Compared to a control behavioral intervention, Contingency Management, an escalating voucher-based incentive system to reinforce stimulant abstinence, better supported MSM stimulant users in PEP course completion, decreased stimulant use, and a trend toward fewer condomless sexual acts.
Keywords: combination prevention, contingency management, HIV prevention, men who have sex with men, postexposure prophylaxis
Abstract
Background. Stimulant-using men who have sex with men (MSM) are at high risk of human immunodeficiency virus (HIV) acquisition. Contingency Management (CM) is a robust substance abuse intervention that provides voucher-based incentives for stimulant-use abstinence.
Methods. We conducted a randomized controlled trial of CM with postexposure prophylaxis (PEP) among stimulant-using MSM. Participants were randomized to CM or a noncontingent “yoked” control (NCYC) intervention and observed prospectively. Generalized linear models were used to estimate the effect of CM on PEP course completion, medication adherence, stimulant use, and sexual risk behaviors.
Results. At a single site in Los Angeles, 140 MSM were randomized to CM (n = 70) or NCYC (n = 70). Participants were 37% Caucasian, 37% African American, and 18% Latino. Mean age was 36.8 (standard deviation = 10.2) years. Forty participants (29%) initiated PEP after a high-risk sexual exposure, with a mean exposure-to-PEP time of 32.9 hours. PEP course completion was greater in the CM group vs the NCYC group (adjusted odds ratio [AOR] 7.2; 95% confidence interval {CI}, 1.1–47.9), with a trend towards improved medication adherence in the CM group (AOR, 4.3; 95% CI, 0.9–21.9).
Conclusions. CM facilitated reduced stimulant use and increased rates of PEP course completion, and we observed a trend toward improved adherence. Participants in the CM group reported greater reductions in stimulant use and fewer acts of condomless anal intercourse than the control group. This novel application of CM indicated the usefulness of combining a CM intervention with PEP to produce a synergistic HIV prevention strategy that may reduce substance use and sexual risk behaviors while improving PEP parameters.
Postexposure prophylaxis (PEP) is an emergency chemoprophylactic intervention deployed after an exposure to viral inoculum [1]. PEP protocols involve administering human immunodeficiency virus (HIV) antiretroviral therapy as quickly as possible after such an exposure, and it is continued for 28 days [1]. PEP is generally considered to be ineffective if administered later than 72 hours after an exposure, and some guidelines recommend an even more conservative “window” in which PEP should be considered [2]. As a result of the limited efficacy data to support administration of PEP, guidelines for the use of PEP have been developed based primarily on expert opinion [2, 3]. Separate guidance is available for occupational exposures (needle-sticks and infectious body fluid-to mucous membrane splashes [4]) and nonoccupational exposures (sexual and injection drug paraphernalia-related exposures [2, 3]).
Stimulant-using men who have sex with men (MSM) are at high risk of HIV acquisition [5, 6] largely as a consequence of increased numbers of condomless sexual encounters while under the influence of stimulant substances [7–9]. MSM represent the majority of incident and prevalent HIV infections in the United States and in Los Angeles County [10]. Attributable risk data suggest that stimulants are driving the HIV epidemic among MSM in Los Angeles and likely other US cities [11, 12]. Stimulant users are a challenging population in which to implement PEP, because each of the critical parameters for PEP efficacy (time from exposure to medication initiation [13], medication adherence [14], and 28-day course completion [15]) may be compromised by their stimulant use [16–18].
Based on the principles of operant conditioning, Contingency Management (CM) is a behavioral therapy intervention that uses positive nondrug reinforcers against drug use [19]. Voucher-based incentive therapy is a form of CM that provides increasing value rewards, in the form of voucher points, for stimulant abstinence. CM has been shown to be a powerful and durable stimulant-use intervention [20, 21]. We previously reported that in a single-arm pilot intervention of PEP combined with CM in methamphetamine-using MSM, time to PEP initiation, adherence, and course completion rates were not different from nonsubstance-using groups of MSM [22]. For this study, we hypothesized that CM would reduce stimulant use and, by association, serve as a mechanism to increase PEP initiation, adherence and completion, and reduce sexual risk behaviors.
METHODS
Study Participants
This study included HIV-uninfected adult males at a single site in Los Angeles, California. Participants self-identified as MSM, reported stimulant use (methamphetamine, amphetamine, and/or powder or crack cocaine) in the past 30 days, and participated in condomless anal intercourse with an HIV-positive or serostatus-unknown partner in the previous 3 months. Participants were required to have evidence of a calculated creatinine clearance ≥30 mL/minute and did not require hemodialysis. Potential participants who had used PEP in the previous 6 months were excluded.
Study Design
This was a randomized, prospective open-label trial of tenofovir/emtricitabine-based (Truvada, Gilead Sciences) PEP for sexual exposures. Two-drug PEP is the standard-of-care for the public health department-funded PEP programs in Los Angeles County, except in documented cases of exposure to resistant virus. Participants were recruited from (1) print and on-line advertising and (2) venue-based outreach at bars, clubs, commercial sex venues, and service locations known to be frequented by stimulant users. Participants were observed for the longer of 6 months after enrollment or for 6 months after the most recent high-risk sexual exposure triggering PEP use. Participants were randomly assigned to receive one of two 8-week behavioral interventions beginning at study entry: CM or a noncontingent “yoked” control (NCYC) group. The CM group provided voucher-based incentives [23] for thrice-weekly (Mondays, Wednesdays, Fridays) urine samples that were stimulant metabolite-free, which included a rapid reset after relapse [24]. Participants earned a voucher worth $2.50 for their first urine sample that was negative for stimulant metabolites. Successive urine samples that were negative for stimulant metabolites increased by $1.25 until the value of a urine sample negative for stimulant metabolites reached $20.00. From that point on, all subsequent negative urine samples were also valued at $20.00. Participants received a $10 bonus for 3 consecutive urine samples negative for stimulant metabolites. The maximal CM payout earned if all 24 urine samples tested negative for stimulant metabolites was $430. Participants receive no vouchers for urine samples positive for stimulant metabolites. Vouchers were redeemable for goods or services that were health-promotional or prosocial (eg, gift cards to grocery stores, department stores, or gas stations; IT support services to repair a computer; payment of a bill). No cash was provided.
To evaluate the efficacy of CM, an NCYC condition was used for comparison; incentives in the NCYC were not tied to substance abstinence. The NCYC group randomly paired study participants to a previously enrolled CM group participant (the “index” participant). Both the NCYC participant and their paired index were unaware of the identity of their pairing assignments. The NCYC participant received voucher-based incentives based solely on the urine metabolite analysis results of the index participant at the corresponding visit in the index's study visit schedule. In this way, the NCYC participant received voucher-based payments independent of his own urine metabolite results.
If participants reported a high-risk exposure to HIV within the 72 hours prior to study entry, “immediate” PEP with tenofovir/emtricitabine was initiated. If such exposure was not reported, participants were provided a 4-day starter-pack of tenofovir/emtricitabine at study entry with which to initiate PEP rapidly in the event of a high-risk exposure to HIV, and they received education on how and in which circumstances PEP should be initiated, according to Centers for Disease Control and Prevention/Department of Health and Human Services Guidelines [3]. “Delayed” PEP, ie, not initiated at baseline, could be initiated at any time during the study through the 6 months postenrollment follow-up. When participants initiated the PEP course via the 4-day starter pack, and they were asked to present to the clinic site within those 4 days of medication “coverage” for PEP baseline assessments and further treatment. Additional medication was not dispensed until the participant presented to the study site for evaluation.
Participants were tested for HIV infection (Oraquick, Orasure Technologies) and sexually transmitted infections (STIs) at baseline, 3 months, and 6 months postenrollment, as well as in a parallel schedule for PEP initiators: HIV and STI testing additionally occurred at PEP initiation and 4–6 weeks, 3 months, and 6 months post-PEP initiation. Sexually transmitted infection testing included rectal, urinary, and pharyngeal nucleic acid amplification testing for Neisseria gonorrhoeae and Chlamydia trachomatis (Aptima, Holistic GenProbe Inc.), as well as serum rapid plasma regain testing (RPR, Arlington Scientific, Inc.). Participants completed a behavioral assessments and substance use assessments at baseline, 3 months, and 6 months postenrollment and the Beck Depression Inventory weekly during the 8-week behavioral intervention.
Statistical Analysis
The primary objective was to evaluate the CM group in PEP-initiators with regard to time from exposure to first dose, PEP medication adherence, and PEP course completion. Time from exposure to first dose was estimated based on participant self-report of the most recent relevant HIV exposure to the time of directly observed first dose of PEP medication at the study site. Medication adherence was estimated from pharmacy dispensation records, returned pill counts, and participant self-report. PEP course completion was assessed from pharmacy dispensation records and participant self-report, reported at the 4- to 6-week post-PEP visit. The target sample size of 70 participants per group would provide 80% power to detect a 39% difference in medication adherence rates between the CM and NCYC groups. This assumed a 32% PEP initiation rate across the study population. Secondary objectives were to evaluate changes in numbers of sexual partners and condomless anal sex during the study period, changes in stimulant use, and an assessment of a CM-based “dose-response” analysis with regard to the primary endpoints.
Descriptive statistics are provided for participant sociodemographics as well as primary and secondary study outcomes (including PEP time-from-exposure-to-initiation, medication adherence, course completion, participant sexual risk-taking, and stimulant use). Sensitivity analyses prompted by inflated dispersion metrics related to the sexual risk-taking data revealed that there was nontrivial influence from outliers in the assessed number of times a participant engaged in condomless anal intercourse in the past 30 days; data related to episodes of sexual risk was thus censored at 30 episodes; this censoring process affected data from 4 participants (2.9%).
Student t tests and χ2 or Fisher's exact analyses were used to test the bivariate associations between primary and secondary outcomes and randomization to receive CM. Robust multivariate binomial logistic (for dichotomous or proportional outcomes) and negative binomial (for counted outcomes) models tested the association between primary or secondary outcomes and randomization to receive CM while controlling for potentially relevant covariates, including the following: race/ethnicity [25], income [26], sexual identity [27–29], and current homelessness [30]. Reported statistical tests are 2-tailed. Analyses were performed with the use of Stata, version 13 (StataCorp, College Station, Texas).
Safety Reporting
Toxicity was assessed by adverse event reporting according to the National Institutes of Health Division of AIDS Adverse Event Grading Scale (2004). Adverse events were managed according to standard clinical care practices. An independent data safety monitoring board reviewed the data on 6 occasions during study conduct; no efficacy or safety concerns were noted.
Human Subjects Considerations
The protocol was approved by the Institutional Review Boards at Friends Research Institute and the University of California, Los Angeles, and all participants provided informed consent before participating in any study procedures. The protocol is registered at clinicaltrials.gov as NCT01140880 and was conducted under US Food and Drug Administration Investigational New Drug Application 101 892.
RESULTS
Study Participants
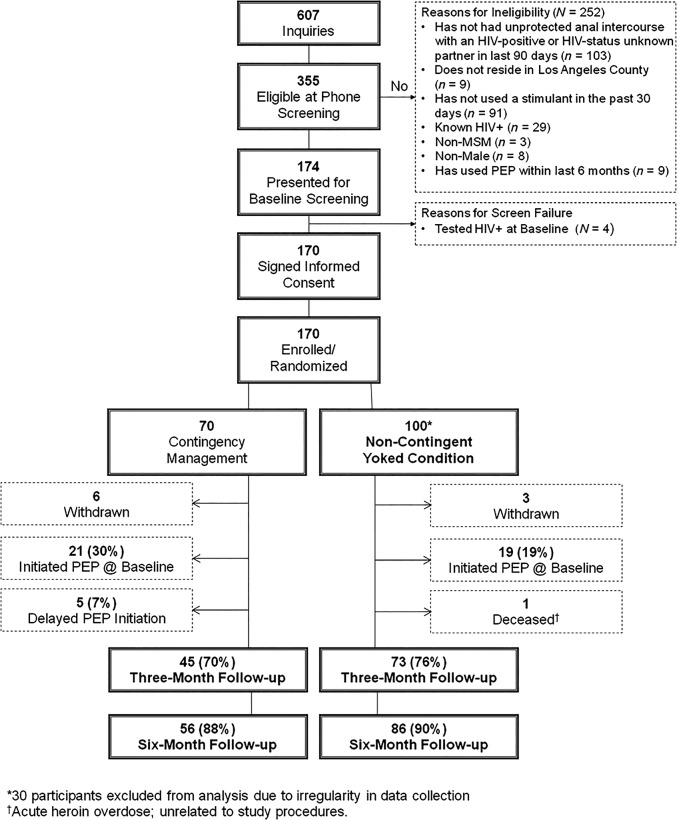
Between June 2010 and June 2012, 607 individuals inquired about the study: 355 were eligible at telephone screening and 174 presented for baseline screening. Four individuals tested HIV-positive at screening and were not enrolled, and they were facilitated into HIV care. One hundred seventy individuals were enrolled and randomized to the CM (n = 70) or NCYC (n = 100) behavioral intervention. Of those, 30 participants in the NCYC group did not have evaluable data due to a problem in data collection that resulted in their permanent loss. These participants were excluded from the intent-to-treat analysis, yielding 140 evaluable participants, 70 in each group (Figure 1). Participants excluded were statistically more likely to be gay-identified and were less likely to be homeless; they were otherwise demographically similar, and they did not differ in their rates of PEP initiation compared with the included population. The additional 30 participants enrolled into the NCYC cohort were enrolled contemporaneously with the remaining CM participants in approximately a 2:1 randomization scheme.
Figure 1.
Study populations, dispositions, and reasons for nonparticipation. Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; PEP, postexposure prophylaxis.
Baseline demographics are shown in Table 1. Participants were 37% Caucasian/white, 37% African American/black, and 18% Hispanic/Latino. Mean age was 36.8 (standard deviation [SD] = 11.1) years, 63% had high school education or less, and 92% reported an annual income ≤$30 000. Participant retention rates at each study visit are shown in Figure 1, and rates were 89% overall at the 6-month follow-up visit.
Table 1.
Participant Sociodemographic Characteristics by Randomized Condition Assignment
| NCYC (n = 70) | CM (n = 70) | Total (N = 140) | ||
|---|---|---|---|---|
| N (%) or Mean (SD) | N (%) or Mean (SD) | N (%) or Mean (SD) | P Valuea | |
| Race/Ethnicity | ns | |||
| Caucasian/White | 27 (38.6%) | 25 (35.7%) | 52 (37.1%) | |
| African American/Black | 27 (38.6) | 25 (35.7%) | 52 (37.1%) | |
| Native American | 1 (1.4%) | 3 (4.3%) | 4 (2.9%) | |
| Asian/Pacific Islander | 2 (2.9%) | 1 (1.4%) | 3 (2.1%) | |
| Hispanic/Latino | 11 (15.7%) | 14 (20.0%) | 25 (17.9%) | |
| Multiracial/Other | 2 (2.9%) | 2 (2.9%) | 4 (2.9%) | |
| Sexual Identity | ns | |||
| Gay | 28 (40.0%) | 37 (52.9%) | 65 (46.4%) | |
| Bisexual | 39 (55.7%) | 31 (44.3%) | 70 (50.0%) | |
| Heterosexual | 2 (2.9%) | 0 (0.0%) | 2 (1.4%) | |
| Other | 1 (1.4%) | 2 (2.9%) | 3 (2.1%) | |
| Age, years | 35.8 (10.3) | 37.9 (11.8) | 36.8 (11.1) | ns |
| Educational Attainment | ns | |||
| Less than HS | 17 (24.3%) | 17 (24.3%) | 34 (24.3%) | |
| HS Diploma/GED | 28 (40.0%) | 26 (37.1%) | 54 (38.6%) | |
| More than HS | 25 (35.7%) | 27 (38.6%) | 52 (37.1%) | |
| Annual incomeb | ns | |||
| Less than $15 000 | 63 (90.0%) | 54 (78.3%) | 117 (84.2%) | |
| $15 001–$30 000 | 4 (5.7%) | 7 (10.1%) | 11 (7.9%) | |
| $30 001–$60 000 | 3 (4.3%) | 3 (4.4%) | 6 (4.3%) | |
| More than $60 000 | 0 (0.0%) | 5 (7.3%) | 5 (3.6%) | |
| Housing Statusc | P ≤ .05 | |||
| Own/Rent House/Apt. | 14 (20.6%) | 26 (37.1%) | 40 (29.0%) | |
| Group Housing/Sober Living | 2 (2.9%) | 6 (8.6%) | 8 (5.8%) | |
| With Family/Friends | 13 (19.1%) | 12 (17.1%) | 25 (18.1%) | |
| No Current Address/Homeless | 39 (57.4%) | 26 (37.1%) | 65 (47.1%) | |
Abbreviations: CM, contingency management; GED, General Education Development; HS, high school; NCYC, noncontingent yoked condition; ns, not significant; SD, standard deviation.
a ns = not significant; all significance tests 2-tailed.
b One participant declined to state annual income.
c Two participants declined to state housing status.
Forty of the 140 enrolled participants (28.6%) initiated PEP after a high-risk exposure during the study period: 26 in the CM group and 14 in the NCYC group. In the CM group, 21 of 26 PEP initiations were at baseline and 5 were delayed. In the NCYC group, all PEP initiators were at baseline. Follow-up rates for the PEP-specific visits at week 4–6 and 3- and 6-months postexposure were 62%, 75%, and 76%, respectively.
Behavioral Intervention
Of the possible 24 study visits for provision of urine samples for the behavioral intervention, mean attendance in the CM and NCYC groups was 7.8 (SD = 8.9) and 6.3 (SD = 6.5) visits, P = .27. Attendance was also not different between groups during the first month of study participation, during which time immediate PEP initiators would have been receiving study drug (data not shown). Voucher-based earnings were not statistically different on average between the 2 groups (CM median [Md] = $22.50, interquartile range [IQR] = $0–$152.50; NCYC Md = $27.50, IQR = $0–$102.50).
Time From Exposure to Postexposure Prophylaxis Initiation, Adherence, and Completion
Primary outcomes are shown in Table 2. Time from exposure to first PEP medication dose was not different between CM and NCYC participants (32.8 hours vs 33.0 hours, P = .98). Thirty of the 40 PEP initiators had evaluable adherence data. Observed PEP medication adherence rates were significantly higher in the CM group compared with the NCYC group (74.7% vs 44.6%, P = .05), and participants in the CM group were also observed to be significantly more likely to successfully complete their full course of PEP medication (70.6% vs 30.8%; P = .03) than participants in the NCYC group. After applying statistical controls, the CM group revealed marginally higher rates of medication adherence (adjusted odds ratio [AOR] = 4.3; 95% confidence interval [CI], 0.9–21.9; P = .08) and significantly higher odds of PEP course completion (AOR = 7.2; 95% CI, 1.1–47.9; P = .04) compared with the NCYC group.
Table 2.
Bivariate and Multivariate Associations Between PEP-Related Outcomes and Conditionsa
| Time to Initiation (NCYC = 14; CM = 26) |
Medication Adherence (NCYC = 13; CM = 17) |
Course Completion (NCYC = 13; CM = 17) |
||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate |
Bivariate |
Bivariate |
||||||
| Student t test |
Student t test |
Pearson's χ2 |
||||||
| NCYC | CM | P Value | NCYC | CM | P Value | NCYC | CM | P Value |
| Mean = 33.0 | Mean = 32.8 | P = .98 | Mean = 0.45 | Mean = 0.75 | P = .05 | n = 4/13 | n = 12/17 | P = .03 |
| SD = 16.1 | SD = 15.1 | SD = 0.39 | SD = 0.40 | 30.8% | 70.6% | |||
| Multivariateb (NCYC = 14; CM = 25) | Multivariateb (NCYC = 13; CM = 16) | Multivariateb (NCYC = 13; CM = 16) | ||||||
| Robust least squares linear regression | Robust binomial logistic regression | Robust binomial logistic regression | ||||||
| Coef. (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| CM | 0.32 (−12.7–13.3) | P = .96 | CM | 4.33 (0.86–21.85) | P = .08 | CM | 7.22 (1.09–47.90) | P = .04 |
Abbreviations: CI, confidence interval; CM, contingency management; Coef., coefficient; NCYC, noncontingent yoked condition; OR, odds ratio; PEP, postexposure prophylaxis; SD, standard deviation.
a All significance tests 2-tailed.
b Controls: race/ethnicity, sexual identity, income, homelessness.
Stimulant Use and Sexual Risk Behaviors
Secondary outcomes are presented in Table 3. Significantly more urine samples negative for stimulant metabolites were submitted by participants in the CM group compared with those in the NCYC group (8.9 [SD = 9.2] vs 6.1 [SD = 6.1], P = .035). After applying sociodemographic controls, CM participants exhibited a significantly greater probability of submitting stimulant-free urine specimens vs NCYC participants (incidence rate ratio [IRR], 1.6; 95% CI, 1.1–2.2). A quantitative difference was sustained at the 6-month follow-up visit with 69.6% of CM participants and 59.3% of NCYC participants producing a stimulant-free urine sample, although the difference did not achieve statistical significance. Student t tests also revealed significant reductions in episodes of condomless anal intercourse from baseline to 6-month follow-up in the CM group (CMBL = 3.8 [SD 5.6]; CM6mFU = 0.8 [SD = 1.8]; P < .001) but not in the NCYC group (NCYCBL = 3.2 [SD = 6.0]); NCYC6mFU = 1.4 [SD = 5.1]; P = ns). When compared with each other, the CM and NCYC groups did not report significantly divergent numbers of condomless anal intercourse episodes by 6-month follow-up at the bivariate level (CM6mFU = 0.8 [SD = 1.8]; NCYC6mFU = 1.4 [SD = 5.1]; P = ns) or after controlling for participant sociodemographics (IRRCM = 0.66; P = ns).
Table 3.
Bivariate and Multivariate Associations Between Sexual Risk-taking/Stimulant-use Outcomes and Conditions
| No. of Male Sexual Partners at 6 m Follow-up |
No. Times Engaged in Condomless Anal Intercourse at 6 m Follow-up |
No. of Stimulant Metabolite-free Urine Samples Submitted During Intervention |
||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate (NCYC = 59; CM = 56) |
Bivariate (NCYC = 59; CM = 56) |
Bivariate (NCYC = 70; CM = 70) |
||||||
| Student t test |
Student t test (Unequal Variance) |
Student t test |
||||||
| NCYC | CM | P Value | NCYC | CM | P Value | NCYC | CM | P Value |
| Mean = 1.48 | Mean = 1.68 | P = .60 | Mean = 1.39 | Mean = 0.82 | P = .43 | Mean = 6.06 | Mean = 8.87 | P = .04 |
| SD = 1.99 | SD = 2.11 | SD = 5.06 | SD = 1.78 | SD = 6.12 | SD = 9.21 | |||
| Multivariatea (NCYC = 57; CM = 55) |
Multivariatea (NCYC = 57; CM = 55) |
Multivariatea (NCYC = 68; CM = 69) |
||||||
| Robust Negative Binomial Regression |
Robust Negative Binomial Regression |
Robust Negative Binomial Regression |
||||||
| IRR (95% CI) | P Value | IRR (95% CI) | P Value | IRR (95% CI) | P Value | |||
| CM | 1.10 (0.67–1.81) | P = .71 | CM | .66 (0.26–1.69) | P = .39 | CM | 1.57 (1.12–2.22) | P = .01 |
All significance tests 2-tailed.
Abbreviations: CI, confidence interval; CM, contingency management; IRR, incidence rate ratio; NCYC, noncontingent yoked condition; SD, standard deviation.
a Controls: race/ethnicity, sexual identity, income, homelessness.
Contingency Management Voucher's Dose Response
No associations were found between the amount of vouchers earned and PEP medication adherence, PEP course completion, or self-reported sexual risk behavior (results not shown).
Incident Human Immunodeficiency Virus and Sexually Transmitted Infections
Prevalent and incident STIs are described in Table 4. One HIV seroconversion (1 of 140; 0.7% 95% CI, 0.1%–0.4%) was observed during the study; the seroconversion occurred in the context of repeat sexual exposures and multiple courses of PEP at other institutions, subsequent to the index exposure reported during study participation.
Table 4.
Prevalent and Incident Sexually Transmitted Infections by Study Time Point
| Infection | Baseline Prevalence | Incident Infection 3 m Follow-up | Incident Infection 6 m Follow-up |
|---|---|---|---|
| n/N (%) | n/N (%) | n/N (%) | |
| Hepatitis B | 0/140 (0.0%) | 0/103 (0.0%) | 0/115 (0.0%) |
| Syphilis | 5/140 (3.6%) | 2/103 (1.9%) | 0/115 (0.0%) |
| Gonorrhea | 0/140 (0.0%) | 1/103 (1.0%) | 1/115 (0.9%) |
| Chlamydia | 12/140 (8.6%) | 0/103 (0.0%) | 3/115 (2.6%) |
DISCUSSION
In addition to the previously noted effects on substance use, monetary incentives have proven highly effective for smoking cessation [31], weight loss [32], and Coumadin adherence [33], as well as children's education, families' preventive healthcare utilization, and parents' employment [34].
In this randomized, controlled trial of stimulant-using, HIV-uninfected MSM, CM (1) facilitated the use of PEP by reducing stimulant use and increasing rates of course completion and (2) suggesting improved adherence, 2 of the 3 critical inputs in PEP efficacy. The ability to optimize these parameters, along with exposure-to-dose interval, may contribute to differential efficacy. Concerns about the use of postexposure strategies with substance users were largely borne out of unsubstantiated perceptions that substance users would be less likely to present within a proscribed efficacy window (primarily due to residual effects of a substance-use episode) and challenges to adherence and follow-up faced by substance users. The absence of a difference in exposure-to-dose time between the CM and control groups in the current study was not unexpected, because the overwhelming majority of PEP initiations in the study occurred at the time of enrollment—before randomization and behavioral intervention initiation. The mean exposure-to-dose time (overall 32.9 hours, for CM participants 32.8 hours) was shorter than the same parameter from our group's single-group pilot intervention (37.8 hours [22]) and similar to other nonsubstance-using cohorts (mean, 33 hours [35]). The consistency of this parameter across multiple studies confirms that PEP initiation within a reasonable window is feasible in populations of stimulant users.
Adherence to PEP, as with ART for HIV treatment, is a challenge to measure; self-report is fraught with social desirability bias and recall bias [36, 37]. Plasma, hair, and intracellular drug levels are the best objective proxy of adherence [38, 39]; cost and turn-around for such assays prevent such biomarkers from being clinically useful in most studies of PEP. For these reasons, triangulation of adherence using pill count and self-report becomes increasingly attractive, and it may be the only available option when PEP is delivered in community-based settings. Substance use has been associated with poor medication adherence and, in HIV-infected populations, worsened clinical outcomes [40], leading many to discount substance users as reasonable PEP candidates. Although limited numbers of PEP initiators in the current study provided reduced power to demonstrate a difference in self-reported medication adherence, the absolute adherence rate of 74.7% observed in the CM group compares extremely favorably with other cohorts and with a recent meta-analysis of adherence across PEP cohorts and studies [41].
The single-arm pilot data suggested exposure-to-dose time, adherence, and course completion parameters were similar to nonsubstance-using populations [22]; however, that study was not designed to estimate the efficacy of CM for reaching those parameters. The current randomized controlled study suggests that the mechanism for those adherence and course completion parameters indeed may be attributable to the CM intervention. The overall low uptake of the CM intervention and lack of an observed dose-response association between negative urine toxicology result frequency and PEP parameters prevent definitive association.
A key attraction of combining CM with PEP is one of HIV prevention synergy: CM is known to reduce substance use with substantial efficacy and durability, even with brief interventions on the order of 8 weeks [21, 42]. Such substance abstinence, in addition to being of general health benefit, would be expected to have HIV-prevention benefits primarily [43]. Stimulants, and methamphetamine in particular, have been found to up-regulate CD4 expression on gastrointestinal lymphocytes and antigen-presenting cells in rectal mucosa (Anton Peter, Personal Communication, May 21, 2014) and CCR5 expression on macrophages [44] that may correlate with increased susceptibility to infection.
Stimulant use is associated with increased transmission risk behavior manifested by increases in numbers of sexual partners and episodes of condomless anal intercourse. In this study, we found some evidence for reduced transmission risk behavior and reduced stimulant use in the CM group but not in the NCYC control group. This finding persists despite an assumed salutary effect of enrollment in a structured behavioral risk-reduction intervention more generally, which would be expected to apply to both intervention groups equally. The ability of PEP to abrogate acute infection risk is extremely attractive to combine with the anticipated more long-term prevention benefits of stimulant abstinence and reduced transmission risk behavior, because it provides immediate protection while awaiting the onset of the more delayed-onset but more durable effects of drug abstinence on biologic susceptibility and behavior change.
The small number of incident STIs seen in this study corroborates aggregate reductions in condomless sex, rather than merely acute HIV protection afforded by the PEP intervention. The single seroconverter in the study, in the context of multiple repeat exposures, and multiple repeat courses of PEP is a sobering reminder that unless behavior change is accomplished, seroconversion is highly likely in those who continue to engage in high-risk sexual behaviors. Such individuals may be better served by more sustained prophylaxis, such as that afforded by pre-exposure prophylaxis (PrEP). Happily, adherence to PrEP does not appear to be negatively affected by noninjecting substance users in a small subset of participants in the open-label iPrEX OLE study [45].
Increased rates of follow-up at 3- and 6-month PEP visits compared with the 4- to 6-week postexposure visit likely reflect the additional incentive provided at 3- and 6-month visits from coincident behavioral assessments that are part of the parent study; such visits occur regardless of PEP initiation.
These findings carry a number of limitations. The first is the obligatory unblinded nature of the intervention: participants randomized to the NCYC group provided fewer urine samples for stimulant metabolite assessment than did CM participants, limiting the power to find association between stimulant abstinence or CM earnings and observed outcomes in a dose-response fashion. The lack of any significant statistical associations between CM earnings and PEP/sexual risk outcomes may result from such a lack of statistical power (ie, a Type-II error), or it may be a function of the fact that improved PEP outcomes and/or reduced sexual risk-taking were not outcomes directly targeted by the CM mechanism in this study. Further research may look to clarify and expand on these potential explanations.
Another limitation was the lower-than-anticipated overall PEP initiation rate: in the pilot study, nearly 66% of participants initiated PEP. The reduced rate of PEP initiation in the current study likely reflects expanded PEP service availability in Los Angeles County in the interval between the pilot and this study: during conduct of the pilot phase, PEP was less widely available, making study participation one of few mechanisms for receiving PEP. By the time of current protocol conduct, PEP had become more widely available outside of the clinical trial context. In addition, the proportion of participants initiating PEP was not balanced between the CM and NCYC groups: the participants who initiated PEP at baseline were deemed PEP eligible or ineligible before their randomization assignments, and therefore the imbalance in at-baseline PEP initiations is purely out of chance (21 vs 14 baseline initiators), but it limits the power to compare the interventions' impact on PEP outcomes. The 5 delayed PEP initiators in the CM cohort may have been facilitated by the CM interventions, and such delayed PEP initiation would be hypothesized to be associated with more strategic use of PEP in the setting of reduced substance use.
Our markers of adherence were self-report and returned pill counts only, which are subject to social desirability bias and potential manipulation, respectively [46, 47]; however, the use of more expensive biomarkers requiring research-based assays would limit the general applicability of the findings to programs and policymakers. Lastly, sexual risk behavior and substance use questionnaires were assessed by face-to-face interview (rather than computer-assisted techniques) and may underestimate such behaviors due to social acceptance bias.
Refining the use of current HIV prevention strategies among those most at risk is a public health priority. MSM stimulant users are particularly at risk for HIV acquisition, due to concomitant sexual risk behaviors facilitated by the stimulant use, and may be driving the HIV epidemic among MSM in Los Angeles County. Yet, stimulant use has the potential to compromise the utility of biomedical prevention strategies. Findings from this study support combining a voucher-based CM intervention with PEP to exploit “prevention synergies” and improved PEP parameters. Despite small numbers of individuals initiating PEP, findings suggest that CM may be a useful support mechanism for the use of PEP and potentially other biomedical prophylactic strategies in stimulant-using MSM.
Acknowledgments
Financial support. This study was supported by the California HIV Research Program, grant number MC08-LA-710. R. J. L. acknowledges additional support from the National Institute of Drug Abuse at the National Institutes of Health (grant K23DA026308), and R. J. L., S. S., and C. J. R. acknowledge additional support from the National Institute of Mental Health at the National Institutes of Health (grant 5P30AI028697).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Landovitz RJ, Currier JS. Clinical practice. Postexposure prophylaxis for HIV infection. N Engl J Med. 2009;361:1768–75. doi: 10.1056/NEJMcp0904189. [DOI] [PubMed] [Google Scholar]
- 2.New York State Department of AIDS Health Institute. HIV prophyalxis following non-occupational exposure including sexual assault. 2010. Available at: http://www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/hiv-prophylaxis-following-non-occupational-exposure-including-sexual-assault/ . Accessed 22 October 2010.
- 3.Smith DK, Grohskopf LA, Black RJ, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the U.S. Department of Health and Human Services. MMWR Recomm Rep. 2005;54:1–20. [PubMed] [Google Scholar]
- 4.Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875–92. doi: 10.1086/672271. [DOI] [PubMed] [Google Scholar]
- 5.Plankey MW, Ostrow DG, Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drumright L, Patterson T, Strathdee S. Club drugs as casual risk factors for HIV acquisition among men who have sex with men: a review. Subst Use Misuse. 2006;41:1551–601. doi: 10.1080/10826080600847894. [DOI] [PubMed] [Google Scholar]
- 7.Purcell DW, Parsons JT, Halkitis PN, et al. Substance use and sexual transmission risk behavior of HIV-positive men who have sex with men. J Subst Abuse. 2001;13:185–200. doi: 10.1016/s0899-3289(01)00072-4. [DOI] [PubMed] [Google Scholar]
- 8.Stall R, Paul JP, Greenwood G, et al. Alcohol use, drug use and alcohol related problems among men who have sex with men: the urban men's health study. Addiction. 2001;96:1589–601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- 9.Colfax G, Coates TJ, Husnik MJ, et al. Longitudinal patterns of methamphetamine, popper (amyl nitrite), and cocaine use and high-risk sexual behavior among a cohort of San Francisco men who have sex with men. J Urban Health. 2005;82(1 Suppl 1):i62–70. doi: 10.1093/jurban/jti025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Division of HIV and STD Programs, Health LACDoP. 2012 Annual HIV Surveillance Report. 2013. Available at: http://publichealth.lacounty.gov/wwwfiles/ph/hae/hiv/2012AnnualHIVSurveillanceReport.pdf . Accessed 25 February 2014.
- 11.Koblin B, Husnik M, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20:731–9. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 12.Ostrow DG, Plankey MW, Cox C, et al. Specific sex drug combinations contribute to the majority of recent HIV seroconversions among MSM in the MACS. J Acquir Immune Defic Syndr. 2009;51:349–55. doi: 10.1097/QAI.0b013e3181a24b20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai CC, Follis KE, Sabo A, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine [see comments] Science. 1995;270:1197–9. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 14.Roland ME, Neilands TB, Krone MR, et al. Seroconversion following nonoccupational postexposure prophylaxis against HIV. Clin Infect Dis. 2005;41:1507–13. doi: 10.1086/497268. [DOI] [PubMed] [Google Scholar]
- 15.Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–73. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celentano DD, Lucas G. Optimizing treatment outcomes in HIV-infected patient with substance abuse issues. Clin Infect Dis. 2007;45(Suppl 4):S318–23. doi: 10.1086/522557. [DOI] [PubMed] [Google Scholar]
- 17.Reback CJ, Larkins S, Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS Care. 2003;15:775–85. doi: 10.1080/09540120310001618621. [DOI] [PubMed] [Google Scholar]
- 18.Scott JD, Bolan RK. Factors associated with poor follow-up to HIV post-exposure prophylaxis; Washington, DC. 2007. 2nd International Workshop on HIV Transmission. Abstract 71. [Google Scholar]
- 19.Hartzler B, Lash SJ, Roll JM. Contingency management in substance abuse treatment: a structured review of the evidence for its transportability. Drug Alcohol Depend. 2012;122:1–10. doi: 10.1016/j.drugalcdep.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–74. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 21.Reback CJ, Peck JA, Dierst-Davies R, et al. Contingency management among homeless, out-of-treatment men who have sex with men. J Subst Abuse Treat. 2010;39:255–63. doi: 10.1016/j.jsat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landovitz RJ, Fletcher J, Inzhakova G, et al. A novel combination HIV prevention strategy: postexposure prophylaxis with contingency management for substance abuse treatment among methamphetamine-using men who have sex with men. AIDS Patient Care STDS. 2012;26:320–8. doi: 10.1089/apc.2011.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins ST, Budney AJ, Bickel WK, et al. Achieving cocaine abstinence with a behavioral approach. Am J Psychiatry. 1993;150:763–9. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- 24.Roll JM, Shoptaw S. Contingency management: schedule effects. Psychiatry Res. 2006;144:91–3. doi: 10.1016/j.psychres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012;380:341–8. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 26.Stratford D, Mizuno Y, Williams K, et al. Addressing poverty as risk for disease: recommendations from CDC's consultation on microenterprise as HIV prevention. Public Health Rep. 2008;123:9–20. doi: 10.1177/003335490812300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palivos C. Compliance and the closet: correlations between HIV medication adherence and subjective measures of sexual identity in men. Los Angeles: Alliant International University; 2010. [Google Scholar]
- 28.Zellner JA, Martinez-Donate AP, Sanudo F, et al. The interaction of sexual identity with sexual behavior and its influence on HIV risk among Latino men: results of a community survey in northern San Diego County, California. Am J Public Health. 2009;99:125–32. doi: 10.2105/AJPH.2007.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders AD. HIV risk factors in African-American men: the relationship between body-mind-spirit well-being, sexual identity and sexual behavior among men who have sex with men. Washington, DC: Howard University; 2009. [Google Scholar]
- 30.Wolitski RJ, Kidder DP, Fenton KA. HIV, homelessness, and public health: critical issues and a call for increased action. AIDS Behav. 2007;11(6 Suppl):167–71. doi: 10.1007/s10461-007-9277-9. [DOI] [PubMed] [Google Scholar]
- 31.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. New Engl J Med. 2009;360:699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 32.Volpp KG, John LK, Troxel AB, et al. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300:2631–7. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volpp KG, Loewenstein G, Troxel AB, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8:272. doi: 10.1186/1472-6963-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riccio J, Dechausay N, Greenberg D, et al. Toward Rescued Poverty across Generations: Early Findings from New York City's Conditional Cash Transfer Program. 2010. Available at http://www.mdrc.org/publications/549/execsum.pdf . Accessed 17 March 2012.
- 35.Kahn JO, BBD W. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–9. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 36.Shuter J, Sarlo JA, Stubbs RO, et al. Sequential antiretroviral adherence measurement using electronic bottle cap monitors in a cohort of HIV-infected adults. J Int Assoc Physicians AIDS Care. 2012;11:94–7. doi: 10.1177/1545109711420498. [DOI] [PubMed] [Google Scholar]
- 37.Nellen JF, Nieuwkerk PT, Burger DM, et al. Which method of adherence measurement is most suitable for daily use to predict virological failure among immigrant and non-immigrant HIV-1 infected patients? AIDS Care. 2009;21:842–50. doi: 10.1080/09540120802612816. [DOI] [PubMed] [Google Scholar]
- 38.Liu AY, Yang Q, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP) PLoS One. 2014;9:e83736. doi: 10.1371/journal.pone.0083736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson P, Glidden DV, Liu AY, et al. Emtricitabine-tenofovir exposure and pre-exposure prophylaxis efficacy in men who have sex with men; Sci Transl Med; 2012. p. 151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howe CJ, Cole SR, Napravnik S, et al. The role of at-risk alcohol/drug use and treatment in appointment attendance and virologic suppression among HIV(+) African Americans. AIDS Res Hum Retroviruses. 2014;30:233–40. doi: 10.1089/aid.2013.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oldenburg C, Bärnighausen T, Harling G, et al. Adherence to post-exposure prophylaxis for non-forcible sexual exposure to HIV: a systematic review and meta-analysis. AIDS Behav. 2014;18:217–25. doi: 10.1007/s10461-013-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoptaw S, Reback CJ, Peck JA, et al. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend. 2005;78:125–34. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher JB, Rusow JA, Le H, et al. High-risk sexual behavior is associated with post-exposure prophylaxis non-adherence among men who have sex with men enrolled in a combination prevention intervention. J Sex Transm Dis. 2013;2013:210403. doi: 10.1155/2013/210403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang H, Wang X, Chen H, et al. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172:1617–24. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant RM, Anderson PL, McMahan V, et al. An observational study of preexposure prophylaxis uptake, sexual practices, and HIV incidence among men and transgender women who have sex with men. Lancet Infect Dis. 2014;14:820–9. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring medication adherence in research. AIDS Behav. 2013;17:284–97. doi: 10.1007/s10461-012-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S79–87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]