Abstract
The use of gene therapy for blinding disease shows growing promise; however, due to an ever-expanding list of disease-causing genes and mutations, the identification of a generic gene-based treatment is urgently needed. In many forms of degenerative retinal disease, there may be a window of opportunity to preserve daylight vision, as the cone photoreceptors degenerate more slowly than do the rods. In this issue of the JCI, Venkatesh et al. and Xiong et al. exploit two different pathways to promote cone cell survival and preserve vision in murine retinal degeneration models. These studies provide hope for developing a universal reagent to treat many different blinding disorders.
Congenital blinding disorders: attractive targets for gene therapy
Given the excellent safety and efficacy data on gene augmentation therapy to target the rare congenital blindness Leber congenital amaurosis (LCA) and the potential approval of a gene therapy reagent for this condition, there is increasing interest in extrapolating these successes to other blinding conditions. There are now more than 260 different genes and associated diseases that could become gene therapy targets (1). These potential candidates include all of the diseases classified as retinitis pigmentosa (RP), a condition that affects 1.5 million people worldwide. Additionally, more prevalent complex blinding conditions, including age-related macular degeneration (AMD) and glaucoma, which affect 8.7% (2) and 3.5% (3) of the world’s population, respectively, also have potential to be targets for gene augmentation therapy. The challenges of developing a gene-based treatment for each specific form of blindness are formidable, as each new transgene must be incorporated into the appropriate virus, the reagent and its safety must be tested in preclinical models, and then the reagent must be rigorously tested in clinical trials before a product can be approved. The total price tag for each potential gene or disease target easily exceeds tens of millions of dollars and is accompanied by indefinite timelines. Therefore, there is a great need for developing a generic strategy to treat congenital blindness that could be applied to a diseased eye or retina, regardless of the molecular cause. In this regard, the studies reported by Xiong et al. (4) and Venkatesh et al. (5) are very exciting in that they describe manipulation of cell survival pathways that could potentially provide reagents to limit disease progression in a very large number of blinding conditions (Figure 1).
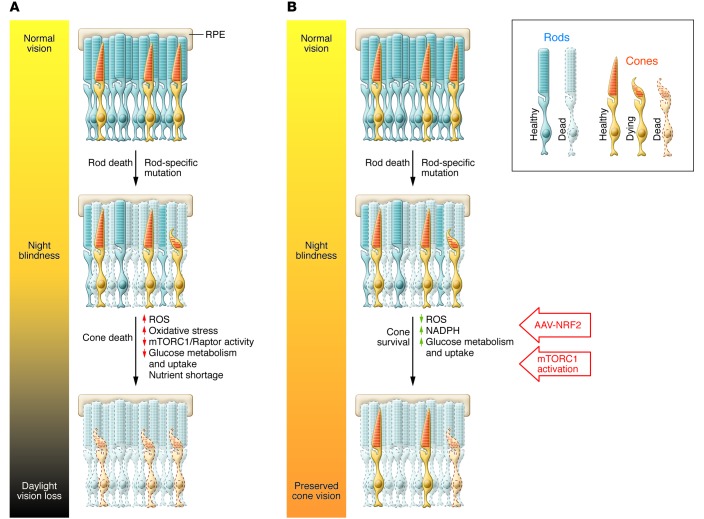
Figure 1. Prolonging cone survival to preserve vision.
(A) In many RP patients, the loss of rod photoreceptors leads to night blindness. Secondary to this effect, there is reduced clearance of ROS, causing increased oxidative stress and nutrient shortage via decreased glucose metabolism in the outer segments. These two factors contribute to cone cell death over time. (B) Since cone cell death occurs slowly, there is a window of opportunity to preserve cone survival and function. The studies by Venkatesh et al. (5) and Xiong et al. (4) demonstrate that constitutive activation of mTORC1 or AAV-mediated NRF2 expression can extend cone survival and preserve cone outer segment morphology and function in mouse models of retinal degeneration. While mTORC1 activation targeted nutrient shortage in the cones by increasing glucose metabolism, NRF2 expression reduced ROS and decreased oxidative stress, thereby preserving cone vision.
Both Venkatesh et al. (5) and Xiong et al. (4) evaluated RP, which is characterized by progressive degeneration of photoreceptors as the primary disease target. In most forms of RP, there is a rapid loss of rods, followed by a slower loss of cones (Figure 1). With artificial lighting in the modern world, most patients with RP can still lead a relatively normal life even after many of the rods have been lost, as cones function in bright light. Cones can persist in affected individuals for a prolonged period before dying; therefore, extending the survival of these photoreceptors could potentially maintain sufficient vision to significantly improve quality of life.
The mechanisms of cone cell death in RP are not fully understood, but a few have been proposed. These include, but are not limited to: a dysregulation of key metabolic genes as the disease progresses, leading to nutrient deprivation (6); an increase in oxidative stress (7); and lack of trophic support as the surrounding rod cells die (8). In developing generic treatments aimed at preventing cone photoreceptor cell death, Venkatesh et al. explored the development of nutrient deprivation (5), while Xiong et al. explored excess oxidative stress (4), as the driver of cone loss.
Applying some m“TORC”1
Photoreceptors rely on glucose metabolism and oxidative phosphorylation for the abundant energy required for visual transduction. Glucose is metabolized in photoreceptor outer segments to produce ATP and NADPH. In the absence of glucose, photoreceptors rapidly degenerate (9). Insulin/mTOR pathways are key in regulating glucose metabolism and uptake and are thus central for cell survival, growth, and homeostasis (10). It has been previously shown that daily systemic administration of insulin increases mTOR activity and extends cone survival in the rd1 mouse model of RP (6). This beneficial effect of insulin is short-lived, leading Venkatesh et al. to hypothesize a feedback inhibition in the mTOR pathway. mTOR interacts with several other proteins to form two distinct complexes, mTORC1 and mTORC2. mTORC1 responds to growth factors, stress, and low energy in the cell to promote protein synthesis, energy metabolism, and inhibit autophagy, while mTORC2 regulates cell survival and cytoskeletal organization (10). To test their hypothesis, Venkatesh et al. constitutively activated components of the mTOR pathway specifically in cone cells in rd1 mice and determined that activation of mTORC1 markedly improved cone survival, function, and morphology (5). Moreover, mTORC1 activation increased expression of the metabolic genes responsible for glucose uptake, retention, and utilization and promoted NADPH production, which likely reduced ROS and prevented apoptosis. Thus, mTORC1 activation acts in several different ways to increase cone survival — by increasing glucose metabolism, reducing oxidative stress, and preventing apoptosis.
An “aye” for NRF2
Xiong and colleagues explored the targeting of ROS as a potential means of prolonging the lives of cones (4). Rods constitute more than 95% of the photoreceptor population, and as these cells die, there is a reduction in both oxygen consumption and ROS removal, which together increase oxidative stress. In rd1 mice, antioxidant administration can prolong cone cell survival, thereby supporting the hypothesis that increased oxidative stress (11) contributes to cone cell death. Systemic delivery of antioxidants can be toxic, and the observed effect on cone cell survival is transient. Xiong et al. avoided systemic exposure by using a recombinant adeno-associated virus (AAV) to deliver a master antioxidant transcription factor, NRF2, specifically to cone cells. AAV-mediated delivery of NRF2 prolonged cone survival in three different RP mouse models (4). NRF2 is known to combat oxidative stress through multiple mechanisms including increased expression of ROS-detoxifying enzymes, such as superoxide dismutase 2 (SOD2) and catalase; upregulation of oxidative stress–reducing factors such as NADPH; and increased redox transport. NRF2 also indirectly regulates autophagy, exerts antiinflammatory functions, regulates the unfolded protein response, and promotes mitochondrial biogenesis (12). The study by Xiong and colleagues (4) is not the first report of the neuronal rescue effects of NRF2, as NRF2 induction was previously found to be neuroprotective in other neurodegenerative models including Parkinson’s disease (13–15); however, this is the first time that such effects have been reported after somatic gene transfer in the eye. In the study by Xiong et al. (4), AAV-mediated delivery of NRF2 resulted in a more durable rescue of the cones than did delivery of catalase and/or SOD2 alone. Visual acuity improved with AAV-NRF2 treatment, as did cone function. Further, AAV-mediated delivery of NRF2 improved cell survival in acute injury models such as optic nerve crush.
Outstanding questions and future directions
An important remaining question is whether mTORC1 activation or NRF2 induction can lead to an increase in cone survival at later stages of the disease process, such as after the rod cells have died. Interestingly, although Venkatesh et al. (5) induced mTORC1 prior to rod cell death, several mTORC1 downstream targets were expressed only at the onset of cone cell death. In the studies by Xiong and colleagues (4), AAV-NRF2 was delivered to retinae of newborn mice, resulting in transgene expression shortly before massive rod cell death would have taken place. Would it be possible to deliver AAV-NRF2 prophylactically in humans with RP? With respect to the mTORC1 studies, what genes or small molecules can be used to activate this pathway? Is there a transcription factor analogous to NRF2 that could be delivered to the appropriate cells to induce mTORC1? Additionally, specificity is an important consideration, as both NRF2 and mTORC1 are master regulators of several downstream processes, and long-term perturbation of these pathways in human patients may not be well tolerated. An inducible system such as the Tet-On/Off or lac operon systems may be beneficial in this scenario, in which activation of the transgene can be controlled externally.
With the reports from Venkatesh et al. (5) and Xiong et al. (4), it is now possible to expand the list of potential gene candidates that could be used to generically enhance cone survival in blinding disease. Somatic gene transfer has been used in recent years to test the ability of the neurotrophic factor rod-derived cone viability factor (RdCVf) to enhance cone survival in both autosomal recessive and autosomal dominant models of RP (16–18).
Regardless of what exact strategy(ies) ultimately serves to sustain cone photoreceptors, it will need to be implemented such that the cones are structurally supported. The rods provide trophic support to cone cells, and, consequently, healthy rod cell transplantation into RP mice lacking rods can delay cone death (8). Providing physical support, for example with the help of biopolymers, may be important for both cone viability and function (19). For end-stage retinal degeneration, in which the residual cone cells are nonfunctional or are lost, strategies such as transplanting photoreceptors (20), implanting electronic prostheses (21), or using optogenetic strategies (22) may be beneficial for restoring navigational vision.
Acknowledgments
This work was supported by grants from the Foundation Fighting Blindness; Research to Prevent Blindness; the NIH (1R24 EY019861-01A1 and 8DP1EY023177); the Mackall Foundation Trust; and the F.M. Kirby Foundation.
Footnotes
Conflict of interest: Jean Bennett serves as a consultant for Spark Therapeutics, is a scientific advisory board member for Avalanche Biotechnologies, and a founder of GenSight Biologics. Spark Therapeutics funds phase I/II and phase III gene therapy clinical trials for LCA2, of which Dr. Bennett is scientific director. Jean Bennett is a coinventor on a patent for a method to treat or slow the development of blindness, but waived any financial interest in this technology in 2002. Jean Bennett is a coinventor on several US provisional patents: “Gene Therapy for Disorders Related to CEP290” (61/847,016); “Compositions and Methods for Correction of Heritable Ocular Disease” (62/081,295); “Vision Test to Determine Retinal Disease Progress” (62/077,286); “Compositions and Methods for Self-regulated Inducible Gene Expression” (62/032,449); and “Proviral Plasmids for Production of Recombinant Adeno-associated Virus” (14/117,312).
Reference information:J Clin Invest. 2015;125(4):1390–1392. doi:10.1172/JCI80821.
References
- 1.Retinal Information Network. American Red Cross Web site. https://sph.uth.edu/retnet/ Accessed February 20, 2015
- 2.Wong WL, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.Tham YC, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125(4):1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatesh A, Ma S, Le YZ, Hall MN, Rüegg MA, Punzo C. Activated mTORC1 promotes long-term cone survival in retinitis pigmentosa mice. J Clin Invest. 2015;125(4):1446–1458. doi: 10.1172/JCI79766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punzo C, et al. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12(1):44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komeima K, et al. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213(3):809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 8.Mohand-Said S, et al. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch Ophthalmol. 2000;118(6):807–811. doi: 10.1001/archopht.118.6.807. [DOI] [PubMed] [Google Scholar]
- 9.Chertov AO, et al. Roles of glucose in photoreceptor survival. J Biol Chem. 2011;286(40):34700–34711. doi: 10.1074/jbc.M111.279752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komeima K, et al. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2006;103(30):11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barone MC, et al. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson’s disease. Dis Model Mech. 2011;4(5):701–707. doi: 10.1242/dmm.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzuferi M, et al. Nrf2 defense pathway: Experimental evidence for its protective role in epilepsy. Ann Neurol. 2013;74(4):560–568. doi: 10.1002/ana.23940. [DOI] [PubMed] [Google Scholar]
- 15.LaPash Daniels CM, et al. Beneficial effects of Nrf2 overexpression in a mouse model of Alexander disease. J Neurosci. 2012;32(31):10507–10515. doi: 10.1523/JNEUROSCI.1494-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leveillard T, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36(7):755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, et al. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther. 2009;17(5):787–795. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne LC, et al. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J Clin Invest. 2015;125(1):105–116. doi: 10.1172/JCI65654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishna Y, et al. Expanded polytetrafluoroethylene as a substrate for retinal pigment epithelial cell growth and transplantation in age-related macular degeneration. Br J Ophthalmol. 2011;95(4):569–573. doi: 10.1136/bjo.2009.169953. [DOI] [PubMed] [Google Scholar]
- 20.Singh MS, et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci U S A. 2013;110(3):1101–1106. doi: 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Cruz L, et al. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br J Ophthalmol. 2013;97(5):632–636. doi: 10.1136/bjophthalmol-2012-301525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagali PS, et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11(6):667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]