Abstract
Background. Tuberculosis (TB) prevalence is high in correctional facilities in southern Africa. With support from local South African nongovernmental organizations, the South African Department of Correctional Services initiated a program of systematically screening newly admitted and current inmates for symptoms followed by GeneXpert Mycobacterium tuberculosis (MTB)/rifampicin (Rif) for microbiologic testing of symptomatic inmates.
Methods. We conducted a program evaluation during a 5-month window describing program reach, effectiveness, adoption within the facilities, cost, and opportunities for sustainability. This evaluation included 4 facilities (2 large and 2 smaller) with a total daily census of 20 700 inmates.
Results. During the 5-month evaluation window from May to September 2013, 7426 inmates were screened at the 4 facilities. This represents screening 87% of all new admits (the remaining new admits were screened by correctional staff only and are not included in these statistics) and 23% of the daily inmate census, reaching 55% of the overall screening target as calculated per annum. The reach ranged from 57% screened during these 5 months at one of the smaller facilities to 13% at the largest facility. Two hundred one cases of pulmonary TB were diagnosed, representing 2.1% of the screened population; 93% had documented initiation of TB treatment. The cost per TB case identified was $1513, excluding treatment costs (with treatment costs it was $1880).
Conclusions. We reached a large number of inmates with high-volume screening and effectively used GeneXpert MTB/Rif to diagnose pulmonary TB and rapidly initiate treatment. The cost was comparable to other screening programs.
Keywords: Africa, correctional facility, implementation, systematic screening, tuberculosis
Correctional facilities are recognized as settings with a high prevalence of tuberculosis (TB) and risk of TB transmission [1]. Recent studies from South Africa and Zambia have reported point prevalence of laboratory-confirmed undiagnosed TB among inmates of 2.9% and 3.8% [2, 3]. In addition to morbidity from the TB, there is a concern that correctional settings may contribute significantly to the wider TB epidemic. Modeling suggests between 2.7% and 17.2% of community TB in low- and middle-income countries is attributable to transmission from individuals infected while detained [1].
In response to a high prevalence of TB in facilities and spread to communities, multiple organizations have called for systematic TB screening on entry into correctional facilities and/or during detention [4, 5]. However, correctional facilities present multiple logistic and organizational obstacles to systematic screening including the focus on security, frequent transfers from facility to facility complicating linking positive results to inmates, competing health and corrections priorities, and perceptions of screening requiring considerable staff time.
Streamlined symptom screening followed by on-site rapid laboratory testing provides a potential option to increase screening in correctional facilities. In particular, symptom screening coupled with GeneXpert Mycobacterium tuberculosis (MTB)/rifampicin (Rif) (Xpert; Cepheid Inc, Sunnyvale, CA) may provide an option to improve TB control. Xpert uses nucleic acid amplification technology for the diagnosis of TB and rifampin resistance from sputa and other specimen types [6]. The system can be placed on a desktop without need for a biosafety cabinet, and it provides results within 2 hours. Compared with the gold standard of sputum culture, the sensitivity of Xpert is approximately 98% for smear-positive and 68% for smear-negative culture positive specimens [7].
To improve implementation of TB screening and control within correctional facilities, the South African Department of Correctional Services (DCS) in partnership with local nongovernmental organizations launched a systematic TB screening and diagnosis program. A major component of this program was systematic symptom screening coupled with the use of on-site Xpert for microbiologic diagnosis for those who were symptomatic. In this study, we evaluate the implementation of this program using a widely used evaluation framework, RE-AIM [8], to describe reach, effectiveness, adoption, implementation (fidelity to strategy and cost), and maintenance in 4 correctional facilities in South Africa.
DESCRIPTION AND METHODS
Setting
Systematic TB screening was implemented at 2 large and 2 smaller facilities with an estimated daily census of 10 300, 7500, 1700, and 1200 inmates, respectively (20 700 total). The facilities had a mix of sentenced and remand inmates and variable local human immunodeficiency virus (HIV) prevalence (Table 1) [9].
Table 1.
Correctional Facility Characteristics
| Facility | Total Facility Population | Remand Inmates | Sentenced Inmates | Facility Characteristics | Human Immunodeficiency Virus Prevalence in Surrounding Communities (%) [9] |
|---|---|---|---|---|---|
| A | 10 300 | 4200 | 6100 | Male medium security, female, juvenile | 16.9 |
| B | 7500 | 3580 | 3920 | Male medium security, male maximum security, female | 12.4 |
| C | 1700 | 0 | 1700 | Male medium security, male maximum security, juvenile | 12.4 |
| D | 1200 | 370 | 830 | Male medium security, male maximum security, female, juvenile | 7.4 |
Screening
The key innovative implementation components of this program are listed in the following sections.
Dedicated On-site Screening Staff
We deployed screening teams paid by the local nongovernmental organization comprised of 3 full-time team members (a nurse, laboratory technician, and screening assistant) to each facility; there were no differences in the constitution or salaries of the teams per facility. These teams worked together with DCS nurses, DCS security officers, and inmate peer educators to organize and perform screening activities (universal screening described below).
Universal Systematic Screening
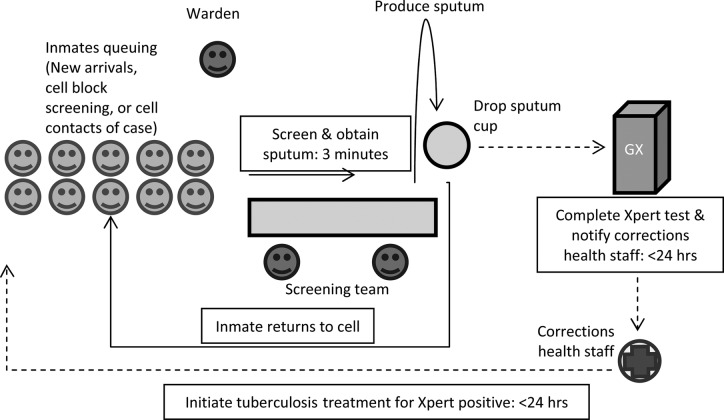
We strengthened prior DCS screening activities by making screening at facility entry a procedure separate from multiple other routine intake activities and by adding systematic cell block by cell block screening among already detained inmates. Our goal was to screen all inmates newly entering the facility (as required by South African law when offered) and to offer screening to all inmates already within the facility at least once every 12 months. In addition, cell-block contacts of an inmate with TB underwent screening. Cell block screening was achieved by inviting all inmates in a cell block to come for screening in nearby area. Cell block screening was performed during a 4-hour window (9:00 AM–1:00 PM) when inmates were allowed to leave their cells. Screening started with inmates queuing near a screening station (Figure 1). Inmates then presented individually for screeners to complete a 1-page questionnaire regarding locator information (name and cell block), TB treatment history, and symptoms. The questionnaire was based on the World Health Organization 4-symptom screen comprising of any of cough, fever, night sweats, or weight loss, to identify individuals for microbiologic testing using Xpert [10]. All inmates with 1 or more of the 4 symptoms were instructed on how to produce a sputum sample, given a sputum bottle, and instructed to go to a ventilated area for sputum production. Human immunodeficiency virus testing was available during TB screening activities, but it has not been included as part of this evaluation of systematic TB case finding.
Figure 1.
Schematic of tuberculosis screening flow (note that human immunodeficiency virus testing was also offered but not represented in this figure).
Mobilization
Peer educators and corrections officers provided information regarding TB disease and TB screening to inmates starting 2–5 days before a screening.
On-site Microbiologic Testing
We installed 1 GeneXpert module-4 machine in each correctional facility to allow for on-site microbiologic testing. The GeneXpert machine was operated by a member of the screening team. Quality assurance procedures were as follows: (1) the GeneXpert machines were calibrated by the manufacturer before installation at the sites; (2) during each test, the GeneXpert machine uses a sample-processing control (included in the Xpert cartridge), which ensures that a specimen is correctly processed; and (3) the trained screening team member further recalibrated the machine after every 2000 tests. In addition, weekly reporting that included Xpert results allowed for rapid identification of fluctuations in Xpert error rates. In one situation, these reports led to identification of a problem and replacement of the GeneXpert machine.
Inmates with positive Xpert results were considered to have pulmonary TB and were referred to the correctional health services for management according to the South African Department of Health Tuberculosis Control Programme Guidelines [11]. Inmates with drug-susceptible TB were transferred to the correctional facility hospital; inmates with drug-resistant TB were transferred to the closest district hospital with a multidrug-resistant TB unit. Inmates with negative Xpert results during the systematic screening had no additional evaluation. Inmates who wished to have diagnosis and management for specific symptoms could access the facility infirmary. Inmates with chronic cough or other chronic TB-related symptoms were referred to the facility infirmary for further evaluation by inmate peer educators.
Oversight
Monthly screening targets were chosen by a team from each correctional facility and the partner nongovernmental organization before the start of full implementation. To perform ongoing program monitoring and optimization, weekly reports of number screened and screening results were generated 2 work days after the end of the week. Program adjustments were made through weekly on-site and telephonic discussions with the screening team and DCS health personnel.
Ethics
We adhered to the principles of the Declaration of Helsinki and to special protections of vulnerable populations (inmates). Because this was an evaluation of implementation without any a priori research questions or other procedures, informed consent was not obtained. All evaluation data were anonymous and analyzed on aggregate. The South African Department of Correctional Services Research Review Board and the University of Witwatersrand Human Research Ethics Committee approved this evaluation.
Evaluation
To assess implementation, we used the multidimensional RE-AIM framework [8]. The RE-AIM components (Table 2) are listed in the sections listed below.
Table 2.
RE-AIM Framework [8]
| Dimension | Unit | Outcome Over 5 Months |
|---|---|---|
| Reach | Individual | 24% screened (range of 5%–57% by facility) |
| Efficacy/Effectiveness | Individual | 2.7% diagnosed with tuberculosis compared with historical prevalence estimate of 2.9% in a South African facility |
| Adoption | Facility | Implemented across the 4 facilities |
| Implementation | Facility/Department of Correctional Services | Maintained high fidelity to tuberculosis screening, testing turnaround, treatment initiation, and weekly reporting. Adaptations to plan occurred due to security issues. The total annual cost was $730 000. The cost per tuberculosis case identified was $1513.27. |
| Maintenance | Facility/Department | There was no attrition in participation by health staff in the systematic screening during the project. The Government of South Africa is expanding program throughout the correctional system |
Reach
We assessed reach by the proportion of the total inmate population who underwent screening and the proportion of those in a cell block who were screened. We did not collect specific data on individuals who declined screening from a cell block; we determined the number declining by subtracting the number screened from the total cell block census. The a priori planned reach was to screen all newly admitted inmates during the arrival processing activities and 100% of current inmates every 12 months.
Effectiveness
Although the use of systematic screening as a component of TB control is supported by evidence and guidelines [5], Xpert has not been previously used for mass screening in a correctional setting. Thus, it was important to evaluate the effectiveness for TB diagnosis in this nonmedical setting of individuals not seeking healthcare. As a proxy for Xpert diagnostic effectiveness, we compared our identified TB cases with a recent liquid-culture based TB prevalence survey from a single correctional facility in South Africa and a point prevalence study from a correctional center in Zambia [2, 3].
Adoption
We have previously observed that lack of ongoing participation from stakeholders impedes adoption. Thus, we have used continued participation in planning meetings by DCS leadership as a proxy of organizational adoption. We did this by retrospectively interviewing the nongovernment organization screening team members regarding availability of DCS staff for scheduled meetings and reviewing the routinely maintained meeting attendance registers.
Implementation
We assessed 2 key components of implementation: fidelity to the program procedures and cost. We measured fidelity as screening by trained professionals, collection of sputum from symptomatic individuals, on-site laboratory testing with 24-hour turnaround for results, and treatment initiation within 24 hours. Cost included overall cost and cost per TB case diagnosed (see below for costing).
Maintenance
We do not have a long-term track record of maintenance of program activities. Thus, we used consistent involvement of DCS health personnel in screening activities during the duration of the program and the continued allocation of funding from the National DCS level as proxy measures to suggest an increased chance of local and policy-level maintenance of the screening activities.
Costs
We assessed the cost for the screening program, including TB treatment from a health system perspective, using a mixed-ingredients approach [12]. Fixed costs (GeneXpert machine, furniture, air conditioner, training) were based on the purchase invoice and were annualized based on expected lifespan, and 3% was discounted for future years. The GeneXpert machine lifespan was estimated to be 5 years, and lifespan of laptop computers and air conditioning units was estimated to be 3 years. Recurring costs were determined using invoices. Recurring costs were split into labor, laboratory consumables, overhead, and project administration. Labor costs included nongovernmental organization head office staff (accountant, program director, and project manager); ie, screening teams, DCS healthcare workers, prison wardens, and inmate peer educators. The DCS labor attributable to the screening program was determined by time-and-motion observations. Staff cost attributable to screening was estimated from the hours worked on the screening activity as a proportion of the estimated total monthly hours worked and monthly salary based on the 50th percentile of the South African Department of Public Service's remuneration rates for each personnel level. Although peer educators were not remunerated, we attributed costs based on community healthcare-worker stipends to account for the resources required for this project. Salaries for staff from the nongovernmental organization were obtained directly from payroll data as total cost to the company. We reported DCS labor costs separately and estimated peer educator costs and nongovernmental organization screening team labor costs. Laboratory costs were estimated to include all the necessary consumables such as Xpert cartridges, sputum bottles, latex gloves, N95 masks, cleaning supplies, waste bags, and sterilizing agents. Tuberculosis treatment costs for drug-sensitive and multidrug-resistant (MDR) TB were identified from the literature and assuming outpatient care [13, 14]. To be conservative, we assumed that all rifampin resistant cases were MDR TB. All costs were adjusted to January 1, 2013 real US dollars.
Cost per participant screened and cost per TB case were calculated based on the total project cost during the 5-month evaluation period and the total number of inmates screened or diagnosed with TB. We completed one-way sensitivity analyses to assess the impact of changes in base-case parameters (consumable, equipment, labor costs, and diagnostic accuracy parameters) on outcome estimates. In addition, we calculated the cost with use of Xpert testing for screening in place of a 2-step process of symptom screen followed by Xpert testing.
RESULTS
Reach
During the evaluation period from May to September 2013, 8602 TB screening episodes occurred among 7426 inmates in the 4 facilities. Of these, 2452 (33%) were among new admissions and 4974 (67%) among current inmates. This quantity represents approximately 87% of the 2824 newly admitted inmates and 23% of the daily inmate census in these facilities during the 5-month evaluation period. Reach varied considerably by facility with 975 of 1700 (57%), 65 of 1200 (5%), 2623 of 7800 (34%), and 1311 of 10 300 current inmates (13%) screened in each of the 4 facilities, respectively. In the facility with 7800 inmates, the screening team only completed cell-block screening and did not perform screening on new admissions because of a request from DCS to focus on current inmates. The facility with only 5% screened was the smallest and had nearly completed all cell-block screening activities by the beginning of the evaluation period because it was the pilot site and began screening before the other sites. Extrapolating to a 12-month period with similar performance for all 4 facilities, we estimate that 55% of all resident inmates would be screened (short of the goal of 100%).
Effectiveness
For those inmates agreeing to screening, 1 or more symptoms were reported during 5350 (62%) screening episodes. Sputum was received for Xpert testing from 5095 of these inmates (95%); the remaining inmates were unable to produce a sample. Of these sputum samples, 150 (2.6%) produced errors when tested; additional sputa was sought from inmates with a test error. Xpert test results were available for 4945 (92.4%) of those with a positive symptom screen. An additional 630 asymptomatic inmates also had Xpert testing because they had contact with a pulmonary TB case. From all the Xpert tests, 201 (2.7%) pulmonary TB cases were identified, 2 (1%) of which were rifampin resistant. The proportion of screened inmates diagnosed with TB varied by facility from 1.3% of those screened in the facility with 7500 inmates to 4.2% in the facility with 1200 inmates. The symptom screen was not highly specific in identifying individuals with a positive Xpert test because there were 201 Xpert positives (4.1%) among 4945 sputa tested. The GeneXpert machines in the 2 larger facilities were in near constant operation during work hours, whereas at the 2 smaller facilities the GeneXpert machines were underutilized. Overall, approximately 50 samples were assayed per work day during this evaluation period.
Adoption
Over this period, during regular debriefings with the coordinator, cooperation of the facility health staff was never raised as a barrier to reaching screening goals, and DCS facility leadership participation was recorded at all planning meetings.
Implementation
The team took a median of 2 minutes (interquartile range [IQR], 2, 3] to administer the symptom screen with the full procedure of screening, obtaining the sputum, and visual inspection of the sputum taking 4 minutes. The median turnaround time between sputum production and Xpert test result was 1 day (IQR, 1, 1) with turnaround extended to 4 days at peak volume as a result of limited throughput of the GeneXpert-4 machine. All test results were verbally relayed to the DCS health staff and accompanied by a paper report on the day the result was available. Among the TB cases, TB treatment was started within 24 hours of a positive Xpert test for 155 (77%) inmates as specified in the screening plan, thus maintaining fidelity 77% of the time. Treatment initiation records were not located for 14 (7%) inmates. Records were not located due to transfer from the facility (4), missing records (3), or not having started treatment at the time of the review (7).
In summary, we diagnosed pulmonary TB among 201 inmates, representing 2.7% of all screened inmates, and documented initiation of TB treatment among 187 (93%). The cost of implementing systematic case finding (excluding treatment costs) with on-site Xpert during the 5-month evaluation period was $304 167 (Table 3). The projected cost for a 12-month program in these 4 correctional facilities is $730 000. The average cost per inmate screened was $35. The cost per TB case identified was $1513. The total cost of the program was $377 951, $73 784 of which was for treatment (20% of the overall cost). Including the treatment costs increased the cost per case identified to $1880.
Table 3.
Implementation Cost During the 5-Month Period May to September 2013
| Cost During the Implementation Period (2013 $) | Cost Extrapolation for 12 Months of Implementation | |
|---|---|---|
| Fixed costs | 9064 | 21 754 |
| GeneXpert-4 machines | 7933a | 19 039 |
| Computers | 590 | 1416 |
| Furniture and air conditioning units | 537 | 1289 |
| Labor | 166 932 | 400 637 |
| Screening teamb | 132 568 | 318 163 |
| Management team | 28 241 | 67 778 |
| DCS staff | 5346 | 12 830 |
| Inmate peer educators | 776 | 1862 |
| Consumables | 99 373 | 238 495 |
| Xpert cartridges | 88 265 | 211 836 |
| Laboratory supplies | 11 107 | 26 657 |
| Overhead and facility costs | 28 798 | 69 115 |
| Information technology support | 6920 | 16 608 |
| Rent, travel, training, monitoring, and administration | 21 878 | 52 507 |
| Drug-sensitive tuberculosis treatment cost | 63 680 | 152 832 |
| MDR tuberculosis treatment cost | 10 104 | 24 250 |
| Implementation cost of screening | 304 167 | 730 000 |
| Implementation cost including tuberculosis treatment | 377 951 | 907 082 |
Abbreviations: DCS, South African Department of Correctional Services; MDR, multidrug-resistant.
a Annualized cost.
b Team paid by the local nongovernmental organization.
Maintenance
Participation by DCS personnel at regional and facility planning meetings for the TB screening program remained consistent during the first year of implementation. At the national level, this screening project continues to receive funding and high-level policy support. Subsequent to the first phase assessed in this analysis, the number of facilities expanded during the second phase.
Cost of Sensitivity Analyses
A one-way sensitivity analysis of costs was relatively insensitive to GeneXpert machine cost, more sensitive to Xpert cartridge cost, and in proportion to those with a positive screening test having a positive Xpert result (Table 4). A 75% reduction in Xpert machine cost would reduce the overall cost by 2%. Finally, screening all individuals directly with Xpert would increase screening costs by 15%, but it would decrease the cost per TB case identified by 4% because fewer TB cases would be missed among asymptomatic individuals.
Table 4.
Effect of Changes to Costs or Screening Performance (Excluding Tuberculosis Treatment Costs)
| Total Estimated Implementation Cost ($) | Change in Cost of Implementation | Cost per Xpert Positive Case ($) | Change in Cost per Tuberculosis Case Identified | |
|---|---|---|---|---|
| Actual cost | 304 167 | 1513 | ||
| Reduce GeneXpert device cost by 75% | 298 217 | −2% | 1483 | −2% |
| Reduce Xpert cartridge cost by 75% | 237 968 | −22% | 1183 | −22% |
| Increase performance of screening strategy to a 10% specificity for a positive Xpert resulta | 237 715 | −22% | 1182 | −22% |
| Use Xpert for screeningb | 349 649 | +15% | 1447 | −4% |
a Assuming a new test with better specificity and no change in sensitivity.
b These costs reflect additional GeneXpert capacity at the larger 2 facilities and increased tuberculosis case detection.
DISCUSSION
We implemented high-volume TB screening and diagnosis in a correctional setting through mass symptom screening and on-site Xpert testing. During the 5-month period chosen for evaluation, we screened 24% of the total population in the participating facilities at a cost of $35 per inmate screened and $1513 per TB case identified. Using an evaluation framework (RE-AIM), we have systematically assessed key dimensions in the implementation process. In our situation, assessing “reach” highlights our success in screening all inmates in a small facility as well as the challenge of achieving mass screening in the larger facilities. We achieved a 100% screening rate of new admissions on team screening days. The staffing level prevented us from screening all admissions every day. On days when the team was not screening admissions, the corrections health staff provided screening; those numbers are not represented in our analysis. Constraints on achieving the target reach among current inmates in the larger facilities were (1) inadequate staffing coupled with (2) limited hours during the day, during which inmates were allowed to leave cell blocks, and (3) the limited capacity of the GeneXpert-4 machine.
We believe these findings have broad applicability to other correctional facilities with high TB prevalence and provide a replicable screening approach and cost. More importantly, our results demonstrate that systematic screening with the use of Xpert for symptomatic individuals was effective in diagnosing a large amount of prevalent pulmonary TB in the correctional setting. However, our finding of TB among 2.7% of screened inmates is likely an underestimate of the true point prevalence given the more limited sensitivity of Xpert when used for systematic screening than when used among ill patients seeking medical care [15]. Based on prior studies, we estimate that we missed 30% of culture-positive pulmonary TB [15]. Based on this finding, a more realistic estimate of point prevalence of TB disease (if culture were used) in these facilities may have been 3.5%. This estimate is in the range of results from a recently reported point prevalence of TB of 2.9% from a single South African correctional facility in which liquid culture of sputum was used [2] and a point prevalence of bacteriologically confirmed TB of 3.8% within a correctional facility in Zambia [3]. We believe that these results should help to allay concerns about the use of Xpert to diagnose TB during systematic screening of a generally healthy population in which most of the TB is subclinical and may be sputum smear microscopy negative [16].
It is notable that, in this program, a majority of inmates reported symptoms (62%). This is consistent with the 34%–62% with symptoms in the above-referenced studies from correctional facilities in South Africa and Zambia [2, 3]. It is higher than the 14%–40% from general and HIV-infected populations, and it presents a limitation of symptom screening when the goal is conserving the use microbiologic testing [10, 17–19]. Alternatives to symptom screening have been suggested to either improve specificity of screening or sensitivity of confirmatory testing. Suggested algorithms for screening that include body mass index or HIV status require more equipment, greater skill, and more time per inmate, potentially impeding a goal of annual or more frequent screening of the correctional population [20]. A combination of clinical, radiological, and laboratory diagnosis such as was used in a program in Zambia involved a 2-day process [3]; this approach may require greater human resources. We did not use chest radiology for screening in this project due to the cost, logistical complexity, and time required.
Additional approaches to program improvement may focus on inmate perceptions regarding TB and TB screening in the facilities. Assessing inmate perceptions may provide valuable information to better educate and mobilize for TB prevention and treatment.
We are unaware of other published reports of the cost of TB screening in correctional facilities with which to compare our findings. When compared with noncorrectional settings, our cost per TB case identified was higher. Studies from Cape Town, South Africa and Cambodia have reported cost per TB case diagnosed of $1117 and $448, respectively [21, 22]. The higher cost per TB case diagnosed in our program compared with the Cambodia program may be attributable to higher personnel costs [23]. An additional important difference between the community studies and our correctional setting is that we (1) sought to screen the entire population in a setting and (2) had limited available hours per day and days per week to perform screening due to facility security regulations.
Our study has the strength of describing a real-world implementation of a program with ambitious targets and constrained resources. Furthermore, data were collected prospectively for use in program management, monitoring, and reporting. Limitations include the nature of program implementation: we did not compare diagnostic approaches and cannot report on the sensitivity of Xpert when compared with liquid culture in this population. In addition, we used historical data on point prevalence of pulmonary TB confirmed by liquid culture as a comparator. Although these historical reports from South Africa and Zambia represent recently completed studies in the region, historical comparisons must be interpreted with caution. We also lacked a comparator group to assess the effect on TB incidence. However, we believe that there is compelling evidence that TB transmission occurs in correctional facilities and that systematic mass case finding is an important component of TB control in these settings [24].
CONCLUSIONS
Additional approaches to achieve TB control in correctional facilities may include treatment of latent TB infection and environmental modifications to reduce transmission risk. Given South Africa's large detained population with significant turnover (to and from the community), TB control in correctional facilities may be an important component for overall national TB control. Systematic TB screening of a correctional population is feasible; scale-up in high TB burden settings is essential.
Acknowledgments
We thank the DCS health staff and wardens for the contributions, team work, and dedication that were essential to deliver TB screening and care to these correctional facilities.
Financial support. C. J. H. is supported by a National Institutes of Health K23 research grant (AI083099). This evaluation describes a project partially funded by the Global Fund to Fight AIDS, Tuberculosis, and Malaria.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Baussano I, Williams BG, Nunn P, et al. Tuberculosis incidence in prisons: a systematic review. PLoS Med. 2010;7:e1000381. doi: 10.1371/journal.pmed.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Telisinghe L, Fielding KL, Malden JL, et al. High tuberculosis prevalence in a South African prison: the need for routine tuberculosis screening. PloS One. 2014;9:e87262. doi: 10.1371/journal.pone.0087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henostroza G, Topp SM, Hatwiinda S, et al. The high burden of tuberculosis (TB) and human immunodeficiency virus (HIV) in a large Zambian prison: a public health alert. PloS One. 2013;8:e67338. doi: 10.1371/journal.pone.0067338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churchyard GJ, Mametja LD, Mvusi L, et al. Tuberculosis control in South Africa: successes, challenges and recommendations. S Afr Med J. 2014;104(3 Suppl 1):244–8. doi: 10.7196/samj.7689. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Geneva: World Health Organization; 2013. Systematic Screening for Active Tuberculosis: Principles and Recommendations. [PubMed] [Google Scholar]
- 6.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;1:CD009593. doi: 10.1002/14651858.CD009593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massyn N, Candy D, Peer N, et al. District Health Barometer. Durban, South Africa: Health Systems Trust; 2014. [Google Scholar]
- 10.Getahun H, Kittikraisak W, Heilig C, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Health (South Africa) Pretoria, South Africa: 2014. National Tuberculosis Management Guidelines. [Google Scholar]
- 12.Sohn H, Minion J, Albert H, et al. TB diagnostic tests: how do we figure out their costs? Expert review of anti-infective therapy. Expert Rev Anti Infect Ther. 2009;7:723–33. doi: 10.1586/eri.09.52. [DOI] [PubMed] [Google Scholar]
- 13.Pooran A, Pieterson E, Davids M, et al. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PloS One. 2013;8:e54587. doi: 10.1371/journal.pone.0054587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinanovic E, Floyd K, Dudley L, et al. Cost and cost-effectiveness of community-based care for tuberculosis in Cape Town, South Africa. Int J Tuberc Lung Dis. 2003;7(9 Suppl 1):S56–62. [PubMed] [Google Scholar]
- 15.Dorman SE, Chihota VN, Lewis JJ, et al. Performance characteristics of the Cepheid Xpert MTB/RIF test in a tuberculosis prevalence survey. PLoS One. 2012;7:e43307. doi: 10.1371/journal.pone.0043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abed Al-Darraji HA, Abd Razak H, Ng KP, et al. The diagnostic performance of a single GeneXpert MTB/RIF assay in an intensified tuberculosis case finding survey among HIV-infected prisoners in Malaysia. PloS One. 2013;8:e73717. doi: 10.1371/journal.pone.0073717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV-prevalence: implications for tuberculosis control. Am J Repir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosimaneotsile B, Talbot EA, Moeti TL, et al. Value of chest radiography in a tuberculosis prevention programme for HIV-infected people, Botswana. Lancet. 2003;362:1551–2. doi: 10.1016/s0140-6736(03)14745-9. [DOI] [PubMed] [Google Scholar]
- 19.Rangaka MX, Wilkinson RJ, Glynn JR, et al. Effect of antiretroviral therapy on the diagnostic accuracy of symptom screening for intensified tuberculosis case finding in a South African HIV clinic. Clin Infect Dis. 2012;55:1698–706. doi: 10.1093/cid/cis775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris JB, Siyambango M, Levitan EB, et al. Derivation of a tuberculosis screening rule for sub-Saharan African prisons. Int J Tuberc Lung Dis. 2014;18:774–80. doi: 10.5588/ijtld.13.0732. [DOI] [PubMed] [Google Scholar]
- 21.Kranzer K, Lawn SD, Meyer-Rath G, et al. Feasibility, yield, and cost of active tuberculosis case finding linked to a mobile HIV service in Cape Town, South Africa: a cross-sectional study. PLoS Med. 2012;9:e1001281. doi: 10.1371/journal.pmed.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadav RP, Nishikiori N, Satha P, et al. Cost-effectiveness of a tuberculosis active case finding program targeting household and neighborhood contacts in Cambodia. Am J Trop Med Hyg. 2014;90:866–72. doi: 10.4269/ajtmh.13-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Bank. WDR2013 Occupational Wages around the World. Available at: http://data.worldbank.org/data-catalog/occupational-wages . Accessed 1 August 2014.
- 24.Johnstone-Robertson S, Lawn SD, Welte A, et al. Tuberculosis in a South African prison—a transmission modelling analysis. S Afr Med J. 2011;101:809–13. [PMC free article] [PubMed] [Google Scholar]