y. Y. (2004). Expression analysis of the pistil genes in controlling self-incompatibility of Brassica campestris by CO2 gas using microarray. Mol. Cells 18 94–99. [PubMed] [Google Scholar]
- Lee J. G., Bonnema G., Zhang N. W., Kwak J. H., De Vos R. C. H., Beekwilder J. (2013). Evaluation of glucosinolate variation in a collection of turnip (Brassica rapa) germplasm by the analysis of intact and desulfo glucosinolates. J. Agric. Food Chem. 61 3984–3993 10.1021/jf400890p [DOI] [PubMed] [Google Scholar]
- Lee S. C., Lim M. H., Kim J. A., Lee S. I., Kim J. S., Jin M., et al. (2008). Transcriptome analysis in Brassica rapa under the abiotic stresses using Brassica 24K oligo microarray. Mol. Cells 26 595–605. [PubMed] [Google Scholar]
- Lee S., Yun S. C. (2006). The ozone stress transcriptome of pepper (Capsicum annuum L.). Mol. Cells 21 197–205. [PubMed] [Google Scholar]
- Leff J. W., Fierer N. (2013). Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS ONE 8:e59310 10.1371/journal.pone.0059310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Gao P., Gjetvaj B., Westcott N., Gruber M. Y. (2009). Analysis of the metabolome and transcriptome of Brassica carinata seedlings after lithium chloride exposure. Plant Sci. 177 68–80 10.1016/j.plantsci.2009.03.013 [DOI] [Google Scholar]
- Li X., Ramchiary N., Dhandapani V., Choi S. U., Lim Y. P. (2013). “Omics applications in Brassica species,” in OMICS Applications in Crop Science ed. Barh D. (Boca Raton, FL: CRC Press) 163–190 10.1201/b16352-7 [DOI] [Google Scholar]
- Li X., Zhang W., Wang Y., Wang Z., Peng Y. (2008). Changes in chloroplast proteome of Chinese cabbage seedlings induced by PS II inhibiting herbicide Atrazine. Acta Agron. Sin. 34 238–242 10.3724/SP.J.1006.2008.00238 [DOI] [Google Scholar]
- Liao C.-S., Yen J.-H., Wang Y.-S. (2006). Effects of endocrine disruptor di-n-butyl phthalate on the growth of Bok choy (Brassica rapa subsp. chinensis). Chemosphere 65 1715–1722 10.1016/j.chemosphere.2006.04.093 [DOI] [PubMed] [Google Scholar]
- Liao C.-S., Yen J.-H., Wang Y.-S. (2009). Growth inhibition in Chinese cabbage (Brassica rapa var. chinensis) growth exposed to di-n-butyl phthalate. J. Hazard. Mater. 163 625–631 10.1016/j.jhazmat.2008.07.025 [DOI] [PubMed] [Google Scholar]
- Ligat L., Lauber E., Albenne C., Clemente H. S., Valot B., Zivy M., et al. (2011). Analysis of the xylem sap proteome of Brassica oleracea reveals a high content in secreted proteins. Proteomics 11 1798–1813 10.1002/pmic.201000781 [DOI] [PubMed] [Google Scholar]
- Lin K. H., Chang L. C., Lai C. D., Lo H. F. (2013). AFLP mapping of quantitative trait loci influencing seven head-related traits in broccoli (Brassica oleracea var. italica). J. Hortic. Sci. Biotechnol. 88 257–268. [Google Scholar]
- Links M. G., Demeke T., Grafenhan T., Hill J. E., Hemmingsen S. M., Dumonceaux T. J. (2014). Simultaneous profiling of seed-associated bacteria and fungi reveals antagonistic interactions between microorganisms within a shared epiphytic microbiome on triticum and Brassica seeds. New Phytol. 202 542–553 10.1111/nph.12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.-S., Ko M.-H., Li H.-C., Tsai S.-J., Lai Y.-M., Chang Y.-M., et al. (2014a). Compositional and proteomic analyses of genetically modified broccoli (Brassica oleracea var. italica) harboring an agrobacterial gene. Int. J. Mol. Sci. 15 15188–15209 10.3390/ijms150915188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Liu Y., Yang X., Tong C., Edwards D., Parkin I. a. P., et al. (2014b). The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 5 3930 10.1038/ncomms4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.-S., Li H.-C., Chang Y.-M., Wu M.-T., Chen L.-F. O. (2011). Proteomic analysis of stress-related proteins in transgenic broccoli harboring a gene for cytokinin production during postharvest senescence. Plant Sci. 181 288–299 10.1016/j.plantsci.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Liu M.-S., Li H.-C., Lai Y.-M., Lo H.-F., Chen L.-F. O. (2013a). Proteomics and transcriptomics of broccoli subjected to exogenously supplied and transgenic senescence-induced cytokinin for amelioration of postharvest yellowing. J. Proteom. 93 133–144 10.1016/j.jprot.2013.05.014 [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Xing J. Y., Liu Z. Y., Feng H. (2013b). Mapping quantitative trait loci for yield-related traits in Chinese cabbage (Brassica rapa L. ssp pekinensis). Euphytica 193 221–234 10.1007/s10681-013-0931-1 [DOI] [Google Scholar]
- Lopez-Sanchez P., De Vos R. C. H., Jonker H. H., Mumm R., Hall R. D., Bialek L., et al. (2015). Comprehensive metabolomics to evaluate the impact of industrial processing on the phytochemical composition of vegetable purees. Food Chem. 168 348–355 10.1016/j.foodchem.2014.07.076 [DOI] [PubMed] [Google Scholar]
- Lopez-Velasco G., Carder P. A., Welbaum G. E., Ponder M. A. (2013). Diversity of the spinach (Spinacia oleracea) spermosphere and phyllosphere bacterial communities. FEMS Microbiol. Lett. 346 146–154 10.1111/1574-6968.12216 [DOI] [PubMed] [Google Scholar]
- Lou P., Xie Q., Xu X., Edwards C. E., Brock M. T., Weinig C., et al. (2011). Genetic architecture of the circadian clock and flowering time in Brassica rapa. Theor. Appl. Genet. 123 397–409 10.1007/s00122-011-1592-x [DOI] [PubMed] [Google Scholar]
- Lu G., Cao J. S., Yu X. L., Xiang X., Chen H. (2008). Mapping QTLs for root morphological traits in Brassica rapa L. based on AFLP and RAPD markers. J. Appl. Genet. 49 23–31 10.1007/BF03195245 [DOI] [PubMed] [Google Scholar]
- Luthria D. L., Lin L. Z., Robbins R. J., Finley J. W., Banuelos G. S., Harnly J. M. (2008). Discriminating between cultivars and treatments of broccoli using mass spectral fingerprinting and analysis of variance-principal component analysis. J. Agric. Food Chem. 56 9819–9827 10.1021/jf801606x [DOI] [PubMed] [Google Scholar]
- Lv H. H., Wang Q. B., Zhang Y. Y., Yang L. M., Fang Z. Y., Wang X. W., et al. (2014). Linkage map construction using InDel and SSR markers and QTL analysis of heading traits in Brassica oleracea var. capitata L. Mol. Breed. 34 87–98 10.1007/s11032-014-0019-1 [DOI] [Google Scholar]
- Maggioni L., Von Bothmer R., Poulsen G., Branca F., Jorgensen R. B. (2014). Genetic diversity and population structure of leafy kale and Brassica rupestris Raf. in south Italy. Hereditas 151 145–158 10.1111/hrd2.00058 [DOI] [PubMed] [Google Scholar]
- Marioni J. C., Mason C. E., Mane S. M., Stephens M., Gilad Y. (2008). RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 18 1509–1517 10.1101/gr.079558.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquès K., Sarazin B., Chané-Favre L., Zivy M., Thiellement H. (2001). Comparative proteomics to establish genetic relationships in the Brassicaceae family. Proteomics 1 1457–1462 [DOI] [PubMed] [Google Scholar]
- Matros A., Kaspar S., Witzel K., Mock H.-P. (2011). Recent progress in liquid chromatography-based separation and label-free quantitative plant proteomics. Phytochemistry 72 963–974 10.1016/j.phytochem.2010.11.009 [DOI] [PubMed] [Google Scholar]
- Matsumoto E., Ueno H., Aruga D., Sakamoto K., Hayashida N. (2012). Accumulation of three clubroot resistance genes through marker-assisted selection in Chinese cabbage (Brassica rapa ssp pekinensis). J. Jpn. Soc. Hort. Sci. 81 184–190 10.2503/jjshs1.81.184 [DOI] [Google Scholar]
- Mewis I., Schreiner M., Nguyen C. N., Krumbein A., Ulrichs C., Lohse M., et al. (2012). UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 53 1546–1560 10.1093/pcp/pcs096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mie A., Laursen K. H., Aberg K. M., Forshed J., Lindahl A., Thorup-Kristensen K., et al. (2014). Discrimination of conventional and organic white cabbage from a long-term field trial study using untargeted LC-MS-based metabolomics. Anal. Bioanal. Chem. 406 2885–2897 10.1007/s00216-014-7704-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K., Shinozaki K. (2011). Advances in omics and bioinformatics tools for systems analyses of plant functions. Plant Cell Physiol. 52 2017–2038 10.1093/pcp/pcr153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad J., Abdel-Farid I. B., Vos R. C. H. D., Jonker H. H., Choi Y., Verpoorte R. (2014). Metabolomic variation of Brassica rapa var. rapa (var. raapstelen) and Raphanus sativus L. at different developmental stages. Pak. J. Bot. 46 1445–1452. [Google Scholar]
- Muller T., Ruppel S. (2014). Progress in cultivation-independent phyllosphere microbiology. FEMS Microbiol. Ecol. 87 2–17 10.1111/1574-6941.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki A., Thorup-Kristensen K., Jensen O. N. (2011). Quantitative proteomics by 2DE and MALDI MS/MS uncover the effects of organic and conventional cropping methods on vegetable products. J. Proteom. 74 2810–2825 10.1016/j.jprot.2011.06.021 [DOI] [PubMed] [Google Scholar]
- Niederhuth C. E., Schmitz R. J. (2014). Covering your bases: inheritance of DNA methylation in plant genomes. Mol. Plant 7 472–480 10.1093/mp/sst165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell P. H. (1975). High-resolution 2-dimensional electrophoresis of proteins. J. Biol. Chem. 250 4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Shinano T., Oka N., Takebe M. (2012). Metabolite profiling of Komatsuna (Brassica rapa L.) field-grown under different soil organic amendment and fertilization regimes. Soil Sci. Plant Nutr. 58 696–706 10.1080/00380768.2012.733924 [DOI] [Google Scholar]
- Park S., Arasu M. V., Jiang N., Choi S. H., Lim Y. P., Park J. T., et al. (2014). Metabolite profiling of phenolics, anthocyanins and flavonols in cabbage (Brassica oleracea var. capitata). Ind. Crops Prod. 60 8–14 10.1016/j.indcrop.2014.05.037 [DOI] [Google Scholar]
- Park S. Y., Lim S. H., Ha S. H., Yeo Y., Park W. T., Kwon D. Y., et al. (2013). Metabolite profiling approach reveals the interface of primary and secondary metabolism in colored cauliflowers (Brassica oleracea L. ssp botrytis). J. Agric. Food Chem. 61 6999–7007 10.1021/jf401330e [DOI] [PubMed] [Google Scholar]
- Parkin I. A. P., Koh C., Tang H. B., Robinson S. J., Kagale S., Clarke W. E., et al. (2014). Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol. 15 18 10.1186/gb-2014-15-6-r77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski T., Rurek M., Janicka S., Raczynska K. D., Augustyniak H. (2005). Preliminary analysis of the cauliflower mitochondrial proteome. Acta Physiol. Plant. 27 275–281 10.1007/s11738-005-0003-9 [DOI] [Google Scholar]
- Pink D., Bailey L., Mcclement S., Hand P., Mathas E., Buchanan-Wollaston V., et al. (2008). Double haploids, markers and QTL analysis in vegetable Brassicas. Euphytica 164 509–514 10.1007/s10681-008-9742-1 [DOI] [Google Scholar]
- Rabbani M. A., Maruyama K., Abe H., Khan M. A., Katsura K., Ito Y., et al. (2003). Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 133 1755–1767 10.1104/pp.103.025742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabilloud T., Chevallet M., Luche S., Lelong C. (2010). Two-dimensional gel electrophoresis in proteomics: past, present and future. J. Proteom. 73 2064–2077 10.1016/j.jprot.2010.05.016 [DOI] [PubMed] [Google Scholar]
- Ramautar R., Demirci A., De Jong G. J. (2006). Capillary electrophoresis in metabolomics. Trends Analyt. Chem. 25 455–466 10.1016/j.trac.2006.02.004 [DOI] [Google Scholar]
- Rastogi G., Coaker G. L., Leveau J. H. J. (2013). New insights into the structure and function of phyllosphere microbiota through high-throughput molecular approaches. FEMS Microbiol. Lett. 348 1–10 10.1111/1574-6968.12225 [DOI] [PubMed] [Google Scholar]
- Rastogi G., Sbodio A., Tech J. J., Suslow T. V., Coaker G. L., Leveau J. H. J. (2012). Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J. 6 1812–1822 10.1038/ismej.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt M., Brown P. D., Schneider B., Oldham N. J., Stauber E., Tokuhisa J., et al. (2002). Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry 59 663–671 10.1016/S0031-9422(02)00014-6 [DOI] [PubMed] [Google Scholar]
- Rochfort S. J., Trenerry V. C., Imsic M., Panozzo J., Jones R. (2008). Class targeted metabolomics: ESI ion trap screening methods for glucosinolates based on MSn fragmentation. Phytochemistry 69 1671–1679 10.1016/j.phytochem.2008.02.010 [DOI] [PubMed] [Google Scholar]
- Ruppel S., Krumbein A., Schreiner M. (2008). Composition of the phyllospheric microbial populations on vegetable plants with different glucosinolate and carotenoid compositions. Microb. Ecol. 56 364–372 10.1007/s00248-007-9354-7 [DOI] [PubMed] [Google Scholar]
- Salmon A., Clotault J., Jenczewski E., Chable V., Manzanares-Dauleux M. J. (2008). Brassica oleracea displays a high level of DNA methylation polymorphism. Plant Sci. 174 61–70 10.1016/j.plantsci.2007.09.012 [DOI] [Google Scholar]
- Schilling G., Gransee A., Deubel A., Lezovic G., Ruppel S. (1998). Phosphorus availability, root exudates, and microbial activity in the rhizosphere. J. Plant Nutr. Soil Sci. 161 465–478 10.1002/jpln.1998.3581610413 [DOI] [Google Scholar]
- Schmidt U. G., Endler A., Schelbert S., Brunner A., Schnell M., Neuhaus H. E., et al. (2007). Novel tonoplast transporters identified using a proteomic approach with vacuoles isolated from cauliflower buds. Plant Physiol. 145 216–229 10.1104/pp.107.096917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhof I., Blankenburg D., Muller S., Krumbein A. (2007a). Sulfur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J. Plant Nutr. Soil Sci. 170 65–72 10.1002/jpln.200620639 [DOI] [Google Scholar]
- Schonhof I., Klaring H. P., Krumbein A., Schreiner M. (2007b). Interaction between atmospheric CO2 and glucosinolates in broccoli. J. Chem. Ecol. 33 105–114 10.1007/s10886-006-9202-0 [DOI] [PubMed] [Google Scholar]
- Schreiner M., Beyene B., Krumbein A., Stutzel H. (2009). Ontogenetic changes of 2-propenyl and 3-indolylmethyl glucosinolates in Brassica carinata leaves as affected by water supply. J. Agric. Food Chem. 57 7259–7263 10.1021/jf901076h [DOI] [PubMed] [Google Scholar]
- Schreiner M., Krumbein A., Knorr D., Smetanska I. (2011). Enhanced glucosinolates in root exudates of Brassica rapa ssp. rapa mediated by salicylic acid and methyl jasmonate. J. Agric. Food Chem. 59 1400–1405 10.1021/jf103585s [DOI] [PubMed] [Google Scholar]
- Schumacher A., Rujan T., Hoefkens J. (2014). A collaborative approach to develop a multi-omics data analytics platform for translational research. Appl. Transl. Genomics 3 105–108 10.1016/j.atg.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian R. L., Howell E. C., King G. J., Marshall D. F., Kearsey M. J. (2000). An integrated AFLP and RFLP Brassica oleracea linkage map from two morphologically distinct doubled-haploid mapping populations. Theor. Appl. Genet. 100 75–81 10.1007/s001220050011 [DOI] [Google Scholar]
- Sepúlveda I., Barrientos H., Mahn A., Moenne A. (2013). Changes in SeMSC, glucosinolates and sulforaphane levels, and in proteome profile in broccoli (Brassica oleracea var. Italica) fertilized with sodium selenate. Molecules 18 5221–5234 10.3390/molecules18055221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro T. A., Fahey J. W., Dinkova-Kostova A. T., Holtzclaw W., Stephenson K. K., Wade K. L., et al. (2006). Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr. Cancer 55 53–62 10.1207/s15327914nc5501_7 [DOI] [PubMed] [Google Scholar]
- Shapiro T. A., Fahey J. W., Wade K. L., Stephenson K. K., Talalay P. (2001). Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol. Biomarkers Prev. 10 501–508. [PubMed] [Google Scholar]
- Shi J. Q., Huang S. M., Zhan J. P., Yu J. Y., Wang X. F., Hua W., et al. (2014). Genome-wide microsatellite characterization and marker development in the sequenced Brassica crop species. DNA Res. 21 53–68 10.1093/dnares/dst040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumin L., Schonhof I., Krumbein A., Long L., Stuetzel H., Schreiner M. (2007). 771 Glucosinolate concentration in turnip (Brassica rapa spp. rapifera L.) roots as 772 affected by nitrogen and sulfur supply. J. Agric. Food Chem. 55 8452–8457 10.1021/jf070816k [DOI] [PubMed] [Google Scholar]
- Sonderby I. E., Geu-Flores F., Halkier B. A. (2010). Biosynthesis of glucosinolates - gene discovery and beyond. Trends Plant Sci. 15 283–290 10.1016/j.tplants.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Song X., Liu G., Duan W., Liu T., Huang Z., Ren J., et al. (2014). Genome-wide identification, classification and expression analysis of the heat shock transcription factor family in Chinese cabbage. Mol. Genet. Genomics 289 541–551 10.1007/s00438-014-0833-5 [DOI] [PubMed] [Google Scholar]
- Stephenson P., Baker D., Girin T., Perez A., Amoah S., King G. J., et al. (2010). A rich TILLING resource for studying gene function in Brassica rapa. BMC Plant Biol. 10:62 10.1186/1471-2229-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. B. (2014). Characterization of the small RNA transcriptome in plant-microbe (Brassica/Erwinia) interactions by high-throughput sequencing. Biotechnol. Lett. 36 371–381 10.1007/s10529-013-1362-8 [DOI] [PubMed] [Google Scholar]
- Sun C., Wang L., Hu D., Riquicho A. R. M., Liu T., Hou X., et al. (2014). Proteomic analysis of non-heading Chinese cabbage infected with Hyaloperonospora parasitica. J. Proteom. 98 15–30 10.1016/j.jprot.2013.11.028 [DOI] [PubMed] [Google Scholar]
- Sun G. (2012). MicroRNAs and their diverse functions in plants. Plant Mol. Biol. 80 17–36 10.1007/s11103-011-9817-6 [DOI] [PubMed] [Google Scholar]
- Takuno S., Kawahara T., Ohnishi O. (2007). Phylogenetic relationships among cultivated types of Brassica rapa L. em. Metzg. as revealed by AFLP analysis. Genet. Resour. Crop Evol. 54 279–285 10.1007/s10722-005-4260-7 [DOI] [Google Scholar]
- Textor S., Gershenzon J. (2009). Herbivore induction of the glucosinolate-myrosinase defense system: major trends, biochemical bases and ecological significance. Phytochem. Rev. 8 149–170 10.1007/s11101-008-9117-1 [DOI] [Google Scholar]
- T’Hoen P. A., Ariyurek Y., Thygesen H. H., Vreugdenhil E., Vossen R. H., De Menezes R. X., et al. (2008). Deep sequencing-based expression analysis shows major advances in robustness, resolution and inter-lab portability over five microarray platforms. Nucleic Acids Res. 36 e141 10.1093/nar/gkn705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick M., Cheung F., Drou N., Fraser F., Lobenhofer E. K., Hurban P., et al. (2009). A newly-developed community microarray resource for transcriptome profiling in Brassica species enables the confirmation of Brassica-specific expressed sequences. BMC Plant Biol. 9:50 10.1186/1471-2229-9-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T. R., James E. K., Poole P. S. (2013). The plant microbiome. Genome Biol. 14 209 10.1186/gb-2013-14-6-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren H., Lentz E., Zainuddin I., Gruissem W. (2013). Proteomics of model and crop plant species: status, current limitations and strategic advances for crop improvement. J. Proteom. 93 5–19 10.1016/j.jprot.2013.05.036 [DOI] [PubMed] [Google Scholar]
- van Hintum T. J. L., De Wiel C., Visser D. L., Van Treuren R., Vosman B. (2007). The distribution of genetic diversity in a Brassica oleracea gene bank collection related to the effects on diversity of regeneration, as measured with AFLPs. Theor. Appl. Genet. 114 777–786 10.1007/s00122-006-0456-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney R. K., Terauchi R., Mccouch S. R. (2014). Harvesting the promising fruits of genomics: applying genome sequencing technologies to crop breeding. PLoS Biol. 12:e1001883 10.1371/journal.pbio.1001883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk R., Schreiner M., Krumbein A., Ciska E., Holst B., Rowland I., et al. (2009). Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 53 S219–S265 10.1002/mnfr.200800065 [DOI] [PubMed] [Google Scholar]
- Vig A. P., Rampal G., Thind T. S., Arora S. (2009). Bio-protective effects of glucosinolates - a review. Lwt-Food Sci. Technol. 42 1561–1572 10.1016/j.lwt.2009.05.023 [DOI] [Google Scholar]
- Vorholt J. A. (2012). Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10 828–840 10.1038/nrmicro2910 [DOI] [PubMed] [Google Scholar]
- Walley P., Carder J., Skipper E., Mathas E., Lynn J., Pink D., et al. (2012). A new broccoli × broccoli immortal mapping population and framework genetic map: tools for breeders and complex trait analysis. Theor. Appl. Genet. 124 467–484 10.1007/s00122-011-1721-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Peng H., Ge T., Liu T., Hou X., Li Y. (2014a). Identification of differentially accumulating pistil proteins associated with self-incompatibility of non-heading Chinese cabbage. Plant Biol. 16 49–57 10.1111/plb.12016 [DOI] [PubMed] [Google Scholar]
- Wang X., Tang D., Huang D. (2014b). Proteomic analysis of pakchoi leaves and roots under glycine–nitrogen conditions. Plant Physiol. Biochem. 75 96–104 10.1016/j.plaphy.2013.12.012 [DOI] [PubMed] [Google Scholar]
- Wang Y. G., Zhang L., Ji X. H., Yan J. F., Liu Y. T., Lv X. X., et al. (2014c). Mapping of quantitative trait loci for the bolting trait in Brassica rapa under vernalizing conditions. Genet. Mol. Res. 13 3927–3939 10.4238/2014.May.23.3 [DOI] [PubMed] [Google Scholar]
- Wang W. X., Huang S. M., Liu Y. M., Fang Z. Y., Yang L. M., Hua W., et al. (2012). Construction and analysis of a high-density genetic linkage map in cabbage (Brassica oleracea L. var. capitata). BMC Genomics 13:523 10.1186/1471-2164-13-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang H., Wang J., Sun R., Wu J., Liu S., et al. (2011). The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 43 1035–1039 10.1038/ng.919 [DOI] [PubMed] [Google Scholar]
- Wang Z., Gerstein M., Snyder M. (2009). RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10 57–63 10.1083/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. J., Xiao M. L., Hayward A., Fu D. H. (2013). Applications and challenges of next-generation sequencing in Brassica species. Planta 238 1005–1024 10.1007/s00425-013-1961-6 [DOI] [PubMed] [Google Scholar]
- Weimer B. C., Slupsky C. (2013). “Metabolomics in food and nutrition,” in Metabolomics in Food and Nutrition eds Weimer B. C., Slupsky C. M. (Cambridge: Woodhead) 237. [Google Scholar]
- White P. J., Hammond J. P., King G. J., Bowen H. C., Hayden R. M., Meacham M. C., et al. (2010). Genetic analysis of potassium use efficiency in Brassica oleracea. Ann. Bot. 105 1199–1210 10.1093/aob/mcp253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner M., Hanschen F. S., Schreiner M., Glatt H., Zrenner R. (2013a). Induced production of 1-methoxy-indol-3-ylmethyl glucosinolate by jasmonic acid and methyl jasmonate in sprouts and leaves of pak choi (Brassica rapa ssp. chinensis). Int. J. Mol. Sci. 14 14996–15016 10.3390/ijms140714996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner M., Zrenner R., Krumbein A., Glatt H., Schreiner M. (2013b). Genotypic variation of the glucosinolate profile in pak choi (Brassica rapa ssp. chinensis). J. Agric. Food Chem. 61 1943–1953 10.1021/jf303970k [DOI] [PubMed] [Google Scholar]
- Wiesner M., Schreiner M., Zrenner R. (2014). Functional identification of genes responsible for the biosynthesis of 1-methoxy-indol-3-ylmethyl-glucosinolate in Brassica rapa ssp. chinensis. BMC Plant Biol. 14:124 10.1186/1471-2229-14-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. R., Marco M. L. (2014). Phyllosphere microbiota composition and microbial community transplantation on lettuce plants grown indoors. Mbio 5 e01564–14 10.1128/mBio.01564-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M. (1988). Plant breeding - importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 75 225–233 10.1007/BF00303957 [DOI] [Google Scholar]
- Wittstock U., Burow M. (2010). Glucosinolate breakdown in Arabidopsis: mechanism, regulation and biological significance. Arabidopsis Book 8 e0134. 10.1199/tab.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzel K., Pietsch C., Strickert M., Matros A., Röder M., Weschke W., et al. (2011). Mapping of quantitative trait loci associated with protein expression variation in barley grains. Mol. Breed. 27 301–314 10.1007/s11032-010-9432-2 [DOI] [Google Scholar]
- Wu Q. J., Yang Y., Vogtmann E., Wang J., Han L. H., Li H. L., et al. (2013). Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann. Oncol. 24 1079–1087 10.1093/annonc/mds601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K. A., Lim C. J., Hong J. K., Jin Z. L., Hong J. C., Yun D. J., et al. (2005). Identification of Chinese cabbage genes up-regulated by prolonged cold by using microarray analysis. Plant Sci. 168 959–966 10.1016/j.plantsci.2004.11.011 [DOI] [Google Scholar]
- Yang Z., Kobayashi E., Katsuno T., Asanuma T., Fujimori T., Ishikawa T., et al. (2012). Characterisation of volatile and non-volatile metabolites in etiolated leaves of tea (Camellia sinensis) plants in the dark. Food Chem. 135 2268–2276 10.1016/j.foodchem.2012.07.066 [DOI] [PubMed] [Google Scholar]
- Yu L. X., Setter T. L. (2003). Comparative transcriptional profiling of placenta and endosperm in developing maize kernels in response to water deficit. Plant Physiol. 131 568–582 10.1104/pp.014365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Chen S., Zhu L., Gao Q., Wang X., Yang X. (2012). Establishment of two-dimensional electrophoresis system of Brassica oleracea L. var. capitata L. stigma protein. China Vegetables 1 30–36. [Google Scholar]
- Zhao J. J., Artemyeva A., Del Carpio D. P., Basnet R. K., Zhang N. W., Gao J., et al. (2010). Design of a Brassica rapa core collection for association mapping studies. Genome 53 884–898. [DOI] [PubMed] [Google Scholar]
- Zhou B., Fan P., Li Y. (2014). High-throughput sequence analysis of small RNAs in skotomorphogenic seedlings of Brassica rapa ssp. rapa. Gene 548 68–74 10.1016/j.gene.2014.07.010 [DOI] [PubMed] [Google Scholar]
- Zhuang J., Zhang J., Hou X. L., Wang F., Xiong A. S. (2014). Transcriptomic, proteomic, metabolomic and functional genomic approaches for the study of abiotic stress in vegetable crops. Crit. Rev. Plant Sci. 33 225–237 10.1080/07352689.2014.870420 [DOI] [Google Scholar]