Abstract
Background. Most inactivated influenza vaccines contain purified and standardized hemagglutinin (HA) and residual neuraminidase (NA) antigens. Vaccine-associated HA antibody responses (hemagglutination inhibition [HAI]) are well described, but less is known about the immune response to the NA.
Methods. Serum of 1349 healthcare personnel (HCP) electing or declining the 2010–2011 trivalent-inactivated influenza vaccine ([IIV3], containing A/California/7/2009 p(H1N1), A/Perth/16/2009 [H3N2], B/Brisbane/60/2008 strains) were tested for NA-inhibiting (NAI) antibody by a modified lectin-based assay using pseudotyped N1 and N2 influenza A viruses with an irrelevant (H5) HA. Neuraminidase-inhibiting and HAI antibody titers were evaluated approximately 30 days after vaccination and end-of-season for those with polymerase chain reaction (PCR)-confirmed influenza infection.
Results. In 916 HCP (68%) receiving IIV3, a 2-fold increase in N1 and N2 NAI antibody occurred in 63.7% and 47.3%, respectively. Smaller responses occurred in HCP age >50 years and those without prior 2009–2010 IIV3 nor monovalent A(H1N1)pdm09 influenza vaccinations. Forty-four PCR-confirmed influenza infections were observed, primarily affecting those with lower pre-exposure HAI and NAI antibodies. Higher pre-NAI titers correlated with shorter duration of illness for A(H1N1)pdm09 virus infections.
Conclusions. Trivalent-inactivated influenza vaccine is modestly immunogenic for N1 and N2 antigens in HCP. Vaccines eliciting robust NA immune responses may improve efficacy and reduce influenza-associated morbidity.
Keywords: antibody, hemagglutinin-inhibition, influenza, neuraminidase-inhibition, vaccine
Influenza vaccination is the most important strategy for protecting against influenza infection, reducing rates of medically attended influenza illness and hospitalization [1, 2]. Most inactivated influenza vaccines (IIV) contain both standardized hemagglutinin (HA) and some neuraminidase (NA) antigens; quantification of the immune response to IIV is typically assessed by hemagglutination-inhibition (HAI) assays alone. In addition, vaccine release criteria are primarily focused on the quantity of HA antigen in the formulation [3].
Relatively little is known about the immune response to the NA component of IIV or of the potential role of NA-specific antibody in vaccine-induced protection. In part, this is due to the difficulty in performing assays of functional NA antibody. We took advantage of the development of the enzyme-linked lectin assay (ELLA) [4–6] to assess NA-inhibition (NAI) antibody responses and the role of NAI antibody in protection in a study of influenza vaccination of healthcare personnel (HCP) conducted during the 2010–2011 influenza season. We explored the NAI antibody response to trivalent IIV (IIV3) and compared this with the HAI antibody response in the same population. We also examined factors associated with the NAI-antibody response to vaccination and to naturally occurring influenza infection.
METHODS
Setting
Samples for this evaluation were collected from an observational cohort study of influenza vaccination in HCP described earlier [7]. In brief, HCP at 2 United States medical centers with voluntary vaccination programs were prospectively enrolled and offered the 2010–2011 IIV3 containing A/California/7/2009 p(H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008 strains. Preseason (S1) sera were collected on all participants, and postvaccination (S2) sera were collected approximately 30 days postvaccination on participants who chose to be vaccinated. End-of-season (S3) sera were also collected on all participants, regardless of vaccination status. Participants were monitored weekly for the development of symptoms of acute respiratory illness (ARI) defined as fever or feverishness and cough, and participants reporting ARI were tested for influenza infection within 7 days of illness onset via real-time reverse-transcriptase polymerase chain reaction (PCR) on nasal, nasopharyngeal, and oropharyngeal swabs. Participants were interviewed approximately 10–14 days after sample collection and asked the date when their illness resolved. Illness duration was defined as the days from self-reported illness onset to illness resolution. For the few HCP who remained ill at the follow-up interview, illness resolution was the date of the interview plus 1 day. We report here the results of NAI-antibody testing of the available S1 and S2 sera and on S3 sera collected from participants with PCR-confirmed influenza illness. We also report HAI-antibody results on the same set of S1 and S2 sera.
Preparation of H5-Pseudotyped Viruses
Because HA-specific antibodies can sterically interfere with NA activity [8], we used pseudotyped viruses that contained an irrelevant HA as the antigens for NAI testing. To generate the pseudotyped viruses, a Madin-Darby Canine Kidney (MDCK) cell line that constitutively expressed the HA (MDCK-HA) of A/Vietnam/1203/04 (H5N1) influenza virus was used [9]. We used single-cycle infectious influenza A viruses (sciIAV) in the background of A/WSN/33 (H1N1), in which the viral HA gene was replaced by that of green fluorescent protein as previously described [10], and the viral NA gene was replaced by either that of the A/California/04/2009 (pH1N1) or the A/Wyoming/03/2003 (H3N2) viruses [11]. Infection of the A/Vietnam/1203/04 H5 MDCK-HA cells with these sciIAV results in the production of H5N1- and H5N2-pseudotyped viruses. Before using these sciIAVs in NAI assays, we confirmed that samples from the study did not have any HAI activity against the H5-pseudotyped sciIAVs.
The NA protein sequences of A/Wyoming/03/2003 used here and A/Perth/16/2009 found in 2010–2011 IIV3 are 97.6% identical, varying in 12 of the 496 amino acids. Murine models of NA DNA vaccines using 2 different but 89% identical N2 subtypes demonstrated cross-protective antibody production between H3N2 influenza strains when challenged [12]. Furthermore, studies using ferret antisera against drifted variants of N2 demonstrated that sera generated using contemporary NA antigens retain NAI activity against older strains [13]. Thus, the NAI specificity demonstrated in our approach likely recapitulates the activity against matched N2 strains.
Neuraminidase Antibody Testing
To measure the anti-NA antibody, we used a modified lectin-based assay described previously [4, 5]. Ninety-six well, flat-bottom immuno-polystyrene plates with Maxisorp surface (Nunc-Immuno) were coated with a fetuin plate-coating solution (50 µg/mL; Sigma-Aldrich). Sera were tested in serial 2-fold dilutions from 1:16 to 1:32 768. Serial dilutions of heat-inactivated sera were combined with each pseudotyped virus and incubated at room temperature for 2 hours. The antibody-antigen volume was then transferred to fetuin-coated plates and incubated for 16–18 hours at 37°C in dry heat, followed by peroxidase-labeled peanut lectin (Sigma-Aldrich) for 2 hours, and bound lectin detected with 3,3′,5,5′ tetramethylbenzidine (Sigma-Aldrich). All sera were tested in duplicate and with each NA antigen. The reciprocal value of the dilution producing a 50% reduction in the optical density of pseudotyped virus alone was defined as the serum's NAI antibody titer. When comparing pre- and postvaccination titers, a 2-fold increase in antibody titer was considered significant, in alignment with previously published lectin-based assays for NA immunogenicity [5, 14].
Hemagglutinin Antibody Testing
Testing for HAI titers to influenza A/California/7/2009 (pH1N1) and A/Perth/16/2009 (H3N2) was performed by Battelle Laboratory (Aberdeen, Maryland) using standard methods (World Health Organization 2011) [15]. An analysis of the pH1N1-specific HAI response in the study population has been reported previously [7]. However, the results shown in the current report are restricted to those samples that were also tested for NAI antibody.
Data Analysis
Because the predominant vaccine at both sites was inactivated, we excluded live-attenuated influenza vaccine (LAIV) recipients. Antibody endpoints are presented as geometric mean titers (GMTs). Due to GMT sample distributions being highly left-skewed, base-2 log-transformed GMT data were used to calculate means and confidence intervals (CIs), and these estimates were then back-transformed to the original GMT scale.
Geometric mean ratios ([GMRs] geometric mean fold ratio of postvaccination S2 titer to pre-vaccination S1 titer) were calculated using multivariate linear mixed-effect models to account for the correlation in repeated measures (S1 and S2). Compound symmetric covariance error structure was assumed. Multivariate analyses adjusted for sex, race, ethnicity, and study site. All multivariate analyses were conducted using base-2 log-transformed GMTs.
Percentage of participants associated with positive seroresponse and respective binomial CIs were calculated by age group and prior vaccination status. In assessing the correlation between duration of influenza illness and NAI or HAI GMTs, a nonparametric measure of correlation (Spearman's rank correlation coefficient) was used because sample sizes were small. All data analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Study Population
Serum samples were available for testing on a total of 1417 participants who were enrolled in the observational study during the 2010–2011 influenza season; 68 who received LAIV were excluded. Of the remaining 1349 tested, 916 (68%) elected vaccination and provided preseason (S1) and postvaccination (S2) sera for NAI antibody testing, and 433 (32%) were not vaccinated and submitted an S1 serum alone. The characteristics of the study population are shown in Table 1. A majority of participants in both groups were white females, with those vaccinated more often age 50 years and older, married, having a high-risk condition, working in outpatient setting, and involved in patient care for >10 years. A greater number of vaccinated than unvaccinated HCP had received the prior seasonal 2009–2010 IIV-3 or monovalent pH1N1 vaccine. The unvaccinated HCP were more often employed in the emergency department. The number of children in the household, occupation, educational level, self-rated health status, or smoking status did not appear to differ substantially between groups.
Table 1.
Descriptive Statistics for Demographic, Occupational, Medical Care Setting, Health Status, and Prior Influenza Vaccination Variable for Healthcare Personnel Population (N = 1349)
| Demographics | Unvaccinated in 2010–2011 n (Col. %) | Vaccinated With 2010–2011 IIV3 n (Col. %) | P Value* |
|---|---|---|---|
| Total | 433 (45) | 916 (55) | |
| Female | 332 (77) | 747 (82) | .05 |
| Age (years) | .0008 | ||
| 18–29 | 82 (19) | 139 (15) | |
| 30–39 | 132 (31) | 238 (26) | |
| 40–49 | 115 (27) | 223 (24) | |
| 50+ | 102 (24) | 316 (35) | |
| Race | |||
| White | 326 (76) | 733 (80) | .07 |
| Ethnicity | |||
| Hispanic | 50 (12) | 109 (12) | .87 |
| Married | 239 (57) | 607 (67) | .0004 |
| Education | .82 | ||
| High school | 36 (8) | 66 (7) | |
| Some college | 112 (26) | 224 (24) | |
| Bachelor degree | 207 (48) | 448 (49) | |
| Masters | 21 (5) | 46 (5) | |
| Advanced graduate | 55 (13) | 133 (15) | |
| Child in household | 167 (39) | 337 (37) | .46 |
| Occupation and medical care setting | |||
| Occupation | .40 | ||
| Physicians | 47 (11) | 120 (13) | |
| PA, NP, and RN managers | 20 (5) | 30 (3) | |
| Nurses | 177 (41) | 398 (43) | |
| Allied Professional | 187 (43) | 368 (40) | |
| Patient care >10 years | 219 (51) | 517 (56) | .05 |
| Medical care settings | |||
| Outpatient | 272 (63) | 629 (69) | .04 |
| Intensive care unit | 170 (39) | 311 (34) | .05 |
| Hospital | 281 (65) | 562 (61) | .17 |
| Emergency department | 140 (32) | 223 (24) | .002 |
| Study site | .40 | ||
| Scott and White Healthcare | 298 (69) | 654 (71) | |
| Kaiser Permanente | 133 (31) | 262 (29) | |
| Health status | |||
| Self-rated very good or excellent | 352 (82) | 742 (81) | .77 |
| Smoker | 26 (6) | 61 (7) | .66 |
| Body mass index | .05 | ||
| normal or lower (<24.9) | 149 (35) | 298 (33) | |
| Overweight (25–29.9) | 161 (37) | 301 (33) | |
| Obese (≥30) | 121 (28) | 317 (35) | |
| High-risk condition | 50 (12) | 155 (17) | .02 |
| Receipt of prior influenza vaccine 2009–10 | |||
| Any seasonal vaccination | 148 (36) | 748 (84) | <.0001 |
| Any pdmH1N1 monovalent vaccination | 69 (17) | 463 (55) | <.0001 |
Data are no. (%) unless otherwise indicated.
Abbreviations: Col., Collective population; IIV3, inactivated influenza vaccine; PA, physician assistant; pdm, pandemic; NP, nurse practitioner; RN, registered nurse;
* χ2 P value; P < .05 is statistically significant and bolded.
Serum Antibody Response to Vaccination
The serum NAI antibody responses to vaccination assessed by ELLA assay, and the serum HAI antibody responses, are shown in Table 2. After vaccination, we observed a 2-fold increase in the serum N1 and N2 NAI antibody titers in 63.7% and 47.3%, and a 4-fold response in 28.4% and 16.7% of vaccine recipients, respectively. After adjusting for sex, race, ethnicity, and study site, we examined the change in NAI-adjusted GMT ratios (aGMRs) across 4 age groups (Table 2) and compared them with the HA aGMRs of the pH1N1 and A(H3N2)Wisconsin by HAI testing. Although baseline N1 GMTs were higher in the HCP aged 50 years and older, in comparison to the 3 younger age groups, after vaccination this older age group showed a smaller aGMR to N1. Baseline GMTs for the pH1N1 HA were relatively similar across age groups, and after vaccination the greatest aGMRs occurred in the youngest age group and declined in a step-wise fashion with each increasingly older age, with overlapping CIs. In contrast to this result, the baseline N2 antibody level and the aGMR were highest in the youngest age group, but they were similar regardless of age.
Table 2.
Response to Seasonal 2010–2011 Trivalent-Inactivated Influenza Vaccine by age Group and HAI and NAI Antibody Titer Types (95% Confidence Intervals)
| Age Group, Years | Antibody Titer Measurement | H1N1a |
H3N2b |
||
|---|---|---|---|---|---|
| HAI | NAI | HAI | NAI | ||
| 18–29 (N = 139) | S1 GMT | 13.2 (10.8, 16.2) | 219.3 (175.6, 274.0) | 13.8 (11.5, 16.6) | 492.0 (413.8, 584.9) |
| S2 GMT | 138.3 (106.6, 179.4) | 509.5 (410.4, 632.4) | 46.9 (37.5, 58.7) | 908.5 (712.1, 1159.1) | |
| aGMR | 10.1 (7.9, 13.0) | 2.32 (2.0, 2.7) | 3.39 (2.9, 4.0) | 1.85 (1.6, 2.2) | |
| Seroresponse (%)c | 75.5 (68.4, 82.7) | 70.5 (62.9, 78.1) | 45.3 (37.0, 53.6) | 53.2 (44.9, 61.5) | |
| 30–39 (N = 238) | S1 GMT | 12.2 (10.6, 14.1) | 189.7 (159.8, 225.1) | 12.2 (10.6, 14.0) | 361.0 (320.0, 407.2) |
| S2 GMT | 97.4 (80.3, 118.1) | 443.9 (380.1, 518.5) | 38.4 (32.9, 44.8) | 655.8 (550.2, 781.7) | |
| aGMR | 8.1 (6.7, 9.8) | 2.3 (2.1, 2.6) | 3.2 (2.8, 3.6) | 1.8 (1.6, 2.0) | |
| Seroresponse (%) | 67.1 (61.1, 73.1) | 70.6 (64.8, 76.4) | 51.1 (44.7, 57.4) | 50.4 (44.1, 56.8) | |
| 40–49 (N = 222) | S1 GMT | 11.7 (10.0, 13.7) | 204.5 (173.1, 241.4) | 10.0 (8.8, 11.3) | 360.9 (318.3, 409.3) |
| S2 GMT | 70.3 (57.9, 85.5) | 461.9 (393.1, 542.7) | 27.5 (23.4, 32.4) | 607.9 (514.8, 717.9) | |
| aGMR | 6.1 (5.0, 7.4) | 2.3 (2.0, 2.5) | 2.8 (2.4, 3.2) | 1.7 (1.5, 1.9) | |
| Seroresponse (%) | 61.3 (54.9, 67.7) | 69.5 (63.5, 75.6) | 41.0 (34.5, 47.5) | 48.4 (41.9, 55.0) | |
| 50 + (N = 316) | S1 GMT | 11.9 (10.6, 13.4) | 427.7 (364.7, 501.7) | 10.9 (9.7, 12.2) | 365.2 (326.9, 408.1) |
| S2 GMT | 52.0 (44.2, 61.2) | 730.5 (628.8, 848.5) | 32.2 (28.1, 37.0) | 555.3 (484.6, 636.3) | |
| aGMR | 4.5 (3.8, 5.3) | 1.7 (1.6, 1.9) | 3.0 (2.7, 3.3) | 1.5 (1.4, 1.7) | |
| Seroresponse (%) | 52.4 (46.9, 57.9) | 57.9 (52.5, 63.4) | 45.7 (40.2, 51.2) | 45.3 (39.8, 50.7) | |
Abbreviations: aGMR, geometric mean fold ratio of the S1 and S2 antibody titer, adjusted for sex, race, ethnicity, and study site; GMT, geometric mean titer; HAI, hemagglutination-inhibition; NAI, neuraminidase-inhibition.
a For H1N1 measurements, the A/California/04/09 virus was used for both HAI and NAI.
b For H3N2 measurements, the HAI used the A/Perth/16/09 virus, whereas NAI was done using the N2 NA from A/Wyoming/03/2003.
c Seroresponse was defined as a 2-fold increase in titer from S1 to S2 for HAI and a 2-fold increase for NAI (see text).
Effect of Prior Vaccination on Antibody Responses
To evaluate the impact of influenza vaccine exposure during the previous season (2009–2010), we compared the HAI and NAI responses of vaccinated participants who had received both the monovalent pH1N1 vaccine and seasonal IIV3 (n = 411) in the previous year with those of participants who had not received either vaccine previously (n = 82). We observed higher baseline N1 antibody levels for those previously vaccinated, and we noted a similar but less substantial trend for N2 (Table 3). However, there was a significantly larger aGMR (P < .01) to both N1 and N2 antigens after the 2010–2011 IIV3 among those who did not receive either IIV3 or pH1N1 vaccine in 2009–2010. Higher baseline HAI titers and lower aGMR among those vaccinated the prior year were also observed for the HAI response.
Table 3.
Hemagglutination-Inhibition and NAI Antibody Response to Seasonal 2010–2011 Trivalent-Inactivated Influenza Vaccine by Prior Season Vaccination Status
| Vaccination Status | Antibody Titer Measurement | H1N1a |
H3N2b |
||
|---|---|---|---|---|---|
| HAI | NAI | HAI | NAI | ||
| No prior 2009–2010 seasonal or Pandemic H1N1 vaccine (N = 82) | S1 GMTc | 9.0 (7.3, 11.0) | 138.0 (102.1, 186.4) | 8.7 (7.2, 10.7) | 285.4 (229.6, 354.6) |
| S2 GMT | 149.7 (111.1, 201.6) | 444.2 (336.3, 586.9) | 37.7 (28.5, 50.0) | 605.1 (450.6, 812.6) | |
| aGMR | 16.7 (12.8, 21.7) | 3.2 (2.7, 3.9) | 4.3 (3.5, 5.4) | 2.1 (1.8, 2.6) | |
| Seroresponse (%)c | 89.0 (82.3, 95.8) | 81.7 (73.3, 90.1) | 58.5 (47.9, 69.2) | 61.0 (50.4, 71.5) | |
| Prior 2009–2010 seasonal and pandemic H1N1 vaccine (N = 411) | S1 GMT | 15.0 (13.5, 16.7) | 331.4 (292.8, 375.0) | 11.6 (10.6, 12.8) | 395.5 (362.6, 431.5) |
| S2 GMT | 49.9 (44.1, 56.5) | 617.6 (548.9, 694.9) | 31.9 (28.5, 35.7) | 602.1 (539.3, 672.2) | |
| aGMR | 3.3 (3.0, 3.7) | 1.9 (1.7, 2.0) | 2.7 (2.5, 3.0) | 1.5 (1.4, 1.6) | |
| Seroresponse (%)c | 40.7 (35.9, 45.5) | 61.6 (56.9, 66.3) | 41.7 (36.9, 46.5) | 45.5 (40.7, 50.3) | |
Abbreviations: aGMR, geometric mean fold ratio of the S1 and S2 antibody titer, adjusted for sex, race, ethnicity, and study site; GMT, geometric mean titer; HAI, hemagglutination-inhibition; NAI, neuraminidase-inhibition.
a For H1N1 measurements, the A/California/04/09 virus was used for both HAI and NAI.
b For H3N2 measurements, the HAI used the A/Perth/16/09 virus, whereas NAI was done using the N2 NA from A/Wyoming/03/2003.
c Seroresponse was defined as a 4-fold increase in titer from S1 to S2 for HAI and a 2-fold increase for NAI (see text).
Comparison of Vaccine and Natural Infection
To compare the response to inactivated vaccine with the response to infection with circulating influenza virus, we assessed pre-exposure antibody (ie, S1 for unvaccinated participants, and S2 for vaccinated participants) and postinfection antibody (ie, S3) levels in participants with PCR-documented influenza. Postseason sera were available on 6 vaccinated and 3 unvaccinated participants who had experienced documented H1N1 illness and on 22 vaccinated and 6 unvaccinated participants who had experienced H3N2 illness. For both viruses, infection stimulated a strong NAI antibody response to the relevant NA antigen. For unvaccinated participants, the GMR comparing S1 to S3 was 10.08 (95% CI = .07, 1452) for N1 in pH1N1 infected and 8.0 (95% CI = 2.2, 29.4) for N2 in participants infected with H3N2. For vaccinated participants, these GMRs were 8.98 (95% CI = 1.9, 42.5) and 3.01 (95% CI = 1.9, 4.82), respectively. Compared with the vaccine responses shown in Table 3, these GMRs are slightly higher than those seen in response to vaccine (no prior vaccine GMR 3.24 for N1 and 2.14 for N2; history of prior vaccine GMR 1.9 for N1 and 1.5 for N2), but the differences were not statistically significant.
Association of Hemagglutination-Inhibition and Neuraminidase-Inhibition Antibody With Risk of Influenza
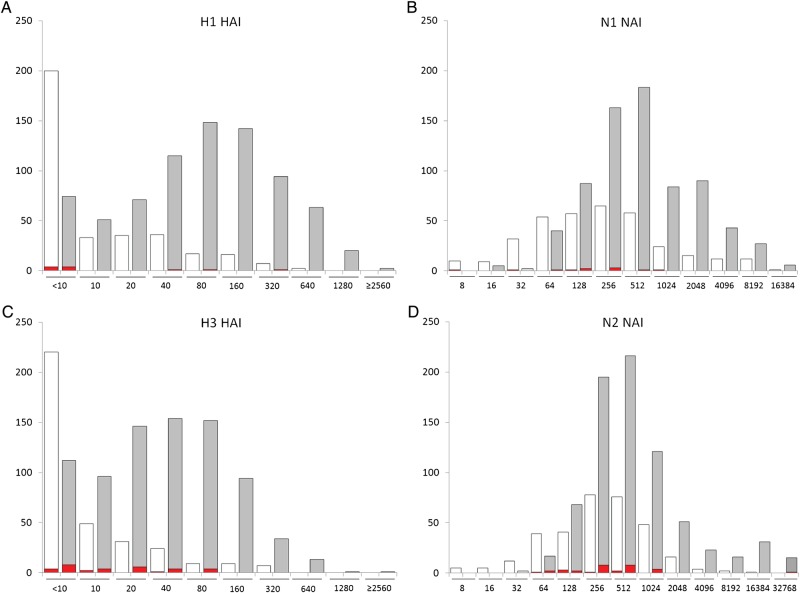
Assessment of the potential role of NAI antibody in protection against influenza was complicated by the relatively small number of laboratory-documented cases that occurred in the study. For this analysis, participants who reported respiratory illness but who tested negative for influenza are removed to avoid possible confusion from false-negative PCR tests. Figure 1 shows the distributions of serum HAI and NAI antibody against H1 and N1, or against H3 and N2 antigens, in the pre-exposure (S1 in unvaccinated and S2 in vaccinated) sera. Although a protective titer in the ELLA assay has not been defined, participants with laboratory-documented influenza had relatively lower pre-exposure serum HAI and NAI antibody; these differences were not statistically significant.
Figure 1.
Number of participants in each strata of pre-exposure serum antibody titer. A and B show titers against H1N1 (left, HAI titer; right, NAI titer), whereas C and D show titers against H3N2 (right, HAI titer; left, NAI titer). In each panel, the white bars show the S1 titers in unvaccinated participants, and the gray bars show S2 titers in vaccinated participants. The red bar indicates those with polymerase chain reaction (PCR)-documented influenza H1N1 (A and B) or H3N2 (C and D) in each strata.
Neuraminidase-Inhibition Antibody Titer and Duration of Influenza Illness
Among the unvaccinated, having higher S1 N1 titers was associated with a shorter duration of influenza illness among all influenza A positives, but this was not seen for N2 (Table 4). However, among the vaccinated participants, although a higher N1 antibody may correlate with a shorter duration of illness for pH1N1 infection, the N2 antibody correlated with a shorter period of illness among all influenza A positives and also for pH1N1 infection, for unclear reasons. It is interesting to note that these correlations were not seen with either H1 or H3 HAI titers.
Table 4.
Spearman Correlations Between N1 and N2 NAI, and A(H1N1)pdm09 HAI, and A(H3N2) HAI Antibody Titers and Duration of Subtypes of Influenza A Virus Illness Among Vaccinated and Unvaccinated Healthcare Personnela
| Spearman Correlations With Duration of Illness |
|||
|---|---|---|---|
| Influenza A Positives | A(H1N1)pdm09 Positives | A(H3N2) Positives | |
| Unvaccinated (N = 10) | (N = 10)b | (N = 7) | |
| N1 NAI titers at S1 (Preseason) | −0.64c | – | −0.46 |
| N2 NAI titers at S1 | −0.13 | – | −0.24 |
| A(H1N1)pdm09 HAI titers at S1 | 0.09 | – | 0.12 |
| A(H3N2) HAI titers at S1 | −0.09 | – | 0.18 |
| Vaccinated (N = 28) | (N = 28)b | (N = 6) | (N = 21) |
| N1 NAI titers at S2 (Postvaccination) | −0.06 | −0.84c | 0.07 |
| N2 NAI titers at S2 | −0.37c | −0.86c | −0.32 |
| A(H1N1)pdm09 HAI titers at S2 | −0.10 | −0.75 | 0.02 |
| A(H3N2) HAI titers at S2 | −0.06 | −0.10 | −0.05 |
Abbreviations: HAI, hemagglutination-inhibition; NAI, neuraminidase-inhibition; pdm, pandemic.
a Duration of illness is days from illness onset to self-reported recovery. Log-transformed titer values were used in Spearman correlations.
b Of 10 influenza positives among unvaccinated, 7 were A(H3N2), 2 were A(H1N1)pdm09, and 1 was A unsubtyped.
c P < .05 is statistically significant and bolded, associated with a shorter duration of illness.
d Includes 1 influenza A unsubtyped, which is not reflected in A(H1N1)pdm09- or A(H3N2)-positive results.
DISCUSSION
The present study explored the NAI antibody response to IIV3 and naturally occurring influenza infection among HCP during the 2010–2011 influenza season and compared this result with the HAI responses in the same cohort. Assays investigating NA antibody responses are challenged by steric hindrance between the HA and NA antibodies targeting adjacent glycoproteins on intact virions [8]. Previously described attempts to avoid this have included examination of circulating influenza infections with novel HA antigens [16], use of a purified NA antigen from a triton split virus [5], reassortant viruses generated by reverse genetics [17], or creation of influenza virus-like particles containing solely NA proteins [6]. We used a modification of a previously described lectin-based assay [4] with HA-pseudotyped sciIAV containing an irrelevant H5 from influenza A/Vietnam/1203/04 H5N1.
We observed a comparable proportion of 2-fold NAI antibody vaccine responses to 4-fold HAI responses to the 2010–2011 seasonal IIV3. Commercially available influenza vaccine doses are based on the amount of HA protein present, but some NA activity and immunogenicity is retained [5]. In a previous study, healthy adults vaccinated with 1 of the 6 licensed 2008–2009 IIV3 showed a 2-fold increase in NA antibody titer ranging from 23% to 57% to the A/Brisbane/59/07 N1 component and 47%–73% to the A/Brisbane/10/07 N2 component [14].
Age seemed to impact vaccine response. The aGMRs after vaccination were lower in the oldest age group, a difference that was more notable for the N1 component compared with the N2; a similar trend was observed in the H1 HA versus the H3. Previous studies describe decreasing HAI responses with age [18], which has prompted some to advocate administration of high-dose influenza vaccine among older adults; indeed, studies have shown a significantly more robust antibody response to both the HA and NA component with the high-dose vaccine in older participants [5, 19, 20].
Age also seemed to impact the baseline NA GMT. Participants aged 50 years and older had higher baseline N1 GMT levels compared with the 3 younger age groups. Other studies have similarly described the presence of pre-existing HAI antibodies to the 2009 pH1N1 influenza virus among older individuals, postulated to be due to previous exposure to circulating influenza viruses with an antigenically similar H1 HA. Hancock et al [21] described HAI antibody titers of ≥1:80 against the pH1N1 in 34% of individuals born before 1950, suggesting that experience with a 1918-like H1N1 virus may stimulate production of a cross-reactive and long-standing antibody response. No such antibody titers were found in younger adults. Investigations of the NA antibody during the 2007–2008 and 2008–2009 influenza seasons showed that although pre-existing serum antibodies to pH1N1 NA were found in both young and older adults, the highest baseline NA titers were observed among adults ≥60 years old [22]. In contrast, levels of N2-specific NAI antibody were slightly higher in the youngest age group (ages 18–29 years) of our cohort, for unclear reasons.
The influence of prior influenza vaccination on baseline antibody levels and vaccine responsiveness is an area of debate [9, 23, 24]. In the current study, those who had received both IIV3 and monovalent pH1N1 vaccines the previous year had remarkably higher baseline N1 and N2 NA antibody levels, and as expected they also had lower aGMR to both N1 and N2 after vaccination compared with those who had not been vaccinated with either vaccine. Within the same cohort of HCP, Gaglani et al [7] also observed that those who previously received the pH1N1 monovalent IIV were less likely to attain HAI titers ≥1:40 postvaccination compared with those who received the 2010–2011 IIV3 alone. Evaluation of 3 consecutive annual influenza vaccinations against A/Taiwan/1/86(H1N1) strain from 1990 to 1993 in healthy elderly (>70 years) and young (<30 years) adults found that baseline HA titers increased 4-fold between the first and third years. However, despite this result, postvaccination HAI titers appeared to decline over successive years [9]. The magnitude of a vaccine response may be related to the level of baseline pre-existing antibody, because high levels of existing circulating antibodies to a specific antigen may result in less antibody production upon subsequent immunization with the same antigen. Although this phenomenon is thought to be due to clearance of the antigen before a secondary immune response can occur [25], the prior study with this cohort found muted HAI response even among vaccinees with no detectable baseline titers [7].
Although there is evidence supporting a role for anti-NA antibodies in protective immunity to influenza [16, 26–28], there is no generally accepted NA antibody level that may confer protection from infection. Therefore, we attempted to correlate pre-exposure NAI antibody levels (ie, S1 in unvaccinated participants and S2 in vaccinated participants) with the risk of development of PCR-documented influenza in follow-up. Because of the small number of PCR-confirmed cases of influenza infection in the study, it is difficult to draw firm conclusions regarding NA antibody levels and protection. However, PCR-documented influenza infection seemed to occur in individuals with lower pre-exposure NAI antibody titers. Spearman correlations also suggested a trend for a shorter duration of illness in cases of H1N1 among those with higher pre-exposure NAI antibody. Neuraminidase inhibitor antiviral therapy is recognized as reducing the severity and duration of symptoms among the influenza-infected [29], and NA antibodies may behave similarly. Murine studies of vaccination with purified HA and NA antigens followed by infection challenge demonstrated that both viral antigens were similarly immunogenic, and although NA antibody responses were infection-permissive, there was an inverse relationship between NAI antibody titer and the amount of viruses isolated in the pulmonary tissue [30]. Augmentation of NA-specific immunity through vaccination may be advantageous, particularly in older adults because this is the population at greater risk of having severe disease and illness-related complications.
Our study has several limitations. First, we were unable to produce an A/Perth/16/2009 N2 sciIV. We instead used a sciIV containing the N2 gene of A/Wyoming/03/2003, which was 97.6% identical, varying in 12 of 496 amino acids. This variance is consistent with the relative conservation of the NA protein among influenza strains with the same NA subtypes [12, 31], and it is consistent with cross-protective antibodies generated in response to vaccination within but not across NA subtypes [12]. Therefore, the majority of N2-specific responses were probably detected in this assay. A second limitation is that several vaccine products were used across the 2 health systems, and the specific NA content of the individual vaccines is not available. Another limitation is the relatively few influenza infections that occurred in the study, hampering our ability to assess the relationship between antibody and protection and severity of illness.
Overall, our study supports the role of NA antibodies in protection against influenza infection by demonstrating a comparable proportion of 2-fold NAI antibody vaccine responses to 4-fold HAI responses to the 2010–2011 seasonal IIV3. Furthermore, similarities in the effect of participant age and vaccination status in prior seasons on NAI and HAI antibody responses were observed. Because NA antigens are described to evolve at a slower rate than the HA [32, 33], and new HA genetic mutations allow progressive antigenic drift between pandemics, there may be less selective pressure on the NA, resulting in structurally similar NA antigens circulating season to season, and for longer periods of time. Vaccines stimulating robust NA antibody production may provide infection-permissive protection extending across influenza seasons, independent of the concurrently predominant HA antigen. In addition, antibodies to the common NA subtypes of human and avian influenza viruses (ie, H1N1 and H5N1) afforded cross-protection in murine studies [32]. Because our findings suggest that the NA component of the vaccine can contribute to vaccine protection, the measurement of NAI antibody responses may be important in the assessment of IIV immunogenicity.
Acknowledgments
Financial support. The laboratory assays were funded by a contract from Abt Associates. S. F. B. is currently supported by National Institutes of Health (NIH) Training Grant T32 AI007285. This research in L. M.-S. laboratory was funded by the National Institute of Allergy and Infectious Diseases Centers of Excellence for Influenza Research and Surveillance (Grant HHSN266200700008C) and by interim funding from the University of Rochester.
Potential conflicts of interest. J. J. T. reports personal fees from Novartis vaccines and diagnostics, personal fees from Merck vaccines, grants from Novartis, grants from Sanofi, grants from CSL, grants from Pfizer, grants from Takeda, and grants from NIH, outside the submitted work. A. N. reports grants from Centers for Disease Control and Prevention, during the conduct of the study, and grants from GlaxoSmithKline, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Schwartz B, Hinman A, Abramson J, et al. Universal influenza vaccination in the United States: are we ready? J Infect Dis. 2006;194:S147–54. doi: 10.1086/507556. [DOI] [PubMed] [Google Scholar]
- 2.Bridges CB, Thompson WW, Meltzer MI, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA. 2000;284:1655–63. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 3.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–9. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 4.Lambre CR, Terzidis H, Greffard A, et al. Measurement of anti-influenza neuraminidase antibody using a peroxidase-linked lectin and microtitre plates coated with natural substrates. J Immunol Methods. 1990;135:49–57. doi: 10.1016/0022-1759(90)90255-t. [DOI] [PubMed] [Google Scholar]
- 5.Cate TR, Rayford Y, Nino D, et al. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine. 2010;28:2076–8. doi: 10.1016/j.vaccine.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavrilov V, Orekov T, Alabanza C, et al. Influenza virus-like particles as a new tool for vaccine immunogenicity testing. J Virol Methods. 2011;173:364–73. doi: 10.1016/j.jviromet.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Gaglani M, Spencer S, Ball S, et al. Antibody response to influenza A(H1N1)pdm09 among healthcare personnel receiving trivalent inactivated vaccine: effect of prior monovalent inactivated vaccine. J Infect Dis. 2014;209:1705–14. doi: 10.1093/infdis/jit825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson BE, Kilbourne ED. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competion. J Virol. 1993;67:5721–3. doi: 10.1128/jvi.67.10.5721-5723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bruijn IA, Remarque EJ, Jol-van der Zijde CM, et al. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J Infect Dis. 1999;179:31–6. doi: 10.1086/314540. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Sobrido L, Cadagan R, Steel J, et al. Hemagglutinin-pseudotyped GFP-expressing influenza viruses for the detection of influenza virus neutralizing antibodies. J Virol. 2010;84:2157–63. doi: 10.1128/JVI.01433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker SF, Guo H, Albrecht RA, et al. Protection against lethal influenza with a viral mimic. J Virol. 2013;87:8591–605. doi: 10.1128/JVI.01081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Kadowaki S, Yoshikawa H, et al. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine. 2000;18:3214–22. doi: 10.1016/s0264-410x(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 13.Sandbulte MR, Westgeest KB, Gao J, et al. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc Natl Acad Sci U S A. 2011;108:20748–53. doi: 10.1073/pnas.1113801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couch RB, Atmar RL, Keitel WA, et al. Randomized comparative study of the serum antihemagglutinin and neuraminidase antibody responses to six licensed trivalent influenza vaccines. Vaccine. 2012;31:190–5. doi: 10.1016/j.vaccine.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Payment P, Trudel M. Methods and Techniques in Virology. New York: Marcel Dekker; 1993. [Google Scholar]
- 16.Murphy BR, Kasel JA, Chanock RM. Association of serum antineuraminidase antibody with resistance to influenza in man. N Engl J Med. 1972;286:1329–32. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- 17.Xie H, Li XJ, Gao J, et al. Revisiting the 1976 "swine flu" vaccine clinical trials: cross-reactive hemaggutinin and neuraminidase antibodies and their role in protection against the 2009 H1N1 pandemic virus in mice. Clin Infect Dis. 2011;53:1179–87. doi: 10.1093/cid/cir693. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 19.Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166:1121–7. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 20.Falsey AR, Treanor JJ, Tornieporth N, et al. Superior immunogenicity of high dose influenza vaccine compared with standard influenza vaccine in adults >65 years of age: a randomized double-blinded controlled phase 3 trial. J Infect Dis. 2008;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 21.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 22.Marcelin G, Bland HM, Negovetich NJ, et al. Inactivated seasonal influenza vaccines increase serum antibodies to the neuraminidase of pandemic influenza A(H1N1) 2009 virus in an age-dependent manner. J Infect Dis. 2010;202:1634–8. doi: 10.1086/657084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner EM, Bernstein ED, Dran S, et al. Characterization of antibody responses to annual influenza vaccination over four years in a healthy elderly population. Vaccine. 2001;19:4610–7. doi: 10.1016/s0264-410x(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 24.Fisher BM, Van Bockern J, Hart J, et al. Pandemic influenza A H1N1 infection versus vaccination: a cohort study comparing immune responses in pregnancy. PLoS One. 2012;7:e33048. doi: 10.1371/journal.pone.0033048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhr JW, Moller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- 26.Monto AS, Kendal AP. Effect of neuraminidase antibody on Hong Kong influenza. Lancet. 1973;7804:623–5. doi: 10.1016/s0140-6736(73)92196-x. [DOI] [PubMed] [Google Scholar]
- 27.Clements ML, Betts RF, Tierney EL, et al. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couch RB, Atmar RL, Franco LM, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. 2013;207:974–81. doi: 10.1093/infdis/jis935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–73. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 30.Johansson BE, Bucher DJ, Kilbourne ED. Purified influenza virus hemagluttinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol. 1989;63:1239–46. doi: 10.1128/jvi.63.3.1239-1246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez C, del Rio L, Partela A, et al. Evolution of the influenza virus neuraminidase gene during drift of the N2 subtype. Virology. 1983;130:539–45. doi: 10.1016/0042-6822(83)90108-3. [DOI] [PubMed] [Google Scholar]
- 32.Sandbulte MR, Jimenez GS, Boon AC, et al. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4:e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westgeest KB, De Graaf M, Fourment M, et al. Genetic evolution of the neuraminidase of influenza A (H3N2) viruses from 1968 to 2009 and its correspondence to haemagglutinin evolotion. J Gen Virol. 2012;93:1996–2007. doi: 10.1099/vir.0.043059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]