Half of all family members of children hospitalized with RSV infection were positive for RSV around the time of the child's admission. In most cases, the likely source of the infant's RSV infection was an older sibling or a parent.
Keywords: bronchiolitis, family transmission, respiratory syncytial virus, respiratory tract infection, vaccination
Abstract
Background. Because the production of an effective respiratory syncytial virus (RSV) vaccine for infants is challenging, vaccination of other family members is one viable alternative to prevent severe RSV illnesses in infants.
Methods. In a prospective study, we enrolled all family members of children who were hospitalized with RSV infection. Nasal swabs for RSV detection were obtained from all participating family members. Data on respiratory symptoms in the family members prior to and after the child's admission were collected using standardized questionnaires.
Results. At the time of or within 1 week after the index child's hospitalization, RSV was detected in 40 (77%) of the 52 families and in 60 (47%) of 129 family members. Forty-nine (82%) of RSV detections in the family members were associated with respiratory symptoms. A sibling or a parent was the probable primary case of RSV in 30 (58%) families. Respiratory syncytial virus loads in the nasal swabs were significantly higher (107.7) in index children than in their parents (105.1, P < .0001).
Conclusions. In most cases, the likely source of an infant's RSV infection is an older sibling or a parent. These findings support the strategy of reducing the burden of RSV in infants by vaccination of their family members.
Since its discovery in the 1950s, respiratory syncytial virus (RSV) has been known as a major cause of acute lower respiratory tract infection in children [1, 2]. According to a recent estimate, the global annual death toll from RSV infection is 66 000–199 000 children <5 years of age, and more than 3 million children in this age group are hospitalized due to RSV [3]. Although RSV infections occur frequently in all age groups, including the elderly [4–6], the burden of this virus is clearly greatest among the youngest infants who experience the highest rates of RSV-associated emergency department visits and hospitalizations [7–9].
The well established clinical burden of RSV has called for the development of safe and effective vaccines, but little progress was seen during the first decades after the discouraging attempts to produce a vaccine in the late 1960s [10]. In recent years, however, a number of different types of candidate RSV vaccines have been developed and are being tested [11]. Although infants <6 months of age are the highest priority target population for an RSV vaccine, there are great challenges in producing an immunogenic vaccine for this age group. Alternative strategies of preventing RSV infections in young infants include the vaccination of mothers during pregnancy and vaccination of older siblings, parents, and other close persons who could transmit the virus to the high-risk infants [11]. Few previous studies have directly examined the transmission of RSV within households [12–16], and more data are needed to guide optimal use of future RSV vaccines. We conducted this prospective study to assess the spread of RSV within families.
METHODS
Study Design
This prospective, single-center study was carried out at the Department of Pediatrics, Turku University Hospital, Finland, during the RSV season of 2005–2006. The study was approved by the Ethics Committee of the Hospital District of Southwest Finland, and it was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the parents of all children.
Participants and Study Procedures
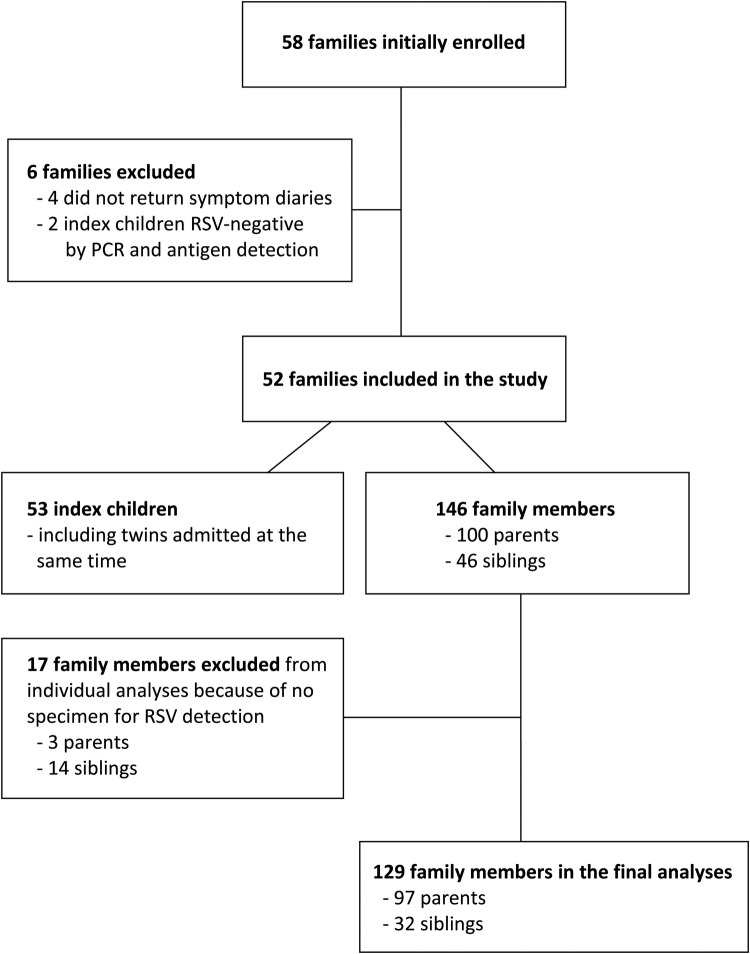
All family members of children who were hospitalized with signs and symptoms of lower respiratory tract infection and who had a positive rapid RSV test on admission were initially enrolled in the study. The index child's RSV infection was confirmed by antigen detection and reverse-transcriptase polymerase chain reaction (RT-PCR) assays. In 2 index children, these confirmatory tests were negative, and these families were excluded from the analyses (Figure 1).
Figure 1.
Flow chart of the study. Abbreviations: PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
Nasal swab samples for RSV detection were obtained from all participating family members either on the day of the index child's admission or on the following day. These samples were taken at the hospital by healthcare professionals. The parents were asked to fill out a standardized questionnaire that inquired about detailed symptoms and timing of respiratory tract infections in each family member during the 2 weeks preceding the index child's admission. The families were also provided with symptom diaries in which they were asked to record daily any symptoms of respiratory tract infection in any family member during the 2 weeks after the index child's admission. In case any sibling or parent developed fever or symptoms of respiratory tract infection during the follow-up period, they were asked to self-sample nasal swabs from the symptomatic subjects by using the kits provided to them and to mail the specimens to the laboratory. The parents were carefully instructed about the sampling procedure by the study personnel.
Virological Methods
Nasopharyngeal aspirates were collected from all index children to confirm RSV infection. The samples were obtained using a disposable catheter that was inserted into a nostril to a depth of 5–7 cm and drawn back while applying gentle suction with an electric suction device [17]. The samples were stored at room temperature and tested for RSV antigen using time-resolved fluoroimmunoassay within 24 h [18].
Nasal swab specimens for RSV detection were obtained from all participating family members, including the index children. The samples were taken with cotton swabs from a depth of 2–3 cm in the nostril and placed in dry sterile vials for storage at −70°C, to allow for all specimens from the members of the same family to be subjected to RT-PCR for RSV at the same time [19]. All virological assays were performed at the Department of Virology, University of Turku, Finland.
For determination of the viral load, nucleic acid extraction and RT-PCR with SYBR Green was performed as described earlier [20] using RSV F protein gene-specific primers [21]. Viral load was estimated by calculating the relative virus copy number in the swab by comparing the threshold cycle value of the sample with the standard curve obtained from a dilution series of 102–107 copies of RSV RNA (Randall strain) per reaction. The specificity of the amplification was confirmed by post-PCR probe hybridisation assay [21]. In addition, RSV A/B strain group was determined using real-time, probe-based RT-PCR [22].
Definitions
In each family, the child who was first hospitalized with RSV was defined as the index case. The primary case of RSV in the family was determined as the first family member with fever, cough, or rhinitis during the 2-week period before the index child's admission. However, if any subject had been asymptomatic for ≥7 days before the onset of symptoms in another family member [23], then the latter family member was considered as the primary case in the family.
Statistical Analyses
One-way analysis of variance, with Tukey–Kramer method for pairwise comparisons, was used for comparing differences in the numbers of RSV copies in nasal swab specimens between the groups. The Mann–Whitney U test was used for comparing medians between 2 groups.
RESULTS
Characteristics of the Families
A total of 52 families consisting of 199 family members (53 index children and 146 other members) were included in the study (Figure 1). The median number of members in the families was 4 (range, 2–7). In 31 (60%) families, the index child had at least 1 sibling. The median age of the index children was 3.7 months; 39 (74%) of them were <6 months of age and 46 (87%) were <1 year of age at admission (Table 1). In all families, the index child was the youngest family member.
Table 1.
Baseline Characteristics of the Study Participants
| Characteristic | Statistic |
|---|---|
| Index children (n = 53) | |
| Age distribution, No. (%) | |
| 0 to <3 mo | 22 (41.5) |
| 3 to <6 mo | 17 (32.1) |
| 6 to <12 mo | 7 (13.2) |
| 1 to <2 y | 5 (9.4) |
| ≥2 y | 2 (3.8) |
| Male sex, No. (%) | 31 (58.5) |
| Siblings (n = 46) | |
| Age, median (range), y | 4.7 (1.4–15.8) |
| Male sex, No. (%) | 23 (50.0) |
| Parents (n = 100) | |
| Age, median (range), y | 30.2 (18.3–50.2) |
| Male sex, No. (%) | 48 (48.0) |
Respiratory Syncytial Virus Illnesses in the Families
The index child was the primary case of RSV in 18 (35%) of the 52 families. In 30 (58%) families, either a parent (n = 15; 7 mothers and 8 fathers) or a sibling (n = 15) was considered as the probable primary case of RSV (Figure 2). The mean age of the siblings who were the primary cases was 4.5 years (range, 1.9–8.9 years); 2 of them were schoolchildren. A nasal swab for RSV detection was obtained from 25 of these 30 primary cases at the time of the index child's admission, and RSV infection could be verified in 12 (48%) of the 25 subjects. Among all family members in the 30 households in which the primary case of RSV was determined to be someone other than the index child (Figure 2), the median time from illness onset to specimen collection was 12 days in RSV-negative subjects and 8 days in RSV-positive subjects (P = .038). The primary case of RSV could not be reliably determined in 4 families.
Figure 2.
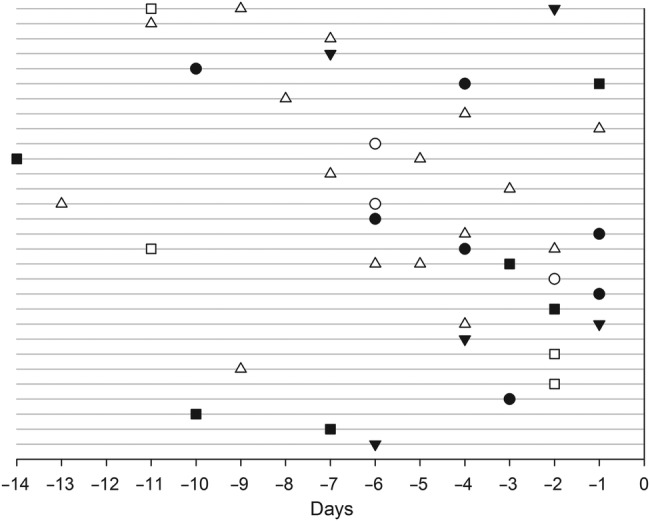
Onset of illness symptoms in household members in 30 families in which a parent or a sibling was considered as the primary case of respiratory syncytial virus (RSV) in the family. Each line represents 1 family, and Day 0 is the day of illness onset in the RSV-positive index child. Squares indicate fathers, circles indicate mothers, and triangles indicate siblings. Filled objects indicate RSV-positive family members and open objects are RSV-negative family members.
In 40 (77%) of the 52 families, at least 1 other family member (in addition to the index child) proved positive for RSV. In 7 of the 12 families in which the index child was the only confirmed case of RSV, the index child had no siblings.
Respiratory syncytial virus group A strains were detected in 51 (98%) of the 52 families and group B strains in 1 (2%) family. This was consistent with the local virologic surveillance data, according to which 96% of all RSV strains in the area during the study season belonged to RSV group A.
Detection of Respiratory Syncytial Virus in the Family Members
Nasal swabs for the detection of RSV by RT-PCR were obtained from all 53 index children and from 129 (88%) of 146 family members (97 parents, 32 siblings). In 114 (88%) of the 129 cases, the specimens from the family members were collected on the same day as those from the index children, and in the remaining 15 (12%) cases, the specimens were obtained on the following day. All index children were RSV-positive by RT-PCR.
Among the 129 family members who were sampled for RSV around the time of the index child's admission, 54 (42%) were positive for RSV. Twenty-seven (50%) of these 54 subjects had concurrent respiratory symptoms at the time of sampling, 9 (17%) developed respiratory symptoms 1–3 days (median, 2 days) after the sampling, and in 7 (13%) cases the symptoms of respiratory infection had disappeared 1–4 days (median, 2 days) before the detection of RSV in the nasal swab. Altogether, direct or indirect evidence for symptomatic RSV infection could be obtained from 43 (80%) of the 54 RSV-positive family members. Neither preceding nor subsequent respiratory symptoms were reported in 11 (20%) family members who were positive for RSV by RT-PCR; 10 of them were parents, and 1 was a 12-year-old sibling.
Fifty-six (43%) of 129 family members had respiratory symptoms at the time of nasal sampling, and 27 (48%) of these 56 subjects were positive for RSV. In 3 symptomatic but initially RSV-negative cases, RSV was detected in another nasal swab obtained 3–5 days later, bringing the total number of confirmed RSV infections to 30 (54%) of 56 symptomatic family members.
Of 46 initially asymptomatic RSV-negative subjects, 4 developed a new respiratory infection during the follow-up period and provided another nasal swab specimen; 3 (75%) of these samples were RSV-positive by RT-PCR.
Overall, RSV could be detected in 60 (47%) of 129 family members at the time of or within 1 week after the hospitalization of the index children. Forty-nine (82%) of these RSV detections were associated with respiratory symptoms, which indicates that 49 (38%) of 129 family members suffered a symptomatic RSV infection during a 2-week period around the hospitalization of the index children.
Respiratory Syncytial Virus Load in Nasal Swabs
Quantification of RSV load was performed on 97 nasal swabs (51 index children, 11 siblings, and 35 parents) obtained at the time of the index children's admission. The mean number of viral copies per swab was highest (107.7) in index children and lowest (105.1) in parents (P < .0001; Figure 3).
Figure 3.
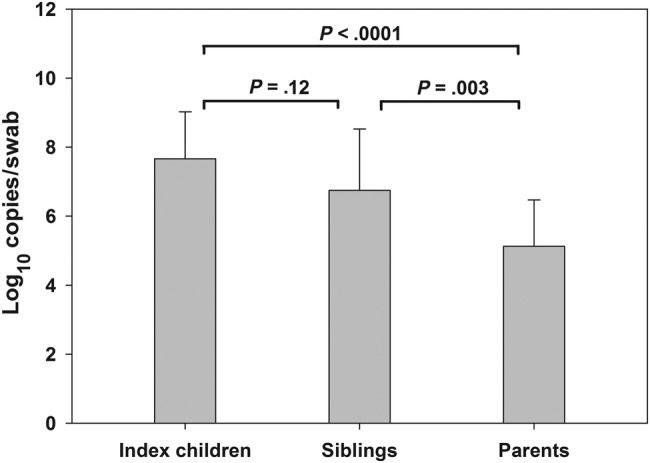
Respiratory syncytial virus loads (mean) in the nasal swabs of the index children (N = 51), their siblings (N = 11), and parents (N = 35). Error bars indicate standard deviations.
DISCUSSION
Although the clinical burden of RSV is undoubtedly greatest on young infants who are frequently hospitalized with a severe form of the illness, RSV is not only a virus of the infant but of the entire family. Our study demonstrates that, in addition to the hospitalized child, approximately three fourths of families were affected by RSV, and half of all family members of the index child were positive for RSV around the time of the child's hospitalization. Most of these family members had a symptomatic RSV infection.
In ∼60% of the families in our study, RSV was most likely introduced into the family by the index child's sibling or parent. This is in agreement with previous studies of RSV transmission in household settings [12–16]. In a follow-up study of 36 families during an RSV outbreak in the United States, RSV was detected in 16 families; the likely source of the virus was a sibling in 8 cases and a parent or another household member in 3 cases [14]. In a prospective study of 44 households in rural Kenya, RSV was detected in 37 households during 1 RSV season [16]. In 54% of RSV infections in infants in that study, the source of infection was a household member, usually a school-aged sibling of the infant. Although all these studies corroborate the view that family members are the primary source of RSV in young infants, the complexity of RSV transmission is highlighted by our finding that the index child was the primary case of RSV in 35% of the families. This indicates that a substantial proportion of RSV infections in infants may be acquired from sources outside of the immediate household.
Knowledge of the transmission dynamics of RSV within families and in the community is of crucial importance for the development of effective vaccination strategies to reduce the burden of RSV in infants. The obvious goal for most stakeholders in the field might be to produce an effective vaccine that could be administered to young infants directly. However, this approach is confronted with several challenges, including the logistic problems of immunizing very soon after birth, immaturity of the immune system of the neonate, presence of maternal antibodies that may reduce the immunogenicity of the vaccine, and concerns about the safety of the vaccine [24]. Alternative strategies of preventing severe RSV illness in young infants include the vaccination of pregnant women and the infants′ close contacts who could transmit the virus to them. If young infants could be protected from RSV during the first 6 months of their lives by vaccination of their close contacts, primarily older siblings, many of the problems associated with the production of a childhood RSV vaccine could be overcome [24].
Some limitations of our study require consideration. Information about respiratory symptoms in the family members during the 2 weeks prior to the index child's hospitalization was based on parental recall, which may not have been fully accurate in all cases. However, we believe that the likelihood of the occurrence of substantial inaccuracies between the reported and actual symptoms within the immediately preceding 2-week period is low. Because we could not obtain viral specimens from the family members before the index child's admission, we were in many cases unable to confirm the viral etiology of the illnesses occurring in the family members before enrollment in this study. Furthermore, it is probable that not all respiratory illnesses in the family members during the follow-up period resulted in self-sampling of nasal swabs for the detection of RSV. Therefore, the observed RSV morbidity in the families is inevitably an underestimate, and it is likely that the total burden of RSV in the families throughout the entire RSV season was higher than reported here.
CONCLUSIONS
In conclusion, our study demonstrates the high extent of RSV transmission between family members at the time of a child's admission for RSV lower respiratory tract infection. In most cases, the likely source of the young child's RSV infection was an older sibling or a parent. These findings indicate that vaccination of the family members and other close contacts of high-risk infants could be an effective strategy to reduce the overall burden of RSV in young infants.
Acknowledgments
We are grateful to Kaisu Kaistinen and the entire staff of the pediatric infectious diseases ward at Turku University Hospital for help in the performance of this study.
Potential conflicts of interest. T. H. has been a consultant to Alios BioPharma on matters outside of this work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 3.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 5.Hall CB, Long CE, Schnabel KC. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792–6. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 6.Falsey AR, McElhaney JE, Beran J, et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis. 2014;209:1873–81. doi: 10.1093/infdis/jit839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgeois FT, Valim C, McAdam AJ, et al. Relative impact of influenza and respiratory syncytial virus in young children. Pediatrics. 2009;124:e1072–80. doi: 10.1542/peds.2008-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis. 2012;54:1427–36. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–8. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 10.Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 11.Anderson LJ, Dormitzer PR, Nokes DJ, et al. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31S:B209–15. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berglund B. Respiratory syncytial virus infections in families. A study of family members of children hospitalized for acute respiratory disease. Acta Paediatr Scand. 1967;56:395–404. doi: 10.1111/j.1651-2227.1967.tb15398.x. [DOI] [PubMed] [Google Scholar]
- 13.Cooney MK, Fox JP, Hall CE. The Seattle Virus Watch. VI. Observations of infections with and illness due to parainfluenza, mumps and respiratory syncytial viruses and Mycoplasma pneumoniae. Am J Epidemiol. 1975;101:532–51. doi: 10.1093/oxfordjournals.aje.a112125. [DOI] [PubMed] [Google Scholar]
- 14.Hall CB, Geiman JM, Biggar R, et al. Respiratory syncytial virus infections within families. N Engl J Med. 1976;294:414–9. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- 15.Crowcroft NS, Zambon M, Harrison TG, et al. Respiratory syncytial virus infection in infants admitted to paediatric intensive care units in London, and in their families. Eur J Pediatr. 2008;167:395–9. doi: 10.1007/s00431-007-0509-9. [DOI] [PubMed] [Google Scholar]
- 16.Munywoki PK, Koech DC, Agoti CN, et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis. 2014;209:1685–92. doi: 10.1093/infdis/jit828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heikkinen T, Marttila J, Salmi AA, et al. Nasal swab versus nasopharyngeal aspirate for isolation of respiratory viruses. J Clin Microbiol. 2002;40:4337–9. doi: 10.1128/JCM.40.11.4337-4339.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waris M, Halonen P, Ziegler T, et al. Time-resolved fluoroimmunoassay compared with virus isolation for rapid detection of respiratory syncytial virus in nasopharyngeal aspirates. J Clin Microbiol. 1988;26:2581–5. doi: 10.1128/jcm.26.12.2581-2585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waris ME, Heikkinen T, Österback R, et al. Nasal swabs for detection of respiratory syncytial virus RNA. Arch Dis Child. 2007;92:1046–7. doi: 10.1136/adc.2006.113514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peltola V, Waris M, Österback R, et al. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197:382–9. doi: 10.1086/525542. [DOI] [PubMed] [Google Scholar]
- 21.Kotaniemi-Syrjänen A, Laatikainen A, Waris M, et al. Respiratory syncytial virus infection in children hospitalised for wheezing: virus-specific studies from infancy to preschool years. Acta Paediatr. 2005;94:159–65. doi: 10.1111/j.1651-2227.2005.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 22.Hu A, Colella M, Tam JS, et al. Simultaneous detection, subgrouping, and quantitation of respiratory syncytial virus A and B by real-time PCR. J Clin Microbiol. 2003;41:149–54. doi: 10.1128/JCM.41.1.149-154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okiro EA, White LJ, Ngama M, et al. Duration of shedding of respiratory syncytial virus in a community study of Kenyan children. BMC Infect Dis. 2010;10:15. doi: 10.1186/1471-2334-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham BS. Protecting the family to protect the child: vaccination strategy guided by RSV transmission dynamics. J Infect Dis. 2014;209:1679–81. doi: 10.1093/infdis/jiu075. [DOI] [PMC free article] [PubMed] [Google Scholar]