Abstract
Aim
This study aimed to assess the diagnostic ramifications of vascular occlusion of the ocular vein and artery as a first thrombotic event associated with factor V Leiden (FVL) and/or prothrombin gene (PTG) heterozygosity.
Methods
Patients with ocular vein (n=191) and artery (n=74) occlusion, free of cardioembolic etiologies, were sequentially referred from vitreoretinal specialists for measurement of thrombophilia-hypofibrinolysis and compared to 110 healthy normal controls.
Results
Of the 265 patients, 29 (11%; 17 women, 12 men) of all referred ocular vascular occlusion (OVO) cases were found to be heterozygous for FVL and/or PTG, including 16 with FVL, 12 with PTG, and 1 with both. Of the 29 cases, 16 had central retinal vein occlusion (CRVO), 2 branch retinal vein occlusion (BRVO), 5 nonarteritic anterior ischemic optic neuropathy (NA-AION), 3 retinal artery occlusion (RAO), 2 amaurosis fugax (AF), and 1 had both CRVO and RAO. Of the 16 FVL cases, 15 (94%) had OVO as a first thrombotic event without prior deep venous thrombosis (DVT) or pulmonary embolism (PE); 6 (38%) also had other thrombotic events, including recurrent miscarriage, osteonecrosis, ischemic stroke, and/or ischemic colitis; and 5 (31%) had immediate family members with previous venous thromboembolism (VTE). Of the 12 PTG cases, 9 (75%) had OVO as a first thrombotic event, 5 (42%) experienced VTE other than DVT or PE, and 6 (50%) had immediate family members with VTE. In one patient with both FVL and PTG, DVT occurred before BRVO. Of the 17 women with FVL and/or PTG mutations, 7 (41%) experienced ≥1 miscarriage, 6 (35%) were on estrogen therapy, and 1 (6%) was on clomiphene.
Conclusion
Of the 265 patients with OVO, 29 (11%) had FVL and/or PTG, and 83% of these 29 cases presented with OVO as their first thrombotic event. By diagnosing thrombophilia as an etiology for OVO, the ophthalmologist opens a window to family screening and preventive therapy.
Keywords: factor V Leiden, prothrombin gene mutation, ocular vascular occlusion, retinal vein occlusion, retinal artery occlusion, anterior ischemic optic neuropathy
Introduction
Retinal vein occlusions (RVOs), retinal artery occlusions (RAOs), and ciliary artery occlusions causing optic nerve infarction or anterior ischemic optic neuropathy (AION) are well-recognized vascular ischemic events that affect the posterior segment of the eye. RVO is widely prevalent,1–4 with 5.20 cases per 1,000 people (95% confidence interval [CI]: 4.40–5.99) in the USA, Europe, Asia, and Australia as in 2010, suggesting that roughly 16 million people may have this disorder.4 AION is less common, with a median age of 62 and a mean annual incidence rate of 2.3 per 100,000 people for nonarteritic AION (NA-AION) and 0.36 per 100,000 people for arteritic AION (A-AION).5 Central retinal artery occlusion (CRAO), which causes retinal infarction, is slightly rarer, estimated to be 1 in 100,000 people and roughly 1 in 10,000 outpatient visits, but it may result in severe vision loss in up to 80% of patients.6
The presentation of ocular vascular occlusion (OVO), including RAO, RVO or AION, is widely variable and may range from an incidental finding in asymptomatic patients to partial visual field loss, diminished or absent central vision, or diminished or complete loss of vision resulting from either one or a combination of the following: optic nerve infarction, retinal infarction, macular edema, macular ischemia, and/or neovascular glaucoma.7,8 Because OVO has the potential for such devastating complications, including permanent vision loss, it is important to identify the etiology of the patient’s OVO to implement the appropriate treatment and to provide available prophylactic measures to prevent subsequent contralateral ocular and/or systemic thrombotic events.
Recognized risk factors for OVO are categorized as systemic or local. Systemic factors include hyperviscosity, myeloproliferative disorders, retro-orbital mass effect, and vasculitis such as Behcet’s disease.7,9 A common local finding predisposing to OVO is open-angle glaucoma. Glaucoma decreases venous outflow through increased intraocular pressure, thus creating vascular stasis and increased risk of occlusion, in accordance with Virchow’s triad.10–13 When commonly recognized etiologies are ruled out, other risk factors for OVO must be assessed, including cardiovascular13–23 and hypercoagulable state9,24,25 risk factors.
The most recognized, but neither sensitive nor specific, cardiovascular risk factors for OVO include age, history of smoking, hypertension, hyperlipidemia, diabetes mellitus, and atherosclerosis.13–23 More recently, however, there has been increased focus on the pathoetiologic role of thrombophilia in OVO. In the absence of a cardioembolic etiology for OVO, thrombophilia is a common, major cause of ocular thrombotic events.9,24 In particular, thrombophilia should be carefully assessed in younger patients, <65 years old, or in patients with a personal or family history of thrombosis.25
Thrombophilia can be heritable – such as in hyperhomocysteinemia, factor V Leiden (FVL) mutation, prothrombin (PTG) G20210A mutation, antithrombin III deficiency, protein C deficiency, or protein S deficiency – or acquired, particularly the lupus anticoagulant found in antiphospholipid syndrome. Of the thrombophilias that are risk factors for OVO,7,16,20,21,24,26–37 hyperhomocysteinemia is the most likely to cause OVO7,20,24,32–37 and is a recognized risk factor for systemic vascular thrombosis, including ischemic heart disease and deep venous thrombosis (DVT).38,39 In addition to hyperhomocysteinemia, FVL and PTG heterozygosity result in a systemic hypercoagulable state and are major risk factors for large-vein thrombosis, and thus their role in OVO deserves careful attention.32,40–43
The FVL mutation involves a G-A substitution at nucleotide 1691 on the factor V gene, resulting in a procoagulant state caused by factor V’s resistance to inactivation by protein C.28,44 This is one of the commonest familial thrombophilias, witĥ ~5% of Caucasian populations being heterozygous for the mutation.45 Multiple studies, including case–control studies and a meta-analysis,21,24,26–28 have shown a significant increase in the prevalence of FVL in patients with OVO.
The PTG mutation is a G-A transition at nucleotide 20210 of the factor II gene and is associated with increased plasma prothrombin, resulting in a procoagulant state.42 Similar to FVL, the PTG mutation has a high-level carrier prevalence of 1%–4% and is more common among people of Caucasian descent.46 In addition, the PTG mutation, similar to the FVL mutation, is associated with venous thromboembolism (VTE),40,47,48 and recent literature has illustrated its significant role in mediating OVO.28,49
Thrombophilia and its role in OVO have received more attention in recent years, particularly in younger patients and in patients without an obvious cardiac, carotid, or embolic etiology. Particularly in Caucasian populations, the FVL and PTG mutations are some of the most frequent thrombophilic contributors to the development of VTE.40,47,50,51 Although the ophthalmologic literature has primarily focused on the pathophysiologic role that these thrombophilias play in ocular vascular thrombosis,8,16,18,21,22,25,30,36 it has not focused on the likelihood that the ocular thrombotic event is the patient’s first thrombotic event when associated with FVL and/or PTG, directing attention to familial thrombophilias in the kindred and prevention of subsequent thrombi in the proband and family. In the current study, our specific aim was to identify OVOs as first thrombotic events facilitated by FVL and/or PTG heterozygosity and to review the role that these heritable thrombophilias play in subsequent treatment, prophylaxis, and ramifications for patient and kindred.
Methods
The study was approved by the Jewish Hospital Institutional Review Board (ID 12-03). Informed consent was obtained from patients after the nature of the study was fully explained.
After NA-AION, branch or central retinal vein occlusion (BRVO, CRVO), RAO, or amaurosis fugax (AF) was diagnosed, 283 patients (172 with CRVO, 19 BRVO, 32 RAO, 41 AF, and 19 NA-AION) were sequentially referred from 1993 to 2015 by vitreoretinal specialists at the Cincinnati Eye Institute to our outpatient thrombosis research center. The diagnoses were established by complete ophthalmological evaluations during which the patients’ histories, visual deficits, and fundus abnormalities were ascertained and found to be typical of the ischemic events.
Patients with NA-AION exhibited segmental edema of the optic nerve head in eyes with little or no preexisting optic nerve cupping. Eyes with RVOs showed dilation of retinal veins (all veins if a CRVO was present and less than all veins if a BRVO was found) associated with intraretinal hemorrhages, retinal edema, and cotton wool spots limited in area by the drainage bed of the affected veins. Eyes with RAOs demonstrated retinal arterial narrowing, segmentation of the arterial blood column in some cases, and whitening of the retina due to opacification and thickening of the inner retina. In the case of a CRAO, a cherry red spot was seen in the macula. The fundus features of acute OVOs are stereotypical to the degree that confirmatory testing may not be necessary. Fluorescein angiography and optical coherence tomography were performed to corroborate the diagnosis depending on the preference of the referring ophthalmologist. In the cases of AION, measurements of erythrocyte sedimentation rates and C-reactive protein levels helped in the elimination of giant cell arteritis as the cause.
All 92 patients referred with ocular arterial disease (NA-AION, RAO, and AF) underwent carotid ultrasound and cardiac echocardiogram studies, and 18 were not entered into our analysis cohort because of documented arterial emboli. Excepting these 18 excluded arterial cases, the analysis cohort was prospectively evaluated in the sequence of their referral and included 265 cases (163 women and 102 men), 191 with ocular venous occlusion and 74 with ocular arterial occlusion.
The analysis cohort was divided into patients with venous (low-pressure, low-velocity) occlusion, including BRVO and CRVO, and those with arterial (high-pressure and high-velocity) occlusion, including NA-AION, RAO, and AF.
At the patients’ initial visit at our center, a detailed history was taken and physical examination was conducted. In particular, presence of hypertension, diabetes mellitus, and hyperlipidemia; reproductive history and pregnancy outcomes; and previous episodes of VTE, DVT, and/or pulmonary embolism (PE) were noted. In addition, the patients’ use of tobacco or hormonal therapy, as well as family history of thrombosis, was obtained. Lastly, the patients’ visual status was assessed at the time of the ocular event and at each subsequent visit with the retinal specialist.
At the initial visit, atherosclerotic risk factors were measured, serologic coagulation assays were done, and polymerase chain reaction (PCR) analyses for thrombophilia and hypofibrinolysis were performed. Atherosclerotic risk factors measured included age, body mass index, smoking history, blood pressure, hemoglobin A1c, glucose, homocysteine, and levels of triglycerides and cholesterol, including HDL and LDL cholesterol.24
PCR measures52 were used to measure G1691A factor V Leiden, G20210A prothrombin, methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C mutations, and the plasminogen activator inhibitor-1 4G/4G mutation. In addition, serologic measures of thrombophilia53 were used, including measurement of anticardiolipin antibodies immunoglobulin G (IgG) and IgM (ACLA IgM), antigenic protein C, total and free protein S, antithrombin III, lupus anticoagulant, factors VIII and XI, and homocysteine. All PCR and serologic measures were done as previously described.24
Healthy normal controls (n=110) were hospital employees, documented by interview and physical examination to be free of acute and chronic disease, including any history or evidence of OVO.
Statistical methods
All statistical analyses were performed using SAS V9.4 (SAS institute Inc, Cary, NC, USA).
Cases were compared to controls by Fisher’s exact test.
Sample size was estimated based on observed data of this report. To detect the difference of 50% having at least one of the seven thrombophilias in cases versus 20% in controls, there should be at least 39 subjects in each group for significance level 0.05 with power 80%.
Results
Central and branch retinal vein occlusion
Of the 191 RVO cases, 172 (90%) had CRVO and 19 (10%) BRVO; there were 116 (61%) women and 75 (39%) men (mean age ± standard deviation [SD]: 57±15 years; median age: 57 years).
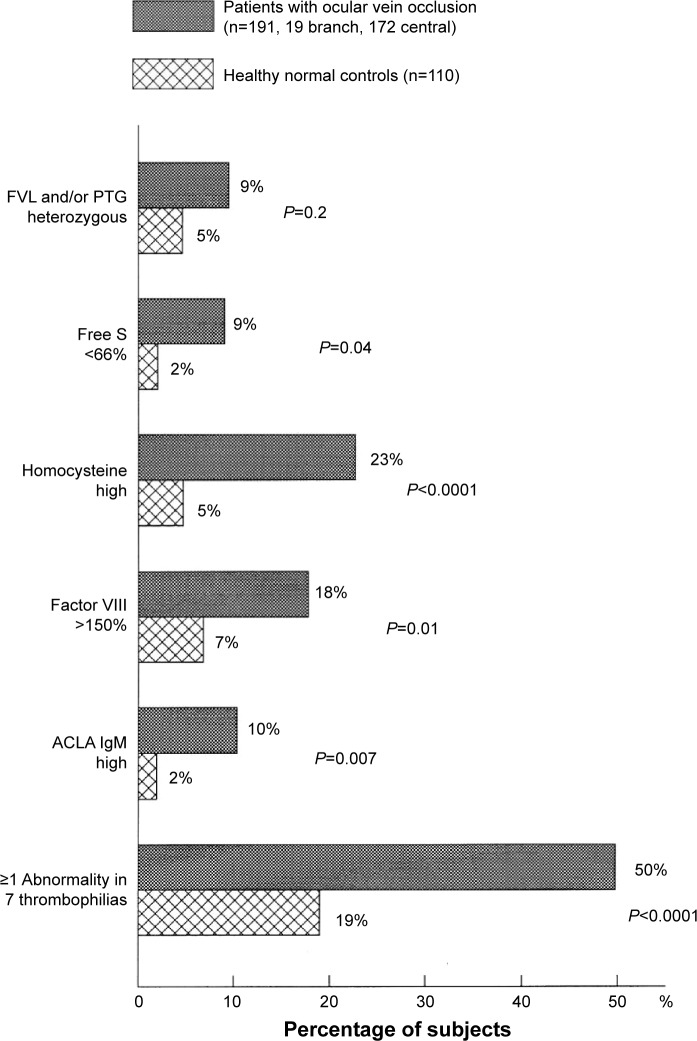
As displayed in Figure 1, the 191 RVO cases differed from controls in terms of low free protein S (9% vs 2%, respectively; P=0.04), high homocysteine (23% vs 5%, respectively; P<0.0001), high factor VIII (18% vs 7%, respectively; P=0.01), and ACLA IgM (10% vs 2%, respectively; P=0.007). Assessing the number of abnormalities in the seven thrombophilias (FVL, PTG, free protein S, homocysteine, factor VIII, factor XI, and ACLA IgM), 50% of the 191 RVO cases had ≥1 thrombophilic abnormality vs 19% of normal controls (P<0.0001, Figure 1). The difference between patients with RVO vs controls in comparing ≥1 abnormality of FVL and PTG mutations was not statistically significant (Figure 1).
Figure 1.
Thrombophilia in 191 patients with ocular vein occlusion (19 branch, 172 central) compared to 110 healthy normal controls without ocular venous or arterial thrombi.
Notes: The seven thrombophilias included FVL, PTG, free protein S, homocysteine, factor VIII, factor XI, and ACLA IgM.
Abbreviations: FVL, factor V Leiden; PTG, prothrombin gene; free S, free protein S; ACLA IgM, anticardiolipin antibody immunoglobulin M.
Retinal artery occlusion
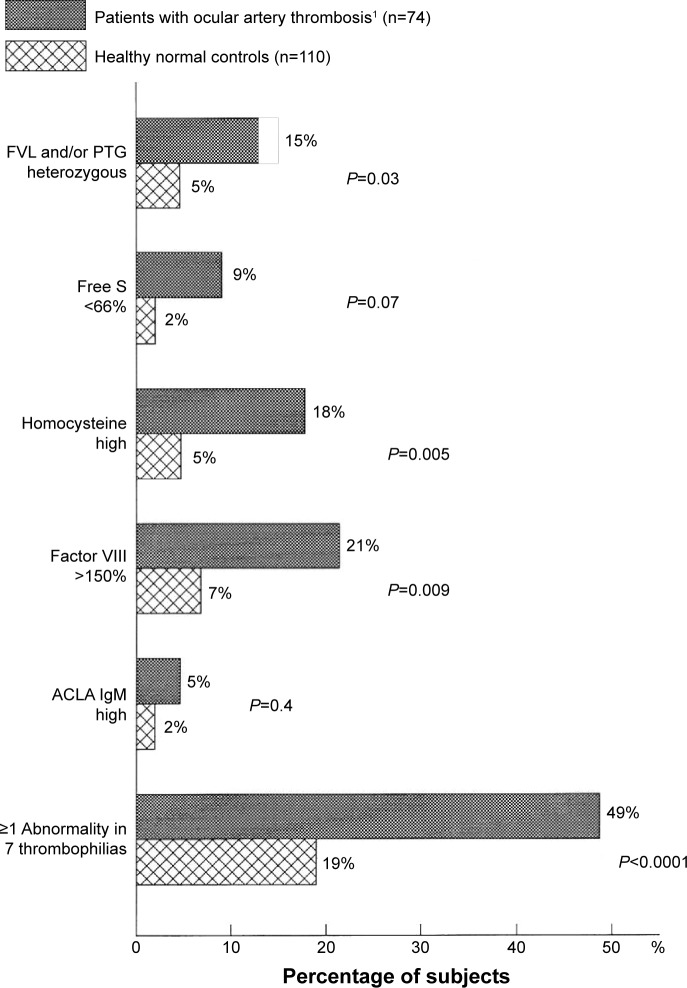
The 74 cases with RAO included 23 with CRAO, 32 AF, and 19 NA-AION, including 47 (64%) women and 27 (36%) men, with mean age ± SD of 54±16 (median: 55 years). As displayed in Figure 2, the 74 RAO cases differed from controls for ≥1 abnormality of FVL and PTG mutations (15% vs 5%, respectively; P=0.03). They also differed in having high levels of homocysteine (18% vs 5%, respectively; P=0.005) and factor VIII (21% vs 7%, respectively; P=0.009), as well as being marginally different in the low levels of free protein S (9% vs 2%, respectively; P=0.07). Assessing the number of abnormalities in the seven thrombophilias (FVL, PTG, free protein S, homocysteine, factor VIII, factor XI, and ACLA IgM), 49% of the 74 RAO cases had ≥1 thrombophilic abnormality vs 19% of normal controls (P<0.0001).
Figure 2.
Thrombophilia in 74 patients with ocular arterial occlusion (23 with central retinal artery occlusion, 32 with amaurosis fugax, and 19 with nonarteritic anterior ischemic optic neuropathy) compared to 110 healthy normal controls without ocular venous or arterial thrombi.
Notes: The seven thrombophilias included FVL, PTG, free protein S, homocysteine, factor VIII, factor XI, and ACLA IgM. 1Central retinal thrombosis (n=23), amaurosis fugax (n=32) and nonarteritic anterior isochemic optic neuropathy (n=19).
Abbreviations: FVL, factor V Leiden; PTG, prothrombin gene; free S, free protein S; ACLA IgM, anticardiolipin antibody immunoglobulin M.
Taken together, of the 265 patients with OVO (191 venous, 74 arterial), 29 (11%) had mutations of FVL, PTG, or both vs 5 (4.5%) of the 110 healthy normal controls (P=0.07).
FVL or PTG mutations
Table 1 shows the 29 (11%) cases with heterozygosity of FVL, PTG, or both, among the total cohort of 265 OVO cases. Among these 29 patients with FVL, PTG, or both, the initial ocular vascular occlusive event occurred before the age of 60 years in 18 (62%) cases, before age 50 years in 10 cases (34%), and before age 40 years in 5 cases (17%). The median age at the time of OVO in these 29 cases was 56 years. Of the 16 cases with FVL heterozygosity, nine (56%) were female and seven (44%) were male (mean age ± SD: 51±13 years; median: 52 years).
Table 1.
Characteristics of 29 patients with ocular vascular occlusion with FVL or PTG G20210A variant
| Serial number | Sex | Age (years) | Type of occlusion | FVL | PTG | First thrombotic event?# | Other thrombotic events?$ | Known family history of thrombosis?† | Visual outcome in given eye? | Estrogen/testosterone/clomiphene use?‡ |
|---|---|---|---|---|---|---|---|---|---|---|
| FVL | ||||||||||
| 1 | Male | 33 | RAO | Yes | No | Yes | No | Decreased | No | |
| 2 | Male | 72 | CRVO | Yes | No | Yes | No | No follow-up | No | |
| 3 | Male | 55 | CRVO | Yes | No | No (DVT) | Yes | No change | No | |
| 4 | Male | 64 | NA-AION | Yes | No | Yes | No | Decreased | No | |
| 5 | Male | 50 | CRVO | Yes | No | Yes | No | Blind | No | |
| 6 | Male | 41 | CRVO | Yes | No | Yes | No | No follow-up | No | |
| 7 | Male | 43 | CRVO | Yes | No | Yes | No | No follow-up | No | |
| 8 | Female | 52 | AF | Yes | No | Yes | Miscarriages | Yes | No change | No |
| 9 | Female | 68 | CRVO | Yes | No | Yes | Miscarriages | No | Decreased | No |
| 10 | Female | 38 | CRVO | Yes | No | Yes | Miscarriages | Yes | No change | Clomiphene |
| 11 | Female | 69 | CRVO | Yes | No | Yes | AVN, miscarriage | No | Blind | No |
| 12 | Female | 52 | CRVO | Yes | No | Yes | Yes | Decreased | No | |
| 13 | Female | 60 | CRVO | Yes | No | Yes | Yes | No change | Estrogen | |
| 14 | Female | 54 | CRVO | Yes | No | Yes | No | No change | Estrogen | |
| 15 | Female | 45 | RAO | Yes | No | Yes | Miscarriages | No | Decreased | No |
| 16 | Female | 25 | CRVO | Yes | No | Yes | Multifocal ON | No | Decreased | Estrogen |
| PTG | ||||||||||
| 1 | Male | 69 | NA-AION | No | Yes | Yes | Stroke | Yes | Decreased | No |
| 2 | Male | 68 | AF | No | Yes | No (DVT) | Yes | Blind | No | |
| 3 | Male | 59 | CRVO | No | Yes | Yes | No | Decreased | Testosterone | |
| 4 | Male | 50 | NA-AION | No | Yes | Yes | TIA | Yes | No change | No |
| 5 | Male | 29 | RAO | No | Yes | Yes | No | No follow-up | No | |
| 6 | Female | 63 | BRVO | No | Yes | Yes | Yes | Decreased | No | |
| 7 | Female | 58 | CRVO | No | Yes | Yes | No | Decreased | No | |
| 8 | Female | 82 | CRVO | No | Yes | Yes | No | Blind | No | |
| 9 | Female | 16 | CRVO | No | Yes | Yes | Yes | Decreased | No | |
| 10§ | Female | 77 80 |
RAO CRVO |
No | Yes | Yes | Miscarriages | No | Blind No change |
Estrogen Estrogen |
| 11 | Female | 75 | NA-AION | No | Yes | No (DVT) | Ischemic colitis | No | Decreased | Estrogen |
| 12 | Female | 56 | NA-AION | No | Yes | No (DVT-PE) | AVN | Yes | Blind | No |
| FVL + PTG | ||||||||||
| 1 | Female | 73 | BRVO | Yes | Yes | No (DVT) | Miscarriages | No | Decreased | No |
Notes:
First thrombotic event in patient, defined as no previous DVT or PE.
Other thrombotic events in patient, defined as events likely resulting from a hypercoagulable state other than DVT or PE, such as miscarriages, AVN, multifocal ON, stroke, or ischemic colitis.
Known family history of thrombosis, defined as an immediate family member with previous DVT or PE.
Use of estrogen, clomiphene, or testosterone therapy at the time of ocular event.
One patient with PTG mutation with two separate ocular events, RAO at the age of 77 years in the left eye and RVO at the age of 80 years in the right eye.
Abbreviations: AF, amaurosis fugax; AVN, avascular necrosis; BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; DVT, deep venous thrombosis; FVL, factor V Leiden; NA-AION, nonarteritic anterior ischemic optic neuropathy; ON, osteonecrosis; PE, pulmonary embolism; PTG, prothrombin gene; RAO, retinal artery occlusion; RVO, retinal vein occlusion; TIA, transient ischemic attack.
Fifteen (94%) of the 16 cases with FVL had OVO as their first thrombotic event, defined by no previous DVT or PE, while 1 (6%) had a previous initial thrombotic event (DVT) (Table 1). Of the 16 FVL heterozygotes, 6 (38%) experienced other thrombotic events, defined as events likely resulting from a hypercoagulable state other than DVT or PE, including recurrent miscarriages, osteonecrosis, ischemic stroke, and/or ischemic colitis (Table 1). Five (31%) of the 16 FVL heterozygotes had an immediate family member with a history of thrombosis, including DVT and/or PE.
At last follow-up visit, 2 (13%) of the 16 cases with FVL had no remaining vision in the affected eye, 6 (38%) had residual vision loss, 5 (31%) returned to baseline vision, and 3 (19%) were lost to follow-up (Table 1).
Of the 12 heterozygous PTG cases, 7 (58%) were female and 5 (42%) were male (mean age ± SD: 59±20 years; median: 61 years). Nine (75%) of the 12 cases had OVO as an initial thrombotic event, while 3 (25%) had a known previous thrombotic event (2 DVT, 1 DVT-PE) (Table 1). Five (42%) of the 12 cases experienced other thrombotic events, and 6 (50%) had an immediate family member with a history of thrombosis. At their last follow-up visit, four (33%) of the 12 cases heterozygous for PTG had no remaining vision in the affected eye, 6 (50%) had residual vision loss, 1 (8%) returned to baseline vision, and 1 (9%) was lost to follow-up (Table 1). One of the 12 PTG heterozygotes (#10) had an additional ocular event (CRVO) in the second eye and returned to baseline vision in that eye at her last follow-up (Table 1).
One case was heterozygous for both FVL and PTG mutations. She had BRVO with previous DVT and recurrent miscarriages. She had residual vision loss at last follow-up (Table 1).
Of the 29 cases with OVO found to have FVL and/or PTG mutations, 24 (83%) had OVO as a first thrombotic event without prior DVT and/or PE, and 12 (41%) had other thrombotic events including miscarriage, osteonecrosis, ischemic stroke, and/or ischemic colitis. Of these 12 cases with prior thrombotic complications, 10 (83%) were female, 7 (70%) of whom had had previous miscarriages.
Seventeen (59%) of 29 OVO cases with FVL and/or PTG mutation were females (Table 1). Of these 17 cases, 7 (41%) had experienced at least one miscarriage for previously unknown reasons, 6 (35%) were on estrogen hormone therapy at the time of the ocular event, and 1 (6%) was on clomiphene. One male patient with PTG heterozygosity (#3, Table 1) developed RVO 3 months after starting testosterone therapy.
Discussion
The ophthalmologist plays a critical role in the disposition and prognosis of patients who present with OVO, particularly when the OVO is the patient’s first thrombotic event. The most frequent pathoetiology for RAO and AF is largely embolic,54,55 which is frequently detectable in branch retinal artery occlusions but often cannot be detected via fundoscopy in ciliary artery occlusions, CRAOs, or AION. The 74 cases with RAO in the current study all had normal carotid and vertebral imaging and normal echocardiography, ruling out overt causes of thromboemboli. There is no easy way to rule out microscopic emboli as a cause of RAO, even when there is no detectable carotid or cardiac abnormality.
While cardioembolic investigation is warranted, there are numerous other contributing etiologies for OVO that must be considered. All patients should have a thorough assessment for cardiovascular and atherosclerotic risk factors, including screening for age, smoking history, hypertension, hyperlipidemia, and diabetes mellitus, all of which have been shown to have a significant, albeit nonspecific and nonsensitive, association with the development of OVO.7,9–23,54–56
Several reports,24,35,57 congruent with our current findings, have demonstrated the significant role of thrombophilia in the development of OVO and emphasize the importance of evaluating for thrombophilia in patients who present with OVO. In the current study, 49% of cases with arterial OVO and 50% with venous OVO had one or more of the seven major thrombophilias vs 19% of 110 healthy normal controls (P<0.0001). In cases of both venous and arterial occlusion, high levels of homocysteine and factor VIII were much more common than in healthy normal controls. Of the multiple thrombophilias, hyperhomocysteinemia has received the most attention as a risk factor for OVO,53 and the relationship between elevated levels of homocysteine and both large-vein thrombosis and OVO is well recognized.5,20,24,32–34,36–39 In addition to hyperhomocysteinemia, genetic mutations of FVL and PTG are major risk factors for large-vein thrombosis.32,40–43 Recent studies have found a high prevalence of FVL21,24,26–28 and PTG mutations58,59 in patients with OVO versus controls; however, some have not found the same.16,29,30–32
An ophthalmologist is in a unique position to diagnose familial and acquired thrombophilias in patients who present with a new OVO. This remains true even when the patient presents with no personal history of prior thrombosis. In the current study, 24 (83%) of 29 OVO patients found to have FVL and/or PTG mutations had no previous DVT, PE, and/or prior OVO. It is critical to diagnose these underlying, heritable thrombophilias in patients who present with OVO as a first thrombotic event to minimize local and systemic thrombotic morbidity and mortality, including significant vision loss. Approximately 7% of patients with RVO may have a contralateral OVO within 4 years,17 and the risk of visual impairment with OVO is evident in the current study, in which 6 (21%) of 29 cases with FVL and/or PTG heterozygosity had no remaining vision in the affected eye, and 13 (45%) had residual vision loss.
In addition, thrombophilia often causes potentially preventable VTE, DVT, PE,60 ischemic cerebral vascular accidents,61 osteonecrosis,62 and pregnancy loss.63 In our study, 12 (41%) of 29 OVO cases with an underlying FVL and/or PTG mutation had some form of VTE other than DVT and/or PE, such as miscarriage, osteonecrosis, ischemic stroke, and/or ischemic colitis. The risk of systemic thrombosis and OVO is particularly high if the patient is exposed to additional known prothrombotic risk factors, including estrogen or testosterone hormone therapy, pregnancy, surgery, immobilization, or smoking.27,40
Of particular note, 7 (41%) of 17 female patients with FVL and/or PTG heterozygosity presenting with OVO shared a history of at least one unexplained miscarriage. Patients with increased exposure to estrogens, including hormone therapy or pregnancy, in combination with thrombophilia, are at a much higher risk of thrombosis. Six (35%) of 17 females in this study who developed OVO were on estrogen therapy, and 1 (6%) was on clomiphene. Case reports have suggested that clomiphene may predispose individuals to RVO, especially patients with underlying risk factors such as thrombophilia.64 Other studies have shown the miscarriage rate in patients with RVO to be 24%–28%, significantly greater compared to the national figure of 15.7%.24,53,65 These findings illustrate the importance of discovering a thrombophilic state for the patient and family to avoid unnecessary risk exposure, such as hormone therapy, and to allow for close monitoring during high-risk periods, including pregnancy.
Most thrombophilic mutations, including FVL and PTG mutations, are autosomal dominant and highly penetrant. Diagnosing an underlying thrombophilia has important implications, not only for the patient, but for the patient’s family as well. Of the 29 OVO cases in this study with the FVL and/or PTG mutation, 11 (38%) had immediate family members with a history of DVT and/or PE. OVOs serve as a gateway for physicians to discover an undiagnosed heritable thrombophilia, with significant implications for the patient and his or her family including the need for treatment, familial screening, and education regarding the avoidance of additional thrombotic risk factors.
In patients who are young, have an unexpected OVO, and/or have a personal or family history of thrombosis, the physician may discover a hypercoagulable state if appropriately evaluated.20 One study found that up to 84% patients with OVO in which noncoagulation etiologies were ruled out were found to have thrombophilia.9 This finding is of major importance for the patient and the patient’s family to ensure that appropriate medical and lifestyle measures are taken to minimize morbidity and mortality.
While elevated homocysteine53 and antiphospholipid syndrome66 have received the most attention regarding thrombophilias associated with OVO, our study demonstrates the importance of testing for the common and highly prothrombotic familial thrombophilias, FVL and PTG mutations, and illustrates how FVL and PTG heterozygosity may present with OVO as a first thrombotic event. It is important for the ophthalmologist to diagnose underlying coagulation disorders as both an etiology for OVO and as a window to family screening and preventive therapy for the family and the proband.
Acknowledgments
This work was supported in part by the Lipoprotein Research Fund of the Jewish Hospital of Cincinnati.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726–732. doi: 10.1001/archopht.124.5.726. [DOI] [PubMed] [Google Scholar]
- 2.David R, Zangwill L, Badarna M, Yassur Y. Epidemiology of retinal vein occlusion and its association with glaucoma and increased intraocular pressure. Ophthalmologica. 1988;197:69–74. doi: 10.1159/000309923. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–518. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 4.Rogers S, McIntosh RL, Cheung N, et al. International Eye Disease Consortium The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–319. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic ischemic optic neuropathy: population-based study in the state of Missouri and Los Angeles County, California. J Neuroophthalmol. 1994;14:38–44. [PubMed] [Google Scholar]
- 6.Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye. 2013;27:688–697. doi: 10.1038/eye.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karia N. Retinal vein occlusion: pathophysiology and treatment options. Clin Ophthalmol. 2010;4:809–816. doi: 10.2147/opth.s7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehak M, Wiedemann P. Retinal vein thrombosis: pathogenesis and management. J Thromb Haemost. 2010;8:1886–1894. doi: 10.1111/j.1538-7836.2010.03909.x. [DOI] [PubMed] [Google Scholar]
- 9.Bick RL, Alfar H, Goedecke C. Thrombophilic causes of retinal vascular thrombosis: etiology and treatment outcomes. Clin Appl Thromb Hemost. 2002;8:315–318. doi: 10.1177/107602960200800402. [DOI] [PubMed] [Google Scholar]
- 10.Beaumont PE, Kang HK. Clinical characteristics of retinal venous occlusions occuring at different sites. Br J Ophthalmol. 2002;86:572–580. doi: 10.1136/bjo.86.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hitchings RA, Spaeth GL. Chronic retinal vein occlusion in glaucoma. Br J Ophthalmol. 1976;60:694–699. doi: 10.1136/bjo.60.10.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dryden RM. Central retinal vein occlusions and chronic simple glaucoma. Arch Ophthalmol. 1965;73:659–663. doi: 10.1001/archopht.1965.00970030661012. [DOI] [PubMed] [Google Scholar]
- 13.Group EDC-CS Risk factors for central retinal vein occlusion. Arch Ophthalmol. 1996;114:545–554. [PubMed] [Google Scholar]
- 14.Mohamed Q, McIntosh RL, Saw SM, Wong TY. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507–519. doi: 10.1016/j.ophtha.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013;120:362–370. doi: 10.1016/j.ophtha.2012.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weger M, Renner W, Pinter O, et al. Role of factor V Leiden and prothrombin 20210A in patients with retinal artery occlusion. Eye (Lond) 2003;17:731–734. doi: 10.1038/sj.eye.6700495. [DOI] [PubMed] [Google Scholar]
- 17.Hayreh SS, Zimmerman B, McCarthy MJ, Podhajsky P. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61–77. doi: 10.1016/s0002-9394(00)00709-1. [DOI] [PubMed] [Google Scholar]
- 18.Prisco D, Marcucci R, Bertini L, Gori AM. Cardiovascular and thrombophilic risk factors for central retinal vein occlusion. Eur J Intern Med. 2002;13:163–169. doi: 10.1016/s0953-6205(02)00025-0. [DOI] [PubMed] [Google Scholar]
- 19.Recchia FM, Brown GC. Systemic disorders associated with retinal vascular occlusion. Curr Opin Ophthalmol. 2000;11:462–467. doi: 10.1097/00055735-200012000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Backhouse O, Parapia L, Mahomed I, Lee D. Familial thrombophilia and retinal vein occlusion. Eye (Lond) 2000;14:13–17. doi: 10.1038/eye.2000.4. [DOI] [PubMed] [Google Scholar]
- 21.Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Thromb Haemost. 2008;99:925–929. doi: 10.1160/TH07-11-0658. [DOI] [PubMed] [Google Scholar]
- 22.Cheung N, Klein R, Wang JJ, et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: the multiethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. 2008;49:4297–4302. doi: 10.1167/iovs.08-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Mahoney P, Wong T, Ray J. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692–699. doi: 10.1001/archopht.126.5.692. [DOI] [PubMed] [Google Scholar]
- 24.Glueck CJ, Wang P, Hutchins R, Petersen MR, Golnik K. Ocular vascular thrombotic events: central retinal vein and central retinal artery occlusions. Clin Appl Thromb Hemost. 2008;14:286–294. doi: 10.1177/1076029607304726. [DOI] [PubMed] [Google Scholar]
- 25.Yau JW, Lee P, Wong TY, Best J, Jenkins A. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38:904–910. doi: 10.1111/j.1445-5994.2008.01720.x. [DOI] [PubMed] [Google Scholar]
- 26.Williamson TH, Rumley A, Lowe GD. Blood viscosity, coagulation, and activated protein C resistance in central retinal vein occlusion: a population controlled study. Br J Ophthalmol. 1996;80:203–208. doi: 10.1136/bjo.80.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson J, Olafsdottir E, Bauer B. Activated protein C resistance in young adults with central retinal vein occlusion. Br J Ophthalmol. 1996;80:200–202. doi: 10.1136/bjo.80.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Ami R, Zeltser D, Leibowitz I, Berliner SA. Retinal artery occlusion in a patient with factor V Leiden and prothrombin G20210A mutations. Blood Coagul Fibrinolysis. 2002;13:57–59. doi: 10.1097/00001721-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Aras S, Yilmaz G, Alpas I, Baltaci V, Tayanç E, Aydin P. Retinal vein occlusion and factor V Leiden and prothrombin 20210 G: A mutations. Eur J Ophthalmol. 2001;11:351–355. doi: 10.1177/112067210101100406. [DOI] [PubMed] [Google Scholar]
- 30.Demirci FY, Güney DB, Akarçay K, et al. Prevalence of factor V Leiden in patients with retinal vein occlusion. Acta Ophthalmol Scand. 1999;77:631–633. doi: 10.1034/j.1600-0420.1999.770605.x. [DOI] [PubMed] [Google Scholar]
- 31.Larsson J, Hillarp A. The prothrombin gene G20210A mutation and the platelet glycopprotein IIIa polymorphism PIA2 in patients with central retinal vein occlusion. Thromb Res. 1999;96:323–327. doi: 10.1016/s0049-3848(99)00111-5. [DOI] [PubMed] [Google Scholar]
- 32.Janssen MCH, den Heijer M, Cruysberg JRM, Wollersheim H, Bredie SJH. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? J Thromb Haemost. 2005;93:1021–1026. doi: 10.1160/TH04-11-0768. [DOI] [PubMed] [Google Scholar]
- 33.Fegan CD. Central retinal vein occlusion and thrombophilia. Eye (Lond) 2002;16:98–106. doi: 10.1038/sj.eye.6700040. [DOI] [PubMed] [Google Scholar]
- 34.Turello M, Pasca S, Daminato R, et al. Retinal vein occlusion: evaluation of “classic” and “emerging” risk factors and treatment. J Thromb Thrombolysis. 2010;29:459–464. doi: 10.1007/s11239-009-0384-5. [DOI] [PubMed] [Google Scholar]
- 35.Sottilotta G, Oriana V, Latella C, et al. Role of hyperhomocystinemia in retinal vascular occlusive disease. Clin Appl Thromb Hemost. 2007;13:104–107. doi: 10.1177/1076029606296423. [DOI] [PubMed] [Google Scholar]
- 36.Cahill MT, Stinnett SS, Fekrat S. Meta-analysis of plasma homocysteine, serum folate, serum vitamin B(12), and thermolabile MTHFR genotype as risk factors for retinal vascular occlusive disease. Am J Ophthalmol. 2003;136:1136–1150. doi: 10.1016/s0002-9394(03)00571-3. [DOI] [PubMed] [Google Scholar]
- 37.Biousse V, Newman NJ, Sternberg PJ. Retinal vein occlusion and transient monocular visual loss associated with hyperhomocystinemia. Am J Ophthalmol. 1997;124:257–260. doi: 10.1016/s0002-9394(14)70800-1. [DOI] [PubMed] [Google Scholar]
- 38.McCully KS. Homocysteine and vascular disease. Nat Med. 1996;2:386–389. doi: 10.1038/nm0496-386. [DOI] [PubMed] [Google Scholar]
- 39.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simsek E, Yesilyurt A, Pinarli F, Eyerci N, Ulus AT. Combined genetic mutations have remarkable effect on deep venous thrombosis and/or pulmonary embolism occurence. Gene. 2013;536:171–176. doi: 10.1016/j.gene.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Seligsohn U, Lubetsky A. Genetic susceptibility to venous thrombosis. N Engl J Med. 2001;344:1222–1231. doi: 10.1056/NEJM200104193441607. [DOI] [PubMed] [Google Scholar]
- 42.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–3703. [PubMed] [Google Scholar]
- 43.Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993;342:1503–1506. doi: 10.1016/s0140-6736(05)80081-9. [DOI] [PubMed] [Google Scholar]
- 44.Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 45.Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA. 1997;277:1305–1307. [PubMed] [Google Scholar]
- 46.Rosendaal FR, Doggen CJ, Zivelin A, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost. 1998;79:706–708. [PubMed] [Google Scholar]
- 47.Zhou X, Qian W, Li J, et al. Who are at risk for thromboembolism after arthroplasty? A systematic review and meta-analysis. Thromb Res. 2013;132:531–536. doi: 10.1016/j.thromres.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Tug E, Aydin H, Kaplan E, Dogruer D. Frequency of genetic mutations associated with thromboembolism in the Western Black Sea Region. Intern Med. 2011;50:17–21. doi: 10.2169/internalmedicine.50.4144. [DOI] [PubMed] [Google Scholar]
- 49.Glueck CJ, Wang P. Ocular vascular thrombotic events: a diagnostic window to familial thrombophilia (compound factor V Leiden and prothrombin gene heterozygosity) and thrombosis. Clin Appl Thromb Hemost. 2009;15:12–18. doi: 10.1177/1076029608321438. [DOI] [PubMed] [Google Scholar]
- 50.Weingarz L, Schwonberg J, Schindewolf M, et al. Prevalence of thrombophilia according to age at the first manifestation of venous thromboembolism: results from the MAISTHRO registry. Br J Haematol. 2013;163:655–665. doi: 10.1111/bjh.12575. [DOI] [PubMed] [Google Scholar]
- 51.de Jong PG, Goddijn M, Middeldorp S. Testing for inherited thrombophilia in recurrent miscarriage. Semin Reprod Med. 2011;29:540–547. doi: 10.1055/s-0031-1293207. [DOI] [PubMed] [Google Scholar]
- 52.Glueck CJ, Freiberg RA, Wang P. Heritable thrombophilia-hypofibrinolysis and osteonecrosis of the femoral head. Clin Orthop Relat Res. 2008;466:1034–1040. doi: 10.1007/s11999-008-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glueck CJ, Hutchins RK, Jurantee J, Khan Z, Wang P. Thrombophilia and retinal vascular occlusion. Clin Ophthalmol. 2012;6:1377–1384. doi: 10.2147/OPTH.S34627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma S, Naqvi A, Sharma SM, Cruess AF, Brown GC. Transthoracic echocardiographic findings in patients with acute retinal arterial obstruction. A retrospective review. Retinal Emboli of Cardiac Origin Group. Arch Ophthalmol. 1996;114:1189–1192. doi: 10.1001/archopht.1996.01100140389004. [DOI] [PubMed] [Google Scholar]
- 55.Sharma S. The systemic evaluation of acute retinal artery occlusion. Curr Opin Ophthalmol. 1998;9:1–5. doi: 10.1097/00055735-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Salomon O, Huna-Baron R, Moisseiev J, et al. Thrombophilia as a cause for central and branch retinal artery occlusion in patients without an apparent embolic source. Eye (Lond) 2001;15:511–514. doi: 10.1038/eye.2001.164. [DOI] [PubMed] [Google Scholar]
- 57.Greiner K, Hafner G, Dick B, Peetz D, Prellwitz W, Pfeiffer N. Retinal vascular occlusion and deficiencies in the protein C pathway. Am J Ophthalmol. 1999;128:69–74. doi: 10.1016/s0002-9394(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 58.Kapur RK, Mills LA, Spitzer SG, Hultin MB. A prothrombin gene mutation is significantly associated with venous thrombosis. Arterioscler Thromb Vasc Biol. 1997;17:2875–2879. doi: 10.1161/01.atv.17.11.2875. [DOI] [PubMed] [Google Scholar]
- 59.Gurgey A, Haznedaroglu IC, Egesel T, et al. Two common genetic thrombotic risk factors: factor V Leiden and prothrombin G20210A in adult Turkish patients with thrombosis. Am J Hematol. 2001;67:107–111. doi: 10.1002/ajh.1087. [DOI] [PubMed] [Google Scholar]
- 60.Rodeghiero F, Tosetto A. Activated protein C resistance and factor V Leiden mutation are independent risk factors for venous thromboembolism. Ann Intern Med. 1999;130:643–650. doi: 10.7326/0003-4819-130-8-199904200-00004. [DOI] [PubMed] [Google Scholar]
- 61.Glueck CJ, Fontaine RN, Wang P. Interaction of heritable and estrogen-induced thrombophilia: possible etiologies for ischemic optic neuropathy and ischemic stroke. Thromb Haemost. 2001;85:256–259. [PubMed] [Google Scholar]
- 62.Glueck CJ, Freiberg RA, Fontaine RN, Sieve-Smith L, Wang P. Anticoagulant therapy for osteonecrosis associated with heritable hypofibrinolysis and thrombophilia. Expert Opin Investig Drugs. 2001;10:1309–1316. doi: 10.1517/13543784.10.7.1309. [DOI] [PubMed] [Google Scholar]
- 63.Glueck CJ, Pranikoff J, Aregawi D, et al. The factor V Leiden mutation, high factor VIII, and high plasminogen activator inhibitor activity: etiologies for sporadic miscarriage. Metabolism. 2005;54:1345–1349. doi: 10.1016/j.metabol.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 64.Viola MI, Meyer D, Kruger T. Association between clomiphene citrate and visual disturbances with special emphasis on central retinal vein occlusion: a review. Gynecol Obstet Invest. 2010;71:73–76. doi: 10.1159/000319497. [DOI] [PubMed] [Google Scholar]
- 65.Ventura SJ, Mosher WD, Curtin SC, Abma JC, Henshaw S. Trends in pregnancies and pregnancy rates by outcome: estimates for the United States, 1976–1996. Vital Health Stat 21. 2000;21:1–47. [PubMed] [Google Scholar]
- 66.Yang P, Kruh JN, Foster CS. Antiphospholipid antibody syndrome. Curr Opin Ophthalmol. 2012;23:528–532. doi: 10.1097/ICU.0b013e328358b937. [DOI] [PubMed] [Google Scholar]