Abstract
This 8-week, multi-center, open-label study assessed the safety and efficacy of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Japanese patients with hypertension and renal dysfunction. Patients (n=32) with mean sitting systolic blood pressure (msSBP) ⩾140 mm Hg (after a 2–5-week washout of previous antihypertensive medications) and estimated glomerular filtration rate (eGFR) ⩾15 and <60 ml min−1 1.73 m−2 received LCZ696 100 mg with an optional titration to 200 and 400 mg in a sequential manner starting from Week 2 in patients with inadequate BP control (msSBP ⩾130 mm Hg and mean sitting diastolic blood pressure (msDBP) ⩾80 mm Hg) and without safety concerns. Safety was assessed by monitoring and recording all adverse events (AEs) and change in potassium and creatinine. Efficacy was assessed as change from baseline in msSBP/msDBP. The mean baseline BP was 151.6/86.9 mm Hg, urinary albumin/creatinine ratio (UACR) geometric mean was 7.3 mg mmol−1 and eGFR was ⩾30 and <60 in 25 (78.1%) patients and was ⩾15 and <30 in 7 (21.9%) patients. Fourteen (43.8%) patients reported at least one AE, which were mild in severity. No severe AEs or deaths were reported. There were no clinically meaningful changes in creatinine, potassium, blood urea nitrogen and eGFR. The geometric mean reduction in UACR was 15.1%, and the mean reduction in msSBP and msDBP was 20.5±11.3 and 8.3±6.3 mm Hg, respectively, from baseline to Week 8 end point. LCZ696 was generally safe and well tolerated and showed effective BP reduction in Japanese patients with hypertension and renal dysfunction without a decline in renal function.
Keywords: angiotensin receptor neprilysin inhibitor, Japanese, LCZ696, renal dysfunction
Introduction
Hypertension is both a cause and a complication of chronic kidney disease (CKD). Hypertension causes functional and structural changes in the kidney and is a major risk factor for cardiovascular complications.1 In Japan, a nationwide database, which included >45 000 patients with CKD, showed a higher prevalence of hypertension in patients with CKD vs. non-CKD (58% vs. 42%).2 As renal disease progression and blood pressure (BP) elevations are closely related to each other, management of hypertension is critical to reduce the risk of further loss of renal function and cardiovascular complications.3
The Japanese Society of Hypertension (JSH 2014) and the Japanese Society of Nephrology (JSN 2013) guidelines recommend that in hypertensive CKD patients with diabetes mellitus or proteinuria a BP target is <130/80 mm Hg and the first choice is an antihypertensive agent acting on the renin–angiotensin system (RAS). In those with neither diabetes mellitus nor proteinuria, a target BP is <140/90 mm Hg and the first-line choice is a calcium channel blocker, RAS inhibitor or diuretic.4, 5 BP lowering and RAS inhibition are important methods for slowing progression of CKD.6 In Japan, despite the availability and use of several classes of antihypertensive agents, BP control was achieved in only 22% of hypertensive patients with comorbid CKD (BP target, <130/80 mm Hg). Not only is the lower BP target more difficult to achieve but the proportion of patients achieving the target BP (<130/80 mm Hg) was significantly lower in CKD patients than in non-CKD patients (35% vs. 44%).2
In hypertensive CKD patients, BP control is not achieved even though most patients take three antihypertensive agents.7 BP control is suboptimal not only in Japan2 but also around the world.8, 9 Salt and water retention,10 increased sympathetic nervous system activity,11 increased activity of RAS12 and reduced biological activity of nitric oxide systems13 are the key promoters of elevated BP in patients with CKD. Even when currently available antihypertensive medications are used, these mechanisms compensate each other to make BP control difficult. Hence, there is a medical need to develop improved antihypertensive agents with favorable efficacy and safety profiles and the ability to slow progression of renal disease.
The natriuretic peptide (NP) system together with the RAS and sympathetic nervous system have an important role in cardiovascular and renal homeostasis.14, 15 Neprilysin (NEP) degrades biologically active NPs, including atrial NP, B-type NP and C-type NP.16 NP levels can be enhanced by inhibiting the enzyme NEP. NPs increase the concentration of cyclic guanosine 3′,5′ monophosphate (cGMP), stimulate diuresis, natriuresis and vasodilation and may have additional antifibrotic and antisympathetic effects.17, 18 However, NEP also contributes to the breakdown of angiotensin.19 Thus NEP inhibition would increase angiotensin levels. Therefore, its full benefits may not be realized unless there is simultaneous suppression of the RAS.
NEP inhibition with simultaneous RAS inhibition increases the plasma concentrations of NPs and inhibits the RAS. These, in turn, regulate water and electrolyte balance by acting on the kidney to promote natriuresis and diuresis.20, 21 In addition to these effects, NPs cause vasodilation by directly relaxing vascular smooth muscle to help further lower BP.22, 23, 24 Additionally, NPs have anti-inflammatory and antifibrotic effects by reducing collagen synthesis.25
LCZ696 is a first-in-class angiotensin receptor NEP inhibitor. After ingestion, LCZ696 delivers systemic exposure to sacubitril (AHU377), a NEP inhibitor pro-drug, and valsartan, an angiotensin receptor blocker (ARB). Sacubitril is then rapidly metabolized by non-specific esterases to the active NEP inhibitor LBQ657. RAS inhibition has beneficial effects on CKD. In addition to lowering BP, RAS inhibition is associated with reducing proteinuria and slowing the decline in glomerular filtration rate (GFR).26 Previous studies with LCZ696 have demonstrated significant reductions in office and ambulatory BP compared with valsartan27 or placebo27, 28 in patients with mild-to-moderate hypertension. Considering the well-known beneficial cardiovascular and renal effects of RAS blockade as well as the potential benefits provided by NEP inhibition, LCZ696 with its multimodal mechanism of action is a promising therapeutic approach in patients with hypertension and renal dysfunction and may offer beneficial renal effects beyond BP lowering. The current study was designed to evaluate the safety and efficacy of LCZ696 in Japanese patients with hypertension and renal dysfunction (CKD, with a confirmed estimated glomerular filtration rate (eGFR) ⩾15 and <60 ml min−1 1.73 m−2).
Methods
Study design
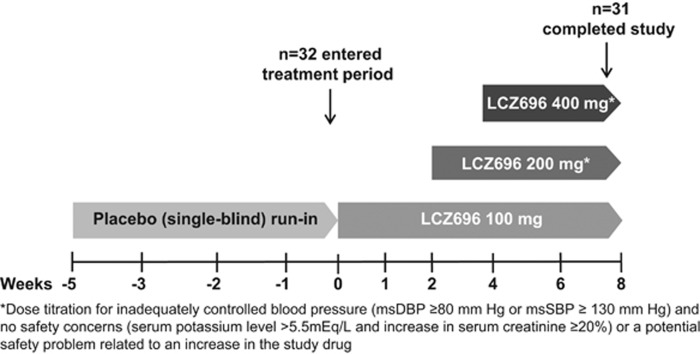
This was an 8-week, multi-center, open-label, phase III study including a placebo run-in period of 2–5 weeks for treated patients (to wash out the effects of earlier antihypertensive agents) and 1–2 weeks for untreated patients and an 8-week treatment period with LCZ696 100 mg, with an optional dose titration to 200 or 400 mg based on the need to achieve BP control (Figure 1). Thirteen Japanese study sites participated in this study. The study protocol was reviewed and approved by the independent institutional review board for each center. The study was performed in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practices, applicable local regulations, and the ethical principles of the Declaration of Helsinki. All participants provided written informed consent prior to study participation (Clinicaltrials.gov NCT01593787).
Figure 1.
Study design.
Patients
Japanese men or women aged ⩾20 years diagnosed with hypertension and moderate-to-severe renal dysfunction (eGFR ⩾15 and <60 ml min−1 1.73 m−2) were selected for the study. Patients with hypertension, either untreated (patients who had not been taking antihypertensive drugs for at least 4 weeks prior to screening) or treated with antihypertensive therapy for at least 4 weeks prior to screening, were included if they had mean sitting systolic blood pressure (msSBP) ⩾140 mm Hg and <180 mm Hg at screening (untreated patients only) and after placebo run-in period (treated and untreated patients). Patients had to achieve a medication compliance rate of ⩾80% during the placebo run-in period.
Patients were excluded if they had severe hypertension (msSBP ⩾180 mm Hg and/or mean sitting diastolic blood pressure (msDBP) ⩾110 mm Hg), secondary hypertension (such as renovascular hypertension except renal parenchymal hypertension), history of angioedema, type 1 or type 2 diabetes mellitus that was not well controlled based on the investigator's clinical judgment, patients on dialysis, eGFR <15 ml min−1 1.73 m−2, patients with acute renal failure or end-stage renal disease. Other exclusion criteria included history of significant cardiovascular/cerebrovascular disease; previous or current diagnosis of heart failure; or any significant laboratory abnormalities at screening such as serum potassium >5.5 or <3.5 mEq l−1, serum sodium <130 mEq l−1 or alanine aminotransferase or aspartate aminotransferase >2 times the upper limit of the normal range.
Treatment
Treatment was initiated with low dose LCZ696 (100 mg) once-daily followed by a stepwise optional dose titration to LCZ696 200 and 400 mg. For patients whose BP was not adequately controlled with LCZ696 100 mg (msBP ⩾130/80 mm Hg) after 2 weeks of treatment and who had no safety concerns (serum potassium >5.5 mEq l−1, increase in serum creatinine by ⩾20% from baseline or a potential safety problem related to an increase in the study drug), LCZ696 was uptitrated to 200 mg once daily and if patients still remained inadequately controlled after 4 weeks of treatment, then the dose was uptitrated to 400 mg once daily.
Doses were taken in the morning, before or after a meal except on the days of scheduled visits when LCZ696 was administered after the completion of all assessments. Patients who were taking two or more antihypertensive agents before the initiation of the study continued to take one agent (other than ARB, angiotensin-converting enzyme inhibitor (ACEI) or a fixed combination containing ARB or ACEI) concurrently, as long as there was no change in the drug or the dosing regimen during the study. Concomitant use of other antihypertensive agents was not permitted from the start of the run-in period (untreated patients) or for 2 weeks after the beginning of the run-in period (treated patients) to the end of the treatment period.
Safety assessments
The primary objective was safety and tolerability, which included monitoring and recording adverse events (AEs), serious AEs, laboratory tests for hematology, blood chemistry and urinalysis and monitoring vital signs, ECG and body weight. Standard laboratory tests were performed at baseline (Week 0) and at Weeks 4 and 8 during the treatment period. In addition, serum creatinine, blood urea nitrogen and serum electrolytes (sodium and potassium) were measured at Weeks 1, 2 and 6 during the treatment period. Random or spot urine samples were collected in the physician's office for urine creatinine and albumin at baseline and at the end of the study for the calculation of the urinary albumin/creatinine ratio (UACR). The eGFR (ml min−1 1.73 m−2) was calculated based on the result of serum creatinine, using the formula eGFR=194 × Cr−1.094 × age−0.287 ( × 0.739; females).29 The incidence of AEs was reported by eGFR category.
Efficacy assessments
The efficacy outcome was the change in msSBP/msDBP from baseline (Week 0) to Week 8 (last observation carried forward). BP was measured at trough (approximately 24 h after dosing after the previous day's dosing and immediately before the same day's dosing) at all scheduled visits. An automated and validated BP measuring device (Omron BP monitor: HEM-7080IC, manufactured by OMRON HEALTHCARE Co., Ltd, Kyoto, Japan) with the appropriate cuff size was used in accordance with British Hypertension Society 2004 guidelines.30 BP measurements were taken four times at 2-min intervals after resting for at least 5 min in the sitting position. The reported BP for the visit was the mean of all these readings. Additional assessments included BP control rate (proportion of patients with BP<130/80 mm Hg), SBP responder rate (proportion of patients with SBP<130 mm Hg or ⩾20 mm Hg reduction from baseline) and DBP responder rate (proportion of patients with DBP<80 mm Hg or ⩾10 mm Hg reduction from baseline). The change from baseline in UACR was also assessed. The efficacy parameters were reported by eGFR category as well.
Pharmacokinetic assessments
LCZ696 delivers systemic exposure to a NEP inhibitor pro-drug AHU377 (which converts to an active form LBQ657) and valsartan. Trough plasma concentrations of valsartan, AHU377 and LBQ657 were measured at the visits when LCZ696 dose was increased and at Week 8 (or discontinuation). Blood samples were drawn from a forearm vein into EDTA-containing polyethylene tubes and were centrifuged at 1500 g for 10 min. Plasma was stored at ⩽−15 °C until the analyses were performed. The concentrations of valsartan, AHU377 and LBQ657 were determined by liquid chromatography/tandem mass spectrometry. The lower limit of quantification was 10.0 ng ml−1 for valsartan, 1.0 ng ml−1 for AHU377 and 20.0 ng ml−1 for LBQ657.
Statistical analyses
A total of 56 enrolled patients were planned to achieve a sample size of 25 patients completing the 8-week treatment period, assuming a drop-out rate of 50% prior to the treatment period and 10% during the treatment period. This sample size was considered sufficient to evaluate the overall safety and tolerability of LCZ696 treatment, with an 84% chance to observe at least one AE, when the true incidence of an individual AE is 7%. Safety analyses were performed on the safety set, consisting of all patients who received at least one dose of LCZ696. Efficacy analyses were performed on the full analysis set, consisting of all patients who entered the treatment period of the study. Descriptive statistics were used for all efficacy and safety variables. Last observation carried forward method was used to assess the changes from baseline to Week 8 end point.
Results
Patient disposition and characteristics
Of the 39 patients who entered the placebo run-in period, 32 patients entered the treatment period and 31 (96.9%) patients completed the study. All 32 patients were exposed to LCZ696 and were evaluable for safety, efficacy and pharmacokinetic analyses. Of the 32 patients, 81% (n=26) of patients had their LCZ696 dose increased from 100 to 200 mg and 56% (n=18) of patients were uptitrated to 400 mg. All the patients were Japanese with a mean age of 65.8 years; 21 patients (65.6%) were elderly (⩾65 years age); 25 patients (78.1%) had stage III (eGFR⩾30 and <60 ml min−1 1.73 m−2); and 7 patients (21.9%) had stage IV (eGFR⩾15 and <30 ml min−1 1.73 m−2) CKD. The mean duration of hypertension was 9.4 years. Patient baseline characteristics are presented in Table 1. During the study, 14 of the 32 patients used antihypertensive medication (other than ARB, ACEI or a fixed combination of ARB and ACEI) at baseline (Day 1) that continued throughout the study; Of these, calcium channel blockers were taken by 12 of the 14 patients and diuretics by 2 of the 14 patients.
Table 1. Patient baseline characteristics.
| Characteristics | N=32 |
|---|---|
| Age (years) | 65.8±9.1 |
| Aged ⩾65 years, n (%) | 21 (65.6) |
| Male, n (%) | 24 (75.0) |
| BMI (kg m−2) | 25.3±3.3 |
| msDBP (mm Hg) | 86.9±10.8 |
| msSBP (mm Hg) | 151.6±10.3 |
| Duration of hypertension (years) | 9.4±6.4 |
| Diabetes, n (%) | 6 (18.8) |
| eGFR (ml min−1 1.73 m−2) | 41.0±10.1 |
| eGFR group, n (%) | |
| ⩾15 to <30 ml min−1 1.73 m−2 | 7 (21.9) |
| ⩾30 to <60 ml min−1 1.73 m−2 | 25 (78.1) |
| UACR (mg mmol−1), geometric mean | 7.3 |
| Creatinine (umol l−1) | 122.4±35.4 |
| BUN (mmol l−1) | 7.1±2.2 |
| Sodium (mmol l−1) | 143.0±1.8 |
| Potassium (mmol l−1) | 4.6±0.3 |
Abbeviations: BMI, body mass index; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; msDBP, mean sitting diastolic blood pressure; msSBP, mean sitting systolic blood pressure; UACR, urinary albumin-to-creatinine ratio.
Data are presented as mean±s.d. unless otherwise specified.
UACR: 1 mg mmol−1=8.85 mg g−1.
Safety and tolerability
LCZ696 was safe and well tolerated during the 8-week treatment in patients with hypertension and renal dysfunction. AEs were reported by 14 patients (43.8%), and all were mild in severity. The most frequently reported AE was nasopharyngitis (6 patients, 18.8%) (Table 2). Two AEs, headache and pruritus, each reported by one patient were considered as possibly related to the study drug, and the patient who reported headache prematurely discontinued the study. No death or serious AE was reported in the study. There were no cases of dizziness, hypotension or other events related to low BP. No cases of angioedema were reported. The incidence of AEs was 57.1% (4/7 patients) and 40.0% (10/25 patients) in patients with eGFR values <30 and ⩾30 ml min−1 1.73 m−2, respectively.
Table 2. Safety and tolerability profile of LCZ696.
| Adverse events | Total (n (%), N=32 |
|---|---|
| Any adverse event | 14 (43.8) |
| Nasopharyngitis | 6 (18.8) |
| Conjunctivitis allergic | 1 (3.1) |
| Constipation | 1 (3.1) |
| Cystitis | 1 (3.1) |
| Gout | 1 (3.1) |
| Headache | 1 (3.1) |
| Oedema peripheral | 1 (3.1) |
| Thermal burn | 1 (3.1) |
| Arthralgia | 1 (3.1) |
| Dyspepsia | 1 (3.1) |
| Pruritus | 1 (3.1) |
| Supraventricular extrasystoles | 1 (3.1) |
| Toothache | 1 (3.1) |
| Clinically notable changes in laboratory values | |
| Potassium (mmol l−1) | |
| ⩾6.0 | 0 (0.0) |
| >5.5 | 1 (3.1) |
| <3.5 | 0 (0.0) |
| Sodium (mmol l−1) | |
| <130 | 0 (0.0) |
| >5% decrease from baseline | 1 (3.1) |
| Blood urea nitrogen (mmol l−1) | |
| >50% increase from baseline | 6 (18.8) |
The mean changes in clinical chemistry parameters from baseline to Week 2 and Week 8 end point were small and not clinically meaningful (Table 3). The median value for changes in laboratory parameters from baseline to Week 2 and Week 8 end point were: serum creatinine (−0.5 μmol l−1 and 2.0 μmol l−1), blood urea nitrogen (0.0 and 0.4 mmol l−1), serum sodium (0.0 mmol l−1), serum potassium (0.0 mmol l−1), and eGFR (0.1 ml min−1 1.73 m−2 and −0.6 ml min−1 1.73 m−2). Clinically notable changes in blood urea nitrogen (>50% increase from baseline) were observed in 6 patients (18.8%); these values remained in the normal range (2.9–8.2 mmol l−1) for 1 patient (3.1%) but were above the normal range for 5 patients (15.6%). A >5% decrease in serum sodium was observed in 1 patient (3.1%). One patient had serum potassium >5.5 mmol l−1 at Week 4, but the serum potassium returned to normal at Week 6 without interrupting study medication (Table 2).
Table 3. Mean change in clinical chemistry from baseline to Week 2 and Week 8 end point.
| Variable | Week 2 end point | Week 8 end point |
|---|---|---|
| Creatinine (umol l−1) | 0.5±8.5 | 1.9±11.5 |
| BUN (mmol l−1) | 0.1±1.4 | 0.2±1.6 |
| Sodium (mmol l−1) | 0.0±1.7 | −0.3±2.1 |
| Potassium (mmol l−1) | 0.0±0.3 | −0.1±0.3 |
| eGFR (ml min−1 1.73 m−2) | −0.2±3.4 | −0.5±5.4 |
Abbreviations: BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate.
Data are presented as mean±s.d.
Efficacy
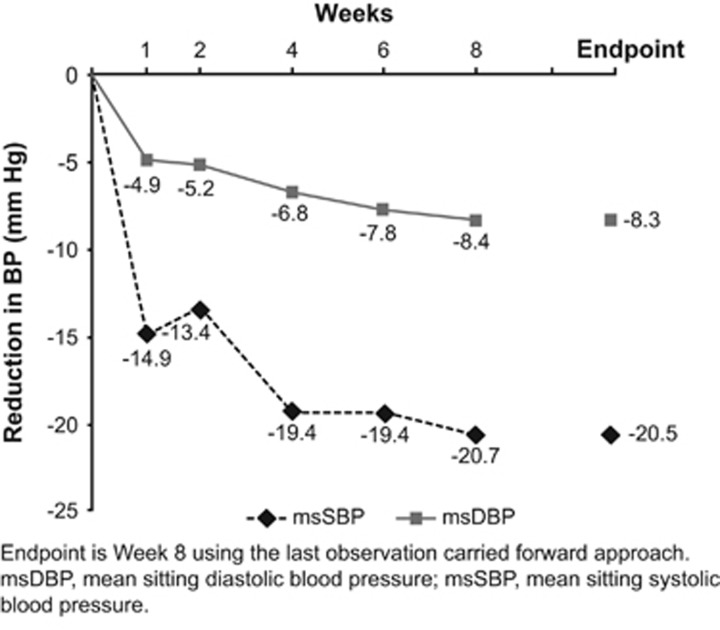
The msSBP±s.d. was reduced from 151.6±10.3 mm Hg at baseline to 138.2±12.1 mm Hg at Week 2, followed by a further decrease to 132.2±10.8 mm Hg at Week 4, which remained stable thereafter at Week 6 (132.5±13.1 mm Hg) and at Week 8 (131.2±11.1 mm Hg). The mean±s.d. decrease in msSBP from baseline to Week 8 end point was 20.5±11.3 mm Hg (Figure 2). The msDBP was reduced from 86.9±10.8 mm Hg at baseline to 81.7±10.1 mm Hg at Week 2, followed by a further decrease to 80.1±10.0 mm Hg at Week 4 and 79.4±10.4 mm Hg at Week 6 and remained stable until Week 8 (78.8±10.7 mm Hg). Mean±s.d. decrease in msDBP from baseline to Week 8 end point was 8.3±6.3 mm Hg (Figure 2).
Figure 2.
Mean change in mean sitting blood pressure from baseline to Week 8 end point with LCZ696.
Baseline msSBP/msDBP were 149.8/83.9 mm Hg for eGFR<30 ml min−1 1.73 m−2 and 152.1/87.7 mm Hg for eGFR⩾30 ml min−1 1.73 m−2. The msSBP/msDBP reduction at Week 8 end point was clinically significant for both subgroups; 17.7/5.5 mm Hg for eGFR<30 ml min−1 1.73 m−2 and 21.3/9.1 mm Hg for eGFR⩾30 ml min−1 1.73 m−2. The overall BP control rate (<130/80 mm Hg) was 25.0% at the Week 8 end point. The SBP and DBP control rates were 50.0% and 46.9% at Week 8 end point. A successful SBP response rate (proportion of patients with SBP<130 mm Hg or ⩾20 mm Hg reduction from baseline) and DBP response rate (proportion of patients with DBP<80 mm Hg or ⩾10 mm Hg reduction from baseline) was achieved by 59.4% and 71.9% of patients, respectively, at the Week 8 end point.
BP control at Week 8 end point was achieved by 28.6% of patients with eGFR<30 ml min−1 1.73 m−2 and by 24.0% of patients with eGFR⩾30 ml min−1 1.73 m−2. The SBP and DBP control rates were 57.1% and 42.9% in patients with eGFR<30 ml min−1 1.73 m−2 while 48.0% of patients with eGFR⩾30 ml min−1 1.73 m−2 achieved both SBP and DBP control. Both SBP and DBP response was achieved by 57.1% of patients with eGFR<30 ml min−1 1.73 m−2, whereas the SBP and DBP response was 60.0% and 76.0% in patients with eGFR⩾30 ml min−1 1.73 m−2.
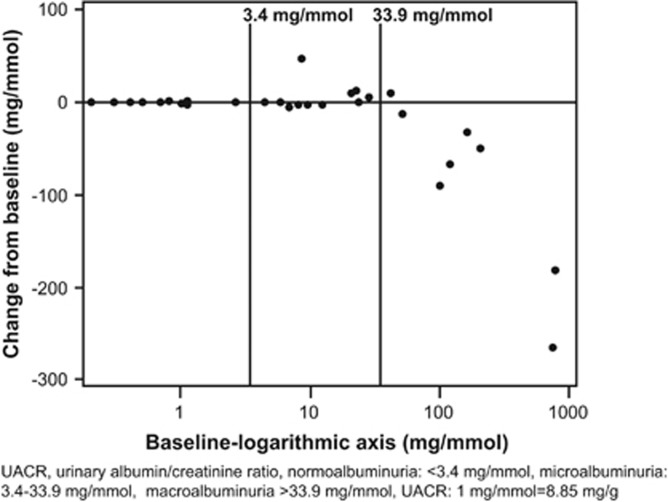
Treatment with LCZ696 showed a geometric mean reduction of 15.1% in UACR from baseline. The geometric mean value of UACR decreased from 7.3 mg mmol−1 at baseline to 6.2 mg mmol−1 at Week 8 end point. Reduction from baseline in UACR in patients with baseline UACR>33.9 mg mmol−1 (macroalbuminuria) was larger than those with baseline UACR<3.4 mg mmol−1 (normoalbuminuria) and 3.4–33.9 mg mmol−1 (microalbumiuria) (Figure 3).
Figure 3.
Scatter plot (baseline vs. change) of UACR.
Pharmacokinetics
A dose-related increase in trough plasma levels of LBQ657 and valsartan were observed from LCZ696 100–400 mg. After the administration of LCZ696 100, 200 and 400 mg, mean trough plasma levels at Week 8 were 2162, 6404 and 8567 ng ml−1 for LBQ657 and 141, 548 and 591 ng ml−1 for valsartan respectively. Dose-normalized trough plasma LBQ657 concentrations tended to increase according to the decrease in eGFR (Supplementary Figure S1). This was not the case for valsartan. The increases of dose-normalized trough plasma LBQ657 concentrations were unaffected by age.
Discussion and Conclusions
Previous data have shown that LCZ696 produced significantly greater reductions in SBP, DBP, pulse pressure and a greater BP control rate and BP response rate than valsartan (ARB) and AHU (NEP inhibitor) in mostly Caucasian patients with mild–moderate hypertension, supporting the complementary mechanism of action of ARB and NEP inhibition in lowering blood pressure.27 This was the first study to evaluate the safety and efficacy of LCZ696 in Japanese patients with hypertension and renal dysfunction. In this patient population, LCZ696 was generally safe and well tolerated during the 8-week treatment, with only one patient discontinuing the study. There were no cases of death, serious AEs, dizziness, hypotension or angioedema during the study. The study demonstrated no clinically important effects of LCZ696 on renal function or serum potassium despite varying levels of renal function and the large reduction in BP during the first weeks of treatment in these hypertensive patients with CKD. The minimal changes in serum potassium observed in this study are consistent with previous LCZ696 studies in patients with hypertension (unpublished data) as well as healthy volunteers and in patients with chronic heart failure.31, 32 The small initial reduction in eGFR that was observed is typically associated with RAS blockade using standard doses of ACEI or ARB.33 The antihypertensive effect of LCZ696 was evident during the first 2 weeks of treatment, and there were further incremental reductions in msSBP and msDBP by Week 4, remaining stable thereafter until the Week 8 end point.
The reduction in UACR observed in the present study is comparable to a previous LCZ696 study in patients with mild-to-moderate hypertension that showed 10, 4 and 12% reductions in UACR with 100, 200 and 400 mg LCZ696 doses, respectively, from baseline to Week 8.27 In the present study, reduction in UACR was greater in patients with macroalbuminuria than in patients with normoalbuminuria or microalbuminuria. This is a similar response to what is reported with RAS inhibitors in subjects with high urinary protein excretion.34 In patients with preserved ejection fraction heart failure, therapy with LCZ696 for 36 weeks preserved renal function (as indicated by a smaller 1.6 ml min−1 1.73 m−2 decrease in eGFR vs. 5.2 ml min−1 1.73 m−2 decrease in the valsartan group). Although this was accompanied by a small (1.0 mg mmol−1) increase in UACR from baseline,35 this increase is well within the variability of the measurement and the day-to-day variability of urinary albumin excretion.
JSH 2014 and JSN 2013 guidelines recommend RAS inhibitors as a first therapeutic choice for CKD patients with diabetes mellitus or proteinuria.4, 5 Several studies have shown that valsartan was well tolerated and effective in treating patients with hypertension and CKD. Treatment with valsartan resulted in slightly greater decreases in total protein, albumin excretion and eGFR as compared with placebo.36 In general, therapy with an ACEI or an ARB is associated with an increase in serum creatinine concentration in patients with renal dysfunction. This is generally attributed to decreased glomerular capillary pressure because of preferential vasodilation of efferent arterioles as compared with afferent arterioles.37 However, in the current study only small and non-progressive increases in the serum creatinine concentration accompanied effective reduction in BP with LCZ696. This may indicate that LCZ696 exerts favorable effects on renal function. These findings suggest that NEP inhibition, in the presence of angiotensin II receptor blockade as provided by LCZ696, preserves GFR by maintaining glomerular capillary pressure despite reductions in BP. This is consistent with an NPs' preferential vasodilator action on the afferent arteriole. NPs also inhibit sodium reabsorption in both the proximal and distal nephron that increases urinary sodium excretion and urine flow. This effect has an important role in regulating tubuloglomerular feedback, which also prevents the decrease in GFR that normally follows increased salt delivery to the distal tubule.20
There is a great need to control the BP in patients with renal dysfunction as previous studies conducted in Asia and Japan have demonstrated a linear and continuous association between BP and renal outcomes even in subjects with prehypertension.38, 39 LCZ696 (100–400 mg) showed clinically significant reductions in msSBP and msDBP from Week 1 to the end of the study. BP control was achieved by 25% of patients, which is in accordance with previous studies from different regions (Spain, US and Norway) where BP<130/80 mm Hg was achieved in 13–37% of patients with CKD. The lack of optimal BP control in these regions was mainly attributed to lack of control of systolic BP.40, 41, 42 In our study, 50% of patients achieved msSBP<130 mm Hg at end point from a baseline msSBP of 151.6 mm Hg. A previous study has shown that, in patients with CKD, SBP is associated with an increased rate of progression of kidney disease, and controlling SBP to 110–129 mm Hg reduces the risk of kidney disease progression.43, 44 Considering the absence of add-on antihypertensive therapy in our study, the BP control rates achieved with LCZ696 compare well with other studies of BP control in patients with CKD.40, 41, 42
We speculate that the excellent BP-lowering effect of LCZ696 may be accompanied by the action of NPs to increase renal medullary blood flow.45 When systemic BP decreases, renal medullary blood flow decreases (unlike cortical blood flow, which is maintained by autoregulation). This decrease in medullary blood flow, in turn, promotes sodium reabsorption, thereby blunting the BP-lowering effects of antihypertensive drugs. Thus LCZ696 may be a unique antihypertensive drug with multiple modes of action, namely inhibiting the RAS, directly vasodilating vascular smooth muscle cells and causing natriuresis induced by both direct tubular actions and increases in medullary blood flow.
In the present study in patients with renal dysfunction, mean trough plasma LCZ696 concentrations increased with dose. A correlation was observed between renal function and systemic exposure to LBQ657 but not to valsartan. This reduction in LBQ657 clearance was expected, because LBQ657 is primarily eliminated in the urine with a circulating half-life of 12 h.31 Although no safety issues were noted in this study, the clinical significance of these increases in LBQ657 exposure in patients with renal dysfunction remains to be determined. The overall safety profile reported in hypertensive patients with moderate or severe renal impairment in the present study treated with LCZ696 doses up to 400 mg were similar to those of patients with mild or moderate hypertension in previous studies.27, 28 Additionally, favorable antihypertensive effects were produced by LCZ696 in hypertensive patients with renal impairment in this study, regardless of the degree of renal impairment. Thus hypertensive patients with renal impairment can be treated with the same dose and regimen used in hypertensive patients with normal renal function.
Our results suggest that LCZ696 may be a promising therapeutic approach in patients with hypertension and renal dysfunction and may offer beneficial renal effects in addition to BP lowering. In addition to the cardiovascular and renal benefits of AT1 receptor blockade, LCZ696 provides NEP inhibition, which enhances the actions of NPs. These peptides promote natriuresis and diuresis and inhibit the sympathetic nervous system and aldosterone secretion. NPs also may provide renal protection in patients with renal dysfunction by reducing intraglomerular pressure and through antiproliferative and antihypertrophic effects. Potential limitations of this study include its open-label design, small sample size and relatively short duration. However, this 8-week study has demonstrated that LCZ696, a first-in-class angiotensin receptor NEP inhibitor, is generally safe and efficacious in Japanese patients with hypertension and renal dysfunction while maintaining renal function. Additional studies are needed to confirm the beneficial effects and safety profile of LCZ696 observed in this trial.
Acknowledgments
Novartis is the sponsor of this study and prepared the study protocol. This study was supported by Novartis. All authors participated in the development and writing of the paper and approved the final manuscript for publication. The authors take full responsibility for the content of the paper and thank Hyosung Kim for statistical analysis (who was an employee of Novartis at the time of study completion and statistical analysis), Sandra Thompson for statistical review (who was an employee of Novartis at the time of statistical analysis) and Dr Madhavi Dokku (Novartis Healthcare) for assisting in the writing of this manuscript, collating comments from all authors and editing the final manuscript. We also thank all the clinical investigators and study co-ordinators at the participating centers (Shinagawa East one Medical Clinic, Miho Clinic, Minamino Heart Clinic, Koukan Clinic, Motomachi Takatsuka Naika Clinic, Anbe Heart Clinic, Ikeoka Clinic, Sapporo Century Hospital, Yoshida Memorial Hospital, Kotoni Medical Support Clinic, Kawahara Nephro-urological Clinic, Tohoku University Hospital, Kawasaki Medical School Hospital) and especially all the patients who participated in the study.
YT, HG, AC, NO, MA and JZ are employees of Novartis Pharmaceutical Corporation and may receive ⩾1 000 000 yen (in one year) for employment. SI received lecture fee and consultant fee from various Japanese pharmaceutical companies, including Novartis. MS has no conflict of interest to disclose.
Footnotes
Supplementary Information accompanies the paper on Hypertension Research website (http://www.nature.com/hr)
Supplementary Material
References
- Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, Flack JM, Carter BL, Materson BJ, Ram CV, Cohen DL, Cadet JC, Jean-Charles RR, Taler S, Kountz D, Townsend R, Chalmers J, Ramirez AJ, Bakris GL, Wang J, Schutte AE, Bisognano JD, Touyz RM, Sica D, Harrap SB. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Konta T, Ikeda A, Ichikawa K, Fujimoto S, Iseki K, Moriyama T, Yamagata K, Tsuruya K, Yoshida H, Asahi K, Kurahashi I, Ohashi Y, Watanabe T. Blood pressure control in a Japanese population with chronic kidney disease: a baseline survey of a nationwide cohort. Am J Hypertens. 2012;25:342–347. doi: 10.1038/ajh.2011.217. [DOI] [PubMed] [Google Scholar]
- Yano Y, Fujimoto S, Sato Y, Konta T, Iseki K, Moriyama T, Yamagata K, Tsuruya K, Yoshida H, Asahi K, Kurahashi I, Ohashi Y, Watanabe T. Association between prehypertension and chronic kidney disease in the Japanese general population. Kidney Int. 2012;81:293–299. doi: 10.1038/ki.2011.346. [DOI] [PubMed] [Google Scholar]
- Japan nephrology society [Special issue: evidence-based practice guideline for the treatment of CKD (Japanese article)] Nihon Jinzo Gakkai Shi. 2013;55:585–860. [PubMed] [Google Scholar]
- Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014) Hypertens Res. 2014;37:253–387. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- Drawz PE, Rosenberg ME. Slowing progression of chronic kidney disease. Kidney Int Suppl (2011) 2013;3:372–376. doi: 10.1038/kisup.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafidis PA, Sharpe CC, Wood E, Blacklock R, Rumjon A, Al-Yassin A, Ariyanayagam R, Simmonds S, Fletcher-Rogers J, Vinen K. Prevalence, patterns of treatment, and control of hypertension in predialysis patients with chronic kidney disease. Nephron Clin Pract. 2012;120:c147–c155. doi: 10.1159/000337571. [DOI] [PubMed] [Google Scholar]
- Fraser SD, Roderick PJ, McIntyre NJ, Harris S, McIntyre CW, Fluck RJ, Taal MW. Suboptimal blood pressure control in chronic kidney disease stage 3: baseline data from a cohort study in primary care. BMC. Fam Pract. 2013;14:88. doi: 10.1186/1471-2296-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga LC, Miller ER, III, Stevens LA, Saran R, Messer K, Flowers N, Geiss L, Powe NR. Blood pressure control among persons without and with chronic kidney disease: US. trends and risk factors 1999-2006. Hypertension. 2009;54:47–56. doi: 10.1161/HYPERTENSIONAHA.109.129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charra B. Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int. 2007;11:21–31. doi: 10.1111/j.1542-4758.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- Klein IH, Ligtenberg G, Neumann J, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol. 2003;14:3239–3244. doi: 10.1097/01.asn.0000098687.01005.a5. [DOI] [PubMed] [Google Scholar]
- Rüster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Böger SM, Haller H, Ritz E. Asymmetric dimethylarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol. 2005;16:2456–2461. doi: 10.1681/ASN.2005020179. [DOI] [PubMed] [Google Scholar]
- Kalra PR, Anker SD, Coats AJ. Water and sodium regulation in chronic heart failure: the role of natriuretic peptides and vasopressin. Cardiovasc Res. 2001;51:495–509. doi: 10.1016/s0008-6363(01)00297-8. [DOI] [PubMed] [Google Scholar]
- Vanderheyden M, Bartunek J, Goethals M. Brain and other natriuretic peptides: molecular aspects. Eur J Heart Fail. 2004;6:261–268. doi: 10.1016/j.ejheart.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett JC., Jr Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34:886–893c. doi: 10.1093/eurheartj/ehs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49:419–426. doi: 10.1161/01.HYP.0000258532.07418.fa. [DOI] [PubMed] [Google Scholar]
- Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- Richards AM, Wittert GA, Crozier IG, Espiner EA, Yandle TG, Ikram H, Frampton C. Chronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin II. J Hypertens. 1993;11:407–416. doi: 10.1097/00004872-199304000-00011. [DOI] [PubMed] [Google Scholar]
- Boerrigter G, Burnett JC., Jr. Recent advances in natriuretic peptides in congestive heart failure. Expert Opin Investig Drugs. 2004;13:643–652. doi: 10.1517/13543784.13.6.643. [DOI] [PubMed] [Google Scholar]
- Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;191:341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkin EM, Tucker VL. Atrial natriuretic peptide as a regulator of transvascular fluid balance. News Physiol Sci. 1996;11:138–143. [Google Scholar]
- Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70:665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- Baxter GF. The natriuretic peptides. Basic Res Cardiol. 2004;99:71–75. doi: 10.1007/s00395-004-0457-8. [DOI] [PubMed] [Google Scholar]
- Chopra S, Baby C, Jacob JJ. Neuro-endocrine regulation of blood pressure. Indian J Endocrinol Metab. 2011;15 (Suppl 4:S281–S288. doi: 10.4103/2230-8210.86860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney Disease Outcomes Quality Initiative (K/DOQI). NKF guidelines – Goals of antihypertensive therapy in CKD. Am J Kidney Dis. 2004;43 (Suppl 1:S65–S73. [Google Scholar]
- Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- Kario K, Sun N, Chiang FT, Supasyndh O, Baek SH, Inubushi-Molessa A, Zhang Y, Gotou H, Lefkowitz M, Zhang J. Efficacy and safety of LCZ696, a first-in-Class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63:698–705. doi: 10.1161/HYPERTENSIONAHA.113.02002. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, McG Thom S. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004;18:139–185. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- Kobalava Z, Pavlikova E, Averkov O, Moiseev V, Albrecht D, Feng A, Chandra P, Jordaan PJ.First experience with concomitant AT1 and neprilysin (NEP 24.11) inhibition with LCZ696 in patients with chronic heart failure Circulation 2010122A19378(Abstract). [Google Scholar]
- Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168–175. doi: 10.1016/j.semnephrol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- Plum J, Bünten B, Nèmeth R, Grabensee B. Effects of the angiotensin II antagonist valsartan on blood pressure, proteinuria, and renal hemodynamics in patients with chronic renal failure and hypertension. J Am Soc Nephrol. 1998;9:2223–2234. doi: 10.1681/ASN.V9122223. [DOI] [PubMed] [Google Scholar]
- Palmer BF. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what to do if the serum creatinine and/or serum potassium concentration rises. Nephrol Dial Transplant. 2003;18:1973–1975. doi: 10.1093/ndt/gfg282. [DOI] [PubMed] [Google Scholar]
- O'Seaghdha CM, Perkovic V, Lam TH, McGinn S, Barzi F, Gu DF, Cass A, Suh I, Muntner P, Giles GG, Ueshima H, Woodward M, Huxley R. Blood pressure is a major risk factor for renal death: an analysis of 560 352 participants from the Asia-Pacific region. Hypertension. 2009;54:509–515. doi: 10.1161/HYPERTENSIONAHA.108.128413. [DOI] [PubMed] [Google Scholar]
- Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41:1341–1345. doi: 10.1161/01.HYP.0000069699.92349.8C. [DOI] [PubMed] [Google Scholar]
- Martinez-Castelao A, Górriz JL, Portolés JM, De Alvaro F, Cases A, Luño J, Navarro-González JF, Montes R, De la Cruz-Troca JJ, Natarajan A, Batlle D. Baseline characteristics of patients with chronic kidney disease stage 3 and stage 4 in Spain: the MERENA observational cohort study. BMC Nephrol. 2011;12:53. doi: 10.1186/1471-2369-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta CA, Hicks LS, Chertow GM, Ayanian JZ, Vittinghoff E, Lin F, Shlipak MG. Control of hypertension in adults with chronic kidney disease in the United States. Hypertension. 2005;45:1119–1124. doi: 10.1161/01.HYP.0000164577.81087.70. [DOI] [PubMed] [Google Scholar]
- Prøsch LK, Saelen MG, Gudmundsdottir H, Dyrbekk D, Hunderi OH, Arnesen E, Paulsen D, Skjønsberg H, Os I. Blood pressure control is hard to achieve in patients with chronic renal failure: results from a survey of renal units in Norway. Scand J Urol Nephrol. 2005;39:242–248. doi: 10.1080/00365590510007810-1. [DOI] [PubMed] [Google Scholar]
- Agarwal R. Blood pressure components and the risk for end-stage renal disease and death in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:830–837. doi: 10.2215/CJN.06201208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta- analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- Kiberd BA, Larson TS, Robertson CR, Jamison RL. Effect of atrial natriuretic peptide on vasa recta blood flow in the rat. Am J Physiol. 1987;252:F1112–F1117. doi: 10.1152/ajprenal.1987.252.6.F1112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.