Abstract
Obesity and type 2 diabetes (T2D) are associated with low-grade inflammation, activation of immune cells, and alterations of the gut microbiota. Mucosal-associated invariant T (MAIT) cells, which are innate-like T cells that recognize bacterial ligands, are present in blood and enriched in mucosal and inflamed tissues. Here, we analyzed MAIT cells in the blood and adipose tissues of patients with T2D and/or severe obesity. We determined that circulating MAIT cell frequency was dramatically decreased in both patient groups, and this population was even undetectable in some obese patients. Moreover, in both patient groups, circulating MAIT cells displayed an activated phenotype that was associated with elevated Th1 and Th17 cytokine production. In obese patients, MAIT cells were more abundant in adipose tissue than in the blood and exhibited a striking IL-17 profile. Bariatric surgery in obese patients not only improved their metabolic parameters but also increased circulating MAIT cell frequency at 3 months after surgery. Similarly, cytokine production by blood MAIT cells was strongly decreased after surgery. This study reveals profound MAIT cell abnormalities in patients harboring metabolic disorders, suggesting their potential role in these pathologies.
Keywords: Endocrinology, Immunology, Metabolism
Introduction
Obesity is associated with low-grade inflammation in adipose tissue (AT) and dysfunctional adipocytes producing inflammatory molecules. The accumulation in AT of immune cells such as macrophages, lymphocytes, neutrophils, and mast cells is thought to participate in obesity and obesity-induced type 2 diabetes (T2D). In contrast, innate immune semi-invariant natural killer T (iNKT) cells are enriched in AT of lean subjects compared with AT of obese patients (1). Indeed, iNKT in AT may exert immunoregulatory functions and influence insulin resistance in murine models (2, 3). Mucosal-associated invariant T (MAIT) cells are a novel subset of innate-like immune cells found in peripheral blood, intestinal mucosa, and abundantly in human liver (4–6). Like iNKT cells, MAIT cells express an invariant T cell receptor α chain, the Vα7.2-Jα33 chain in humans. MAIT cells can produce IFN-γ, granzyme B (GrB), and IL-17 (4); are restricted by the major histocompatibility complex class I–related molecule MR1 (7); and are activated by cells infected by different microorganisms (8). Vitamin B2 (riboflavin) metabolites produced by bacteria and yeasts are required to generate MAIT cell–activating ligands (9–12). It has been recently shown that host-derived small molecules, such as methylglyoxal and glyoxal, form with the bacterial riboflavin metabolite 5-A-RU, a potent MAIT cell ligand (13). Interestingly, methylglyoxal levels are increased in diabetic patients (14).
The absence of MAIT cells in the intestinal lamina propria of germ-free mice (7) underlies the role of the gut commensal flora in MAIT cell expansion and/or survival in the periphery. The composition of the gut microbiota is altered in obesity (15, 16) and diabetes (17, 18), impacting the riboflavin pathway, among others. Two conditions that result in weight loss and improved metabolic status, i.e., diet (16) and gastric bypass (19–21), induce a shift of the gut microbiota.
Given the homing capacity of MAIT cells to the gut and their activation by bacterial products, one can speculate that changes in gut microbiota would in turn influence the MAIT cell compartment. Here, we analyzed MAIT cell frequency and cytokine production in T2D patients and in severely or morbidly obese patients before and after weight loss induced by bariatric surgery.
Results
Blood MAIT cell frequency is decreased in T2D and obesity.
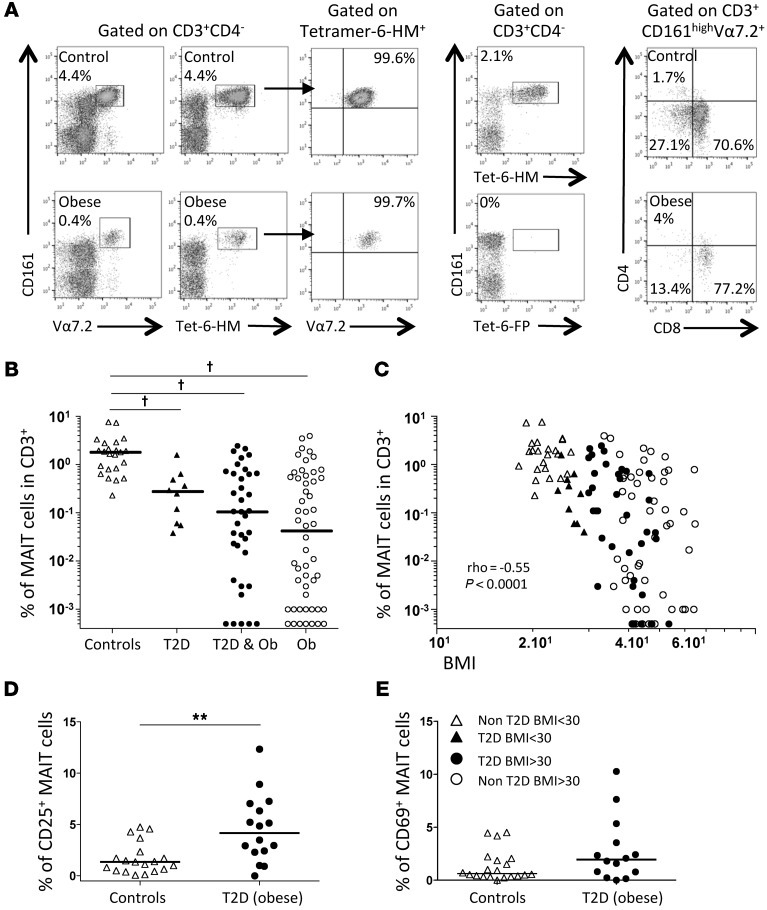
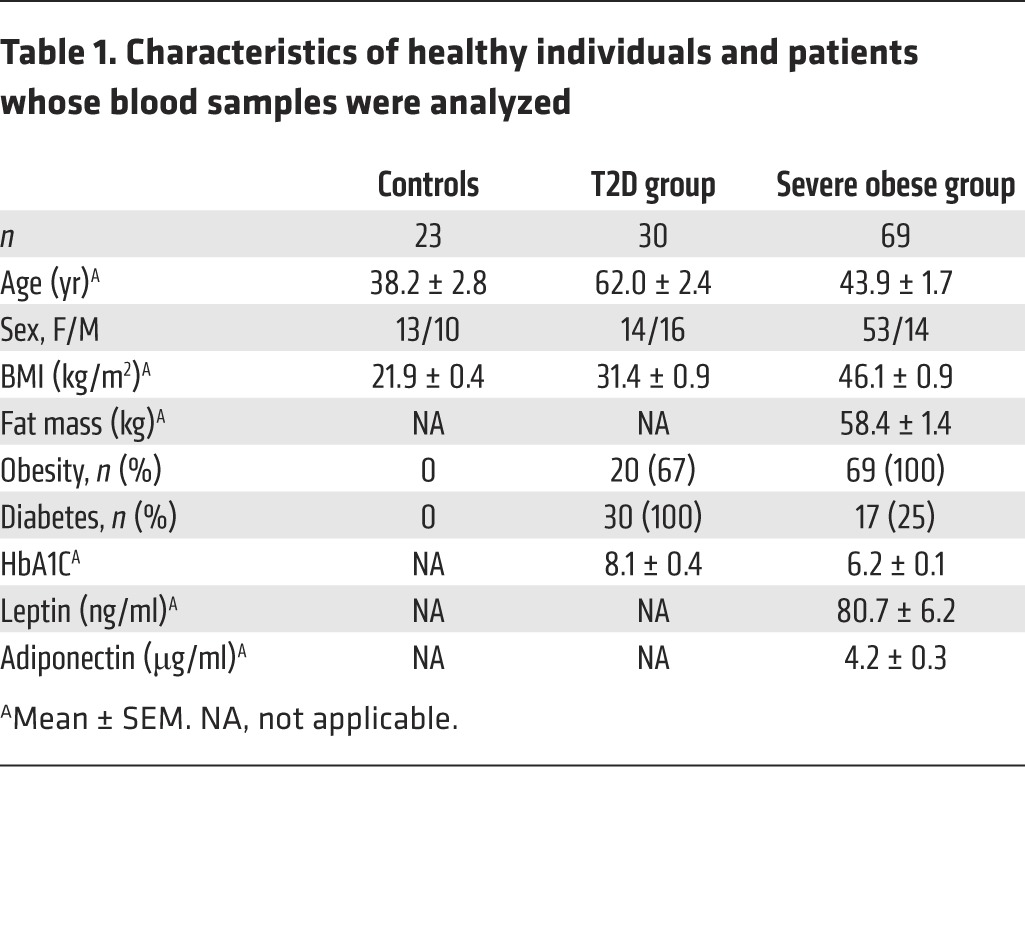
MAIT cells are characterized by the high expression of CD161; thus, we identified MAIT cells as CD3+CD4–CD161hiVα7.2+ lymphocytes. The development of MR1-6-HM tetramers specifically detecting MAIT cells (11) allowed us to confirm that CD3+CD4–CD161hiVα7.2+ cells identified all MAIT cells in healthy individuals and obese patients. Ten individuals with high and low frequencies of MAIT cells were analyzed with MR1-6-HM tetramers (with the MR1-6-FP tetramer used as negative control). With the use of peripheral blood mononuclear cells (PBMCs) that were costained with MR1-6-HM tetramers and anti-Vα7.2 antibodies, our results showed that MR1-6-HM tetramers identified all CD3+CD161hiVα7.2+ cells (Figure 1A). Conversely, all CD3+CD4–CD161hiVα7.2+ cells were stained by MR1-6-HM tetramers (data not shown). The majority of MAIT cells were either CD8α+ or double negative (Figure 1A). We then analyzed MAIT cell frequency in two cohorts of patients: T2D subjects, some of whom were obese (BMI ≥30 kg/m2, T2D group); and patients with severe obesity (BMI ≥35 kg/m2), candidates for bariatric surgery. These severely obese subjects included some patients with associated T2D. Healthy individuals were also part of the study. Table 1 summarizes clinical characteristics in the three groups. The range of circulating MAIT cell frequency in the healthy controls ranged from 0.2% to 7.6% of CD3+ cells, with a median frequency of 1.82% (Figure 1B), comparable to previous reports (22–25). Compared with controls, the frequency of circulating MAIT cells in patients was significantly lower. It was even below the detection limit (0.001% of CD3+ cells) in 12 of 69 (17%) subjects with severe obesity. In patients with detectable MAIT cells, the median MAIT cell frequency was 0.27% in nonobese T2D, 0.10% in obese T2D, and 0.04% in obese non-T2D subjects (P < 0.0001 compared with controls, Figure 1B). Interestingly, the frequency of circulating MAIT cells was negatively associated with subjects’ BMI (ρ = –0.55, P < 0.0001, Figure 1C) and, in severely obese patients, positively associated with serum levels of adiponectin, an insulin-sensitizing adipokine (n = 57, ρ = 0.29, P < 0.05, data not shown).
Figure 1. Decreased frequency of circulating MAIT cells in T2D and obesity.
(A) Costaining of lean and obese adults’ PBMCs with anti-Vα7.2 and anti-CD161 antibodies and with the 6-HM–loaded MR1 tetramer. All the cells binding to the MR1-6-HM tetramer were CD161hiVα7.2+, and MAIT cells were stained only by the MR1 tetramer loaded with the 6-HM ligand, and not the 6-FP ligand. MAIT cells were either CD8+ or double negative. (B) Lower frequencies of circulating MAIT cells were detected in nonobese T2D (n = 10), obese (Ob) T2D (n = 37), and non-T2D obese patients (n = 52) as compared with nondiabetic, nonobese healthy controls (n = 23). Note that in 12 obese patients, circulating MAIT cell frequency was below detection limit (<0.001%). Frequencies below 0.001% were arbitrarily displayed at 0.0005 but not included in the median and statistical calculations. †P < 0.0001. Adjusting for age and sex in a linear regression model did not change the significance. (C) Correlation between BMI and MAIT cell frequency (n = 122). (D) CD25 expression on MAIT cells in controls (n = 19) and T2D obese patients (n = 16). (E) CD69 expression on MAIT cells in controls (n = 20) and T2D obese patients (n = 14). **P < 0.003. Mann-Whitney U test and Spearman’s correlation. Straight lines represent medians. BMI is shown as kg/m2.
Table 1. Characteristics of healthy individuals and patients whose blood samples were analyzed.
Since the decreased circulating MAIT cell frequency may result from activation-induced cell death, we analyzed the expression of activation markers in the T2D group. The expression of CD25 was upregulated in obese T2D patients as compared with healthy controls (median of 4.2% versus 1.3%, P < 0.003) (Figure 1D). Of note, there was also a trend toward increased expression of CD69 in T2D patients as compared with controls (median of 1.9% versus 0.6% of MAIT cells) (Figure 1E). Thus, the decreased frequency of MAIT cells in patients was accompanied with an activated phenotype suggesting an abnormal activation of MAIT cells in these metabolic diseases.
Blood MAIT cells in T2D and severe obesity display a Th17 profile.
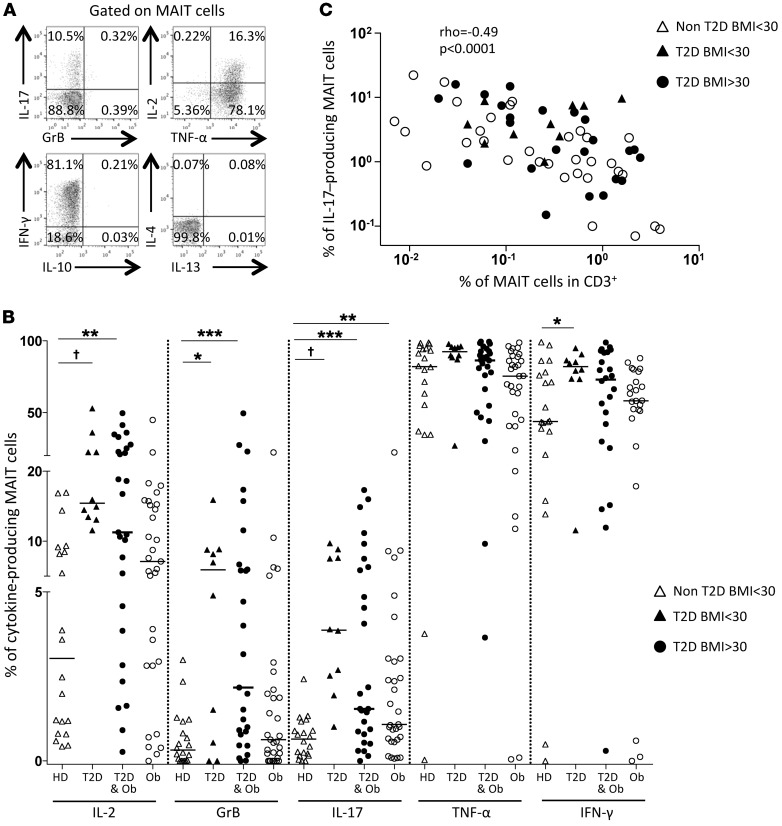
We investigated the cytokines IL-17, IL-2, TNF-α, IFN-γ, IL-10, IL-4, IL-13, and GrB produced by MAIT cells by intracytoplasmic staining (Figure 2A and Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI78941DS1) upon in vitro stimulation either with PMA and ionomycin or with MAIT cell ligands. After PMA-ionomycin stimulation, MAIT cells from T2D patients showed the highest levels of IL-2, GrB, IL-17, IFN-γ, and TNF-α production as compared with healthy controls and with obese patients (Figure 2B). In comparison with controls, nonobese T2D patients displayed higher frequencies of MAIT cells producing IL-2 (15.4% vs. 3.0%), GrB (5.9% vs. 0.3%), IL-17 (3.9% vs. 0.7%), and IFN-γ (82.1% vs. 43.8%). Significantly increased production of these inflammatory cytokines was also observed in obese T2D patients. However, in obese non-T2D patients, only IL-17 production was significantly increased. In contrast, the frequencies of MAIT cells producing IL-13, IL-10, and IL-4 remained low (median <0.5%) in both controls and patients (Supplemental Figure 1). Of note, there was a negative correlation between the frequency of MAIT cells among CD3+ cells and the frequency of IL-17–producing MAIT cells (Figure 2C).
Figure 2. Cytokine production by circulating MAIT cells in T2D and severe obesity.
(A and B) Analysis of circulating MAIT cell cytokine production by flow cytometry, after PMA-ionomycin stimulation. (A) Representative dot plot from an obese patient. (B) MAIT cell cytokine production in healthy donors (HD) (n = 20), nonobese T2D (n = 10), obese T2D (n = 27), and non-T2D obese patients (n = 31). *P ≤ 0.04, **P ≤ 0.007, ***P = 0.005, †P < 0.001. (C) Correlation between the frequency of IL-17–producing MAIT cells and MAIT cell frequency in patients (n = 67). Mann-Whitney U test and Spearman’s correlation.
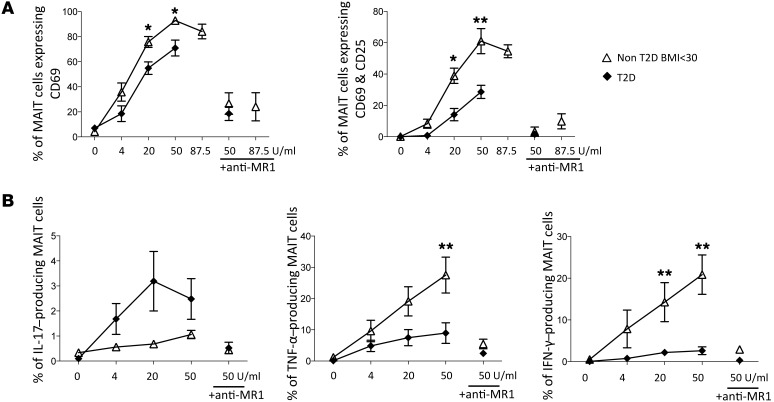
Interestingly, after specific TCR activation, MAIT cells from T2D patients compared with healthy individuals displayed a less-activated phenotype, as shown by a lower expression of CD69 and CD25 (Figure 3A). Moreover, MAIT cells from patients exhibited impaired production of IFN-γ and TNF-α. In contrast, the production of IL-17 was higher in patients than in controls. This response was specific for MAIT cells, since it was blocked by MR1 mAb. Together, these results revealed a strong Th17 bias of circulating MAIT cells in both T2D and obese patients (Figure 3B).
Figure 3. Defective activation of T2D patients’ MAIT cells after TCR triggering.
(A and B) ON stimulation with MAIT ligand at various concentrations (0–87.5 U/ml). (A) CD25 and CD69 expression on MAIT cells from controls (n = 5) and T2D patients (n = 7). (B) Cytokine production by MAIT cells from controls (n = 8) and T2D patients (n = 8). Blocking MR1 Ab was added when indicated. *P < 0.05, **P ≤ 0.005. Mann-Whitney U test.
Recruitment of MAIT cells in AT.
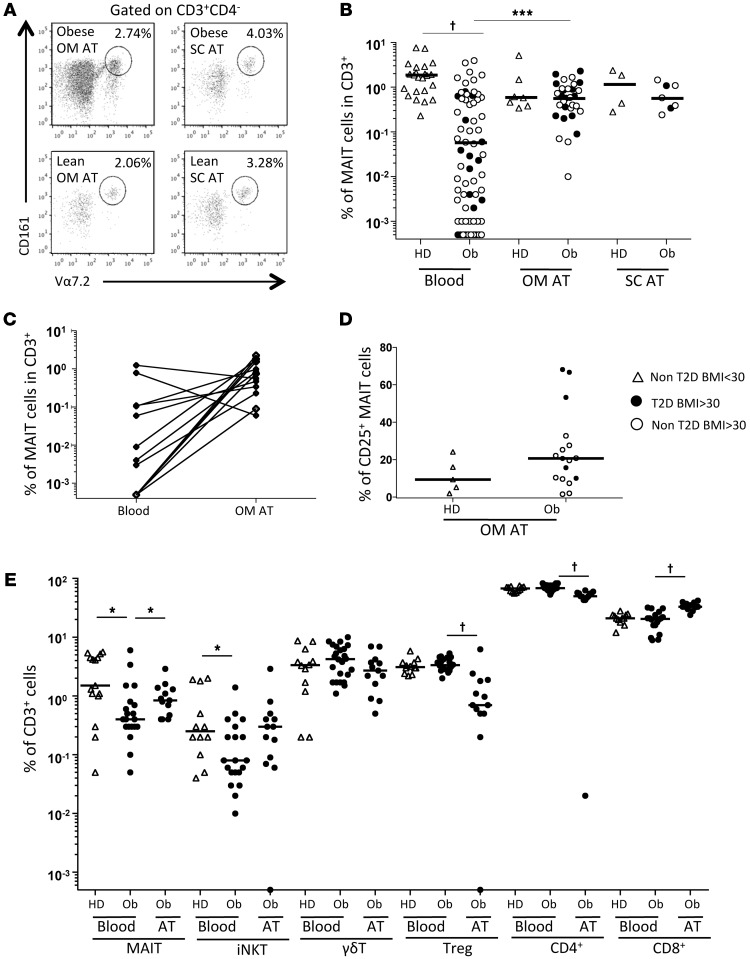
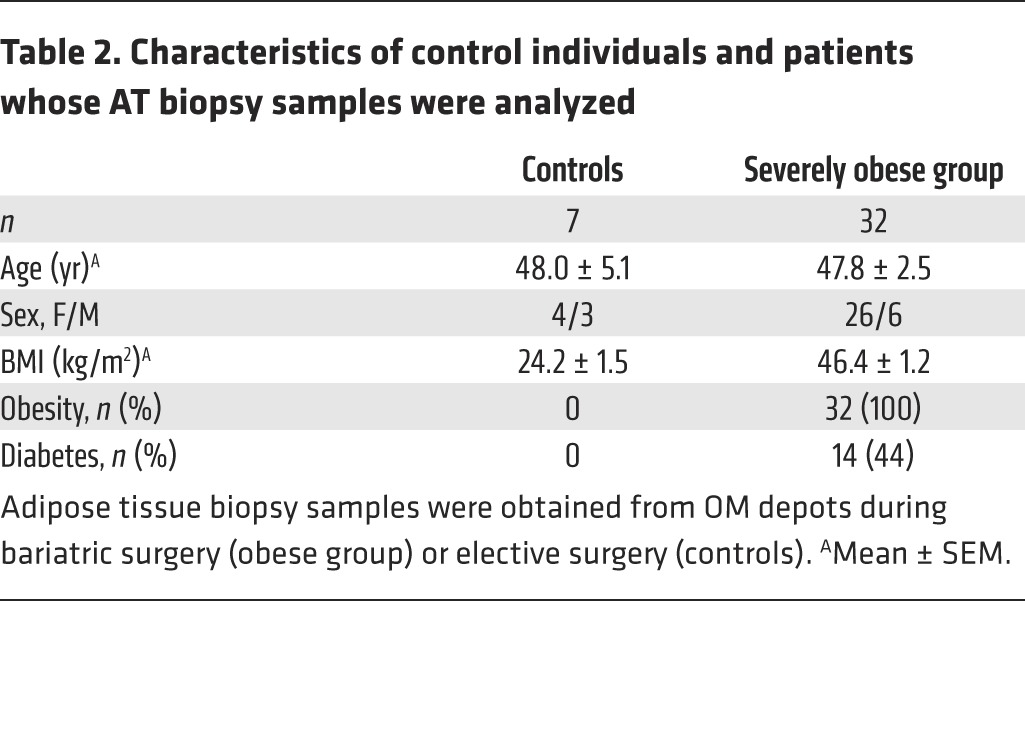
Because of the diminished MAIT cell frequency in metabolic disorders, we hypothesized that MAIT cells could be recruited at inflammatory sites. We analyzed MAIT cells in subcutaneous (SC) and omental (OM) AT of obese patients and healthy lean subjects (Figure 4A and Table 2). MAIT cell frequency among CD3+ cells between obese and lean OM and SC AT was not significantly different (Figure 4B). Interestingly, MAIT cell frequency in obese patients was significantly higher in the OM AT than in peripheral blood (0.59% vs. 0.06%) (Figure 4B). In most cases, except for 13 obese patients, we could not obtain paired peripheral blood and AT samples. Five patients who did not have detectable circulating MAIT cells exhibited MAIT cells in the OM AT (frequency of 1.6%). For 6 of the 8 remaining patients, MAIT cell frequency was higher in OM AT than in blood (Figure 4C). Of note, the median frequency of MAIT cells expressing CD25 in OM AT, even though not reaching statistical significance, was higher in obese patients as compared with lean subjects (20.7% vs. 9.4%, respectively, Figure 4D). To compare MAIT cells with other T cell subsets, we also analyzed iNKT cells, γδT cells, Tregs, and conventional CD4+ and CD8+ T cells in smaller patient groups (Figure 4E and Supplemental Table 1). This analysis confirmed the decreased circulating MAIT cell frequency in obese patients versus controls; and, as described previously (2), we observed a diminished frequency of circulating iNKT cells in obese patients, whereas there was no differences in the frequency of the other T cell subsets. In OM AT from obese patients, the frequency of MAIT and CD8+ T cells was significantly increased as compared with the frequency observed in the blood of obese patients, with a similar trend for iNKT cells. Conversely, CD4+ cells, Tregs and γδT cells were less frequent in OM AT than in the blood of obese patients. These data support a preferential recruitment of MAIT cells in AT of obese patients.
Figure 4. MAIT cells in the AT of obese patients.
(A and B) Flow cytometry analysis of MAIT cells detected in the OM and SC AT. (A) Representative dot plots. (B) MAIT cell frequency in the blood, OM, and SC AT of healthy donors (n = 23, 7, and 4, respectively) and obese patients (n = 69, 31, and 7, respectively). †P < 0.0001. (C) MAIT cell frequency in paired blood and OM AT from 13 obese patients. (D) CD25 MAIT cell expression in OM AT of control individuals and obese patients (n = 5 and 17, respectively). (E) Frequency of T cell subsets in blood from control individuals (n = 15) and obese patients (n = 20) and in OM AT from obese patients (n = 13). *P < 0.04, †P < 0.0001. Mann-Whitney U test.
Table 2. Characteristics of control individuals and patients whose AT biopsy samples were analyzed.
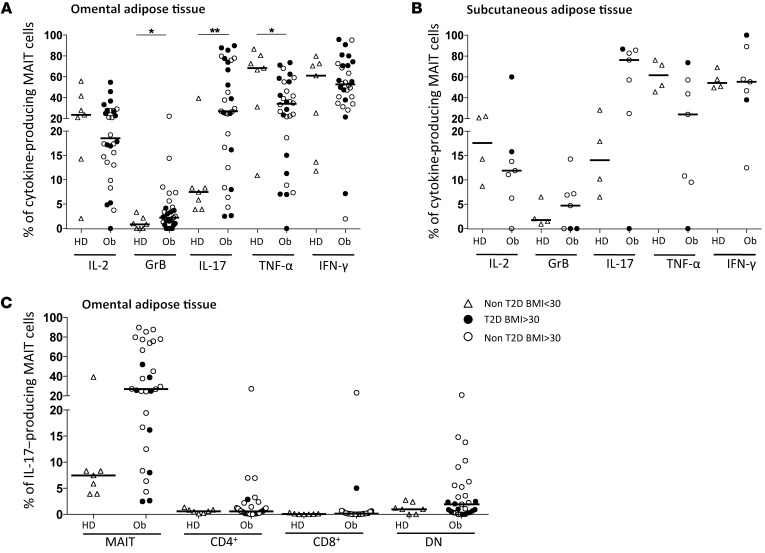
Elevated production of IL-17 by MAIT cells from AT of obese patients.
As performed in PBMCs, we analyzed MAIT cell cytokine production in AT. Within OM AT, in obese versus lean samples, the frequency of MAIT cells producing GrB (2.2% vs. 0.8%, P = 0.04) and, even more strikingly, IL-17 (26.9% vs. 7.5%, P = 0.009) was higher, but it was lower for TNF-α (34.0% vs. 68.3%, P = 0.02) (Figure 5A). Similar differences were observed in MAIT cell cytokine production between obese and lean SC AT (Figure 5B). Despite the limited number of subjects, we observed that a majority of MAIT cells produced IL-17 in the SC AT of obese patients (76% vs. 14% in lean SC AT). In both OM and SC AT, the frequency of MAIT cells producing IL-2, IFN-γ, IL-10, and IL-13 (data not shown) was not significantly different between lean and obese patients. Of note, in the OM AT of obese patients, the frequency of MAIT cells producing IL-4 was higher (5.0% vs. 0.7%) than in lean subjects (data not shown). The frequency of MAIT cells in OM AT producing IL-17 was much higher than what was observed for CD4+, CD8+, and double-negative T cells, which included γδT cells (Figure 5C). Together, these data suggest that MAIT cells are recruited to AT in obese patients, where they display a strong IL-17 profile.
Figure 5. Cytokine production by MAIT cells in the AT of severely obese patients.
Analysis of T cell cytokine production by flow cytometry, after PMA-ionomycin stimulation. (A and B) MAIT cell cytokine production in (A) the OM AT of healthy donors (n = 7) and obese patients (Ob) (n = 31; *P < 0.05, **P < 0.01) and (B) the SC AT of healthy donors (n = 4) and obese patients (n = 7). (C) IL-17 production by various T cell subsets from OM AT from healthy donors and obese patients shown in A. Mann-Whitney U test.
Influence of AT on MAIT cell phenotype.
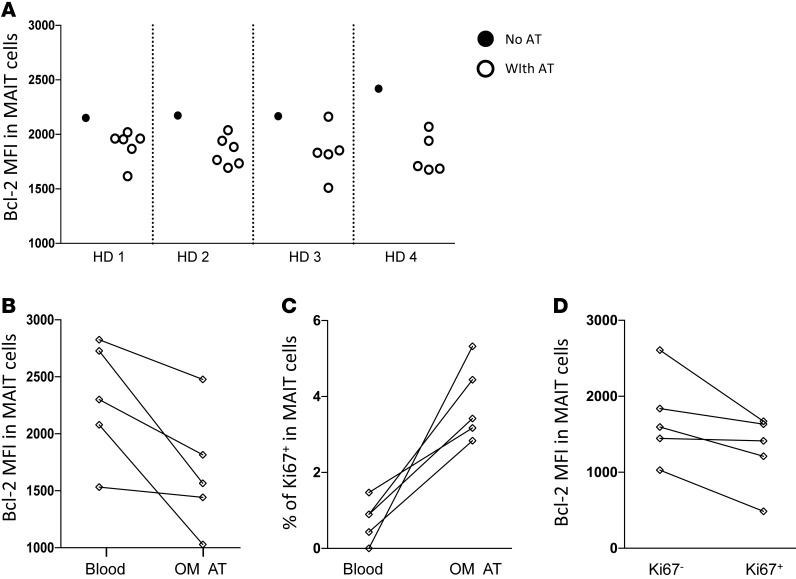
In order to better understand the homeostasis of MAIT cells in AT, we analyzed the expression of several markers by MAIT cells after coculture of PBMCs of healthy donors with AT samples from obese patients. Interestingly, the presence of AT dampened the expression level of the survival molecule Bcl-2 in MAIT cell AT in 21 of 22 cocultures performed (Figure 6A). The comparison of Bcl-2 expression in MAIT cells from the blood and the AT of five obese patients showed a similar decrease in Bcl-2 in MAIT cells from AT (Figure 6B). These results indicate that in AT, MAIT cells might be more susceptible to cell death. Interestingly, in the same obese patient samples, we also observed an increased frequency of proliferating MAIT cells (Ki-67 expression) in AT as compared with blood (Figure 6C). In AT MAIT cells, Bcl-2 MFI was lower in Ki-67+ as compared with Ki-67– cells (Figure 6D). Together, these data showing that proliferation of MAIT cells increased and their expression of Bcl-2 decreased in, or in the presence of, AT suggest that chronic activation of the MAIT cell population takes place locally.
Figure 6. Influence of AT samples on MAIT cell phenotype.
(A) PBMCs from healthy donors were cocultured with AT samples from obese patients, followed by flow cytometric analysis of Bcl-2 expression in MAIT cells. (B–D) Flow cytometric analysis of Bcl-2 and Ki-67 expression in MAIT cells. (B and C) Bcl-2 MFI and percent of Ki-67 expression in MAIT cells from peripheral blood and OM AT of 5 obese patients. (D) Bcl-2 MFI in Ki-67– and Ki-67+ MAIT cells from AT. MFI, median fluorescence intensity.
Attenuation of MAIT cell abnormalities after weight loss induced by bariatric surgery.
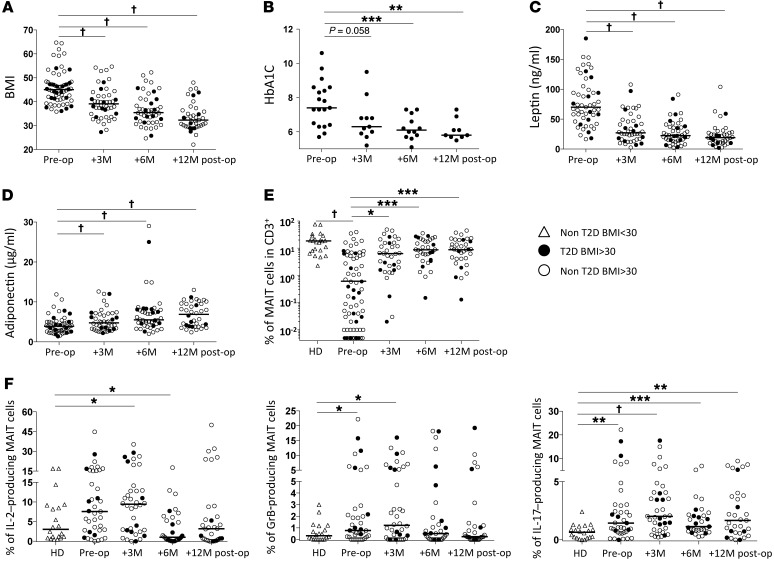
We next analyzed MAIT cells in the peripheral blood of the obese patients who underwent bariatric surgery (up to 12 months after surgery), which as expected improved metabolic and hormonal parameters (Figure 7, A–D). In agreement with previous reports (19, 21), BMI, glycated hemoglobin (HbA1C), and serum leptin diminished after the surgery, whereas serum adiponectin increased. Concomitantly, at 3 months after surgery, the MAIT cell frequency had already increased as compared with the initial frequency (0.66% vs. 0.06%, P = 0.01, Figure 7E). Of note, before surgery MAIT cells were below detection in 12 of 69 of the patients, while these cells were detected in all subjects after surgery. Six months after surgery, MAIT cell frequency further increased, reaching 0.9% of CD3+ cells, and then remained at the same level at 12 months after surgery. However, MAIT cell frequency was still significantly lower in obese patients who underwent surgery (but remained obese) than the frequency detected in lean controls (P = 0.04). The profile of MAIT cell cytokine production was also assessed at 3, 6, and 12 months after surgery (Figure 7F and Supplemental Figure 2). Three months after surgery, the frequency of MAIT cells producing the cytokines IL-2, GrB, and IL-17 was still higher than in healthy controls. However, at 6 months after surgery, the frequency of MAIT cells producing GrB was comparable in patients and control individuals and was even lower for IL-2 in patients than controls. In contrast, IL-17 production remained increased as compared with controls at 6 and 12 months after surgery (1.1% and 1.5%, respectively, vs. 0.7%). There was no difference between diabetic and nondiabetic obese patients.
Figure 7. Increased frequency of circulating MAIT cells after bariatric surgery.
(A) Obese patients’ BMI before (pre-op; n = 62) and at 3, 6, and 12 months after surgery (post-op; n = 46, 47, and 39, respectively). †P < 0.0001. (B) HbA1c levels of obese patients with T2D before surgery (n = 19) and at 3, 6, and 12 months after surgery (n = 11, 12, and 9, respectively). **P = 0.003, ***P = 0.0005. (C and D) Circulating leptin and adiponectin levels in obese patients before (n = 52) and at 3, 6, and 12 months after surgery (n = 44, 46, and 39, respectively). †P < 0.0001. (E) Circulating MAIT cells frequency before (n = 69) and at 3, 6, and 12 months after surgery (n = 35, 34, and 35, respectively). (Control individuals, n = 23.) *P = 0.01, ***P < 0.002, †P < 0.0001. Circulating MAIT cell frequency was significantly lower in obese patients at each time point compared to control individuals (P < 0.05). (F) Cytokine production after PMA-ionomycin stimulation of MAIT cells from healthy individuals (n = 20) and obese patients before surgery (n = 39) and 3, 6, and 12 months after surgery (n = 38, 33, and 31, respectively). *P < 0.04, **P < 0.004, ***P = 0.0008, †P < 0.0001. Mann-Whitney U or Wilcoxon test.
Discussion
Recent advances in understanding the pathophysiology of obesity and related T2D have established the involvement of the immune system, and the present study reveals major MAIT cell alteration in T2D and obese patients. The frequency of circulating MAIT cells is decreased in T2D patients and even more dramatically in severe obesity. This diminished frequency is associated with increased production of IL-17 by circulating MAIT cells, and this Th17 shift was exacerbated in AT from obese patients. Importantly, bariatric surgery restored circulating MAIT cell frequency and dampened their functional alteration. These data highlight the potential role of MAIT cells in these metabolic diseases.
The decreased frequency of circulating MAIT cells detected as Vα7.2+ and CD161hi was confirmed with human MR1 tetramers. Moreover, we did not observe an increase in frequency of CD161–Vα7.2+ T cells, excluding the possibility that the decreased MAIT cell frequency was due to the downregulation of CD161 surface expression. Of note, a recent study in patients with HIV confirmed that CD161–Vα7.2+ T cells do not bind MR1 tetramers and are not MAIT cells (26). Decreased circulating MAIT cell frequency has been described in different diseases: hepatitis C, HIV, tuberculosis, inflammatory bowel diseases, and severe infections (23, 24, 27–30). However, the MAIT cell frequency observed in T2D and obese patients was much lower than previously described, and in 12 of 69 (17%) severely obese patients, it was below our detection limit (<0.001% of CD3+ cells).
The decrease in circulating MAIT cell frequency in T2D and obese patients was accompanied by an increase in their activation status as defined by CD25 and CD69 expression. Similar observations were reported for patients with inflammatory bowel diseases (29) and with HIV (24). Moreover, the loss of circulating MAIT cells in T2D and obese patients was also associated with high levels of IL-17 production by MAIT cells, as previously reported in inflammatory bowel diseases (29). Similarly, in patients who underwent allogeneic stem cell transplantation — and thus were prone to graft-versus-host disease resulting in an excessive inflammatory environment — CD161hiCD8+ cells, most likely mainly MAIT cells, were less frequent and produced more IL-17 (31). In the present study, MAIT cells exhibited an inflammatory profile (particularly Th17) in both groups of patients, yet with differences. The pattern of MAIT cell activation could reflect a proinflammatory environment as well as modified hormonal and metabolic conditions. The fact that MAIT cell production of IL-2, GrB, and IL-17 was markedly increased in T2D patients suggests that, in T2D and severe obesity, the degree and/or mediators of inflammatory environments and possibly other metabolic mediators resulting in MAIT cell activation may differ.
The frequency of IL-17 production by MAIT cells was markedly elevated in AT as compared with the level measured in peripheral blood in severe obesity. Moreover, in AT of obese patients, there was a substantially increased frequency of MAIT cells producing IL-17 (OM: 26.9%, SC: 76.2%) as compared with conventional CD4+ T cells (OM: 0.6%, SC: 4.7%), CD8+ T cells (OM: 0.14%, SC: 0.69%), and double-negative T cells (OM: 1.9%, SC:8.5%) (AT SC data not shown). While all T cell subsets analyzed (CD8+ cells, CD4+ cells, Tregs, and γδT cells) expressed a very high level of CD69 in AT (data not shown), MAIT cells seemed particularly activated toward an IL-17 profile as compared with other T cell subsets. IL-12 and IL-18 have been shown to activate MAIT cells in an MR1-independent manner (32), indicating that not only MAIT cell–specific ligands, but also inflammatory molecules can activate them. MAIT cells express high levels of IL-7R (CD127), and IL-7 can promote IL-17 production by MAIT cells and license MAIT cell activation in the liver (5). In OM AT from obese patients, IL-7 among other adipokines was oversecreted by stromal vascular cells, a heterogeneous population of stem, progenitor, and differentiated cells (33). In addition to cytokines, MAIT cells could be activated through their TCR by specific ligands generated in the presence of bacterial riboflavin metabolites and methylglyoxal. Changes in the gut microbiota composition that occur in obesity and T2D may impact gut permeability and the presence of bacterial products in AT (34), and methylglyoxal might accumulate in AT, as described in obese rats (35). Whatever the mechanism leading to the high IL-17 production by MAIT cells, this proinflammatory cytokine could influence and participate in the local inflammation of AT. IL-17 can activate JNK, which in addition phosphorylates IRS1, inducing insulin resistance (36).
In obese patients, in contrast to the low frequency of circulating MAIT cells, in AT their frequency was 10-fold higher. There was a similar trend for iNKT cells. The decreased frequency of circulating MAIT cells could reflect their increased cell death and/or their recruitment to AT in obese patients, as previously suggested for other immune cells, such as conventional CD8+ T cells (37). The impaired activation capacity of circulating MAIT cells from T2D patients in the presence of specific ligands revealed an exhausted status, which could represent a transition toward cell death. This exhaustion associated with a decline in MAIT cells was previously observed in patients with chronic HIV infection (24). The level of CCR5 and CCR6 (data not shown), which are highly expressed by MAIT cells (28), was comparable in healthy controls and patient groups, suggesting that circulating MAIT cells in obese and T2D patients have intact tissue-homing capacities. Here, MAIT cell frequency in AT remained comparable in obese and lean subjects. This can imply either that MAIT cells in AT are protected from the mechanisms responsible for their depletion observed in the peripheral blood, or that MAIT cells are also activated in AT and, although they may die, they are continuously recruited, explaining in part the lower frequencies in peripheral blood. This latter hypothesis is supported by several in vitro and ex vivo observations. In cocultures, AT from obese patients promoted MAIT cell activation in the presence of ligand as shown by their CD25 and CD69 upregulation (Supplemental Figure 3), and there was a higher frequency of Ki-67+ MAIT cells from AT than from blood in five pairs of obese patient samples. In cocultures, AT from obese patients decreased MAIT cell Bcl-2 expression, and in obese patients Bcl-2 expression in MAIT cells was lower in AT than in the blood. Thus, the AT environment seems to influence in various ways the homeostasis of MAIT cells.
Bariatric surgery–induced weight loss and associated amelioration of patients’ metabolic and inflammatory status was accompanied by a significant increase of blood MAIT cell frequency already found at 3 months after surgery. Interestingly, using a linear mixed model, we found a kinetic association between the change in MAIT cell abundance and circulating levels of adiponectin (P = 0.036) that increased after the surgical procedure. This might suggest a possible relationship between increased MAIT cells and an improved metabolic and inflammatory status, since adiponectin is also considered to be a hormone with anti-inflammatory properties. In contrast, the association with circulating leptin that drastically diminished after surgery-induced weight loss was negative (P = 0.008, data not shown). Bariatric surgery is accompanied by changes in proinflammatory cytokine levels and vitamin status, as well as in carbohydrate intake, which is drastically reduced at 3 months after gastric bypass surgery, returning progressively to pre-surgery levels after 6 months (38). It is then tempting to speculate that these changes after surgery may influence the MAIT cell compartment and may in part be responsible, directly or not, for the increased frequency of MAIT cells. Whether and which changes in food intake or nutrient and related modification of gut microbiota (21) might influence MAIT cell abundance is an open question.
Taken together, our data revealed for the first time to our knowledge that T2D and obesity have a major impact on circulating and AT MAIT cell frequency and function: circulating MAIT cell frequency was profoundly decreased, and they produced more cytokines, such as IL-2, GrB, and IL-17, with exacerbated IL-17 production in AT. After bariatric surgery, MAIT cell abnormalities were markedly attenuated. The impact of the gut microbiota and the inflammatory environment on MAIT cells in patients with T2D and obese patients needs to be further explored. This article paves the way for deeper investigation of the mechanisms responsible for the prolonged Th17 shift of MAIT cells observed after bariatric surgery and whether gut microbiota, MAIT cell–specific ligands, MR1 tissue expression, and inflammatory mediators play a role.
Methods
Healthy controls and patients.
Blood samples were collected from control individuals who did not report T1D or T2D and who were nonobese, i.e., had a BMI <30 kg/m2. Patients from the T2D group were T2D outpatients consulting at the Hôtel-Dieu hospital in Paris with no acute event. Their mean HbA1C was 8.1%. Most received metformin (73%), and 43% also received insulin. The severe obesity cohort consisted of 69 subjects involved in a bariatric surgery program at the ICAN at Pitié-Salpêtrière hospital in Paris. Thirty-nine patients were examined at 3, 6, and 12 months after bariatric surgery. These subjects met the criteria for obesity surgery (BMI ≥40 kg/m2, or ≥35 kg/m2 with at least one obesity comorbidity). Subjects were weight stable (±3 kg) for at least 3 months before the surgery. Seventeen (25%) of the subjects had T2D with a fasting glycemia >7 mmol/l and/or the use of an antidiabetic drug (n = 13, 19%). Patients were exempt from antibiotic treatment 3 months prior to the intervention and during the follow-up. They only received an i.v. flash of antibiotics during the intervention as part of the routine surgical protocol. They benefited from a detailed bioclinical exploration as described previously (39). Briefly, body composition was determined by dual-energy X-ray absorptiometry (DXA, Hologic). In a subset of subjects, paired biopsies of abdominal periumbilical SC and OM AT were obtained during the surgical procedure. Blood samples were taken after 12 hours of overnight fasting before the surgery and 3, 6, 12 months afterward. Clinical variables were measured as described elsewhere (39). We also collected AT biopsy samples from nonobese subjects who did not report T1D or T2D and underwent elective surgery (e.g., hernia and Nissen fundoplication). These were used as controls. Clinical characteristics of the patients and controls are summarized in Tables 1 and 2 and Supplemental Table 1.
PBMC and AT preparation.
Cells of the stromal vascular fraction (SVF) were obtained by collagenase digestion of AT as previously reported (40) and were resuspended in endotoxin-free PBS supplemented with 2% FCS and 1 mM EDTA. SVF cells and freshly isolated PBMCs were stained either directly after isolation, or for detection of cytokine production after stimulation in RPMI medium supplemented with 10% fetal bovine serum (Life Technologies) with PMA and ionomycin (Sigma-Aldrich) at 25 ng/ml and 1 μg/ml, respectively, in the presence of brefeldin A at 10 μg/ml (Sigma-Aldrich) for 6 hours at 37°C. For stimulation with MAIT cell–specific ligand, HeLa cells and PBMCs from T2D patients were plated, at a final concentration of 106 cells/ml each, in RPMI medium supplemented with 10% fetal bovine serum in the presence of various concentrations of MAIT ligand semi-purified bacterial fraction and incubated overnight. For ligand preparation, E. coli were cultured to the stationary phase, washed in PBS, and resuspended into water at 4°C for 6 days. Supernatant was filtered through an 0.22 μM filter and ultra-filtrated through a 3-kDa centrifugal filter unit (Amicon-Millipore). The flow-through was then lyophilized. Fractions are expressed in arbitrary units with 1 arbitrary unit being equivalent to 25 μl of bacterial supernatant. For cytokine detection, BD GolgiPlug (BD Biosciences) was added 1 hour after the beginning of the stimulation. Anti-MR1 blocking mAb (clone 26.5, provided by Ted Hansen, Washington University School of Medicine, St. Louis, Missouri, USA) was added in some wells. The coculture experiments on PBMCs from healthy donors with AT samples from obese patients were done in RPMI medium supplemented with 10% fetal bovine serum; the AT was removed after 24 hours, and flow cytometric analysis was performed 48 hours later. In some experiments MAIT ligand (1 U/ml) was added for a further 48 hours, followed by flow cytometric analysis.
Flow cytometric analysis.
The following antibodies were used: anti-CD195 (2D7/CCR5), anti–IFN-γ (4S.B3), anti–IL-17a (N49-653), anti–IL-10 (JES3-19F1), anti–TNF-α (6401.1111), anti-CD25 (M-A251), anti–IL-4 (8D4-8), anti–IL-2 (MQ1-17H12), anti-CD8α (SK1), anti-CD69 (FN50), anti-GrB (GB11), anti-TCRγδ (B1), anti–IL-13 (JES10-5A2), and anti–Ki-67 (B56) from BD Biosciences; anti-CD69 (FN50), anti-Vα24Jα18 (6B11), anti-CD4 (OKT4), and anti-CD161 (HP-3G10) from eBioscience; anti-Vα7.2 (3C10), anti-CD4 (OKT4), anti-CD3 (OKT3), anti-CD161 (HP-3G10), anti-CCR6 (G034E3), and anti–Bcl-2 (clone 100) from BioLegend; anti-CD56 (N901) and anti-CD127 (R34.34) from Beckman Coulter; and anti-CD3 (BW264/56) and anti-CD56 (AF12-7H3) from Miltenyi Biotec. In some experiments biotinylated anti-Vα7.2 (3C10) and streptavidin Qdot 605 (Life Technologies) were used. Biotinylated human MR1 tetramers loaded with the active ligand rRL-6-CH2OH (6-HM) were used to specifically identify MAIT cells; biotinylated MR1 tetramers loaded with the non-activating ligand 6-formyl-pterin (6-FP) were used as a negative control (11). MR1 tetramers were coupled to streptavidin PE (BD Biosciences). Data acquisition was performed using a BD Biosciences LSRFortessa cytometer, and results were analyzed using FlowJo analysis software (Tree Star).
Statistics.
Categorical variables are expressed as numbers (n) and percentages (%). Nonparametric tests were performed using Mann-Whitney U or Wilcoxon test, as appropriate. Correlation analyses were performed using Spearman’s correlation. All P values are 2-sided, and P values less than 0.05 were considered to be statistically significant. Kinetic analyses were made using a linear mixed-effects model (for paired data). Analyses were performed using GraphPad software (GraphPad Prism) and R statistical software, version 3.0.1 (http://www.r-project.org/).
Study approval.
The Ethics Committee (Comité de protection des personnes [CPP] Ile-de-France) approved the clinical investigations for both obese and nonobese individuals. All subjects provided written informed consent when included in the surgery program. The study was conducted in accordance with the Helsinki Declaration, and was registered in a public trial registry: ClinicalTrials.gov number NCT00476658.
Supplementary Material
Acknowledgments
We thank all the patients and their physicians, the nurse and technician staff (Rohia Alili) who helped with the study. We are grateful to Yannick Simoni for discussion and Michael Gesnon for technical help. We thank Antoine Soprani, INSERM, UMRS 1138, Geoffroy Saint-Hilaire Clinic, Paris, for providing human AT for in vitro cultures. We thank the Direction of Clinical Research (CRC) for support of this clinical investigation (PHRC 02076 and PHRC microbaria), as well as the Fondation pour la Recherche Médicale (FRM, no. DEQ20140329520 to A. Lehuen and DEQ20120323701 to K. Clément), the National Agency of Research (ANR Fibrota and ObeMAIT projects), the French national program “Investissements d’Avenir” with the reference ANR-10-IAHU-05, the LabEx INFLAMEX with the reference ANR-11-IDEX-0005-02, and Département Hospitalo-Universitaire (DHU) AutHors (Autoimmune and Hormonal Diseases).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(4):1752–1762. doi:10.1172/JCI78941.
References
- 1.Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39(7):1893–1901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 2.Lynch L, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37(3):574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schipper HS, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122(9):3343–3354. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dusseaux M, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 5.Tang XZ, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190(7):3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 6.Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32(5):212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Treiner E, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 8.Le Bourhis L, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 9.Kjer-Nielsen L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 10.Patel O, et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun. 2013;4:2142. doi: 10.1038/ncomms3142. [DOI] [PubMed] [Google Scholar]
- 11.Reantragoon R, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210(11):2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birkinshaw RW, Kjer-Nielsen L, Eckle SBG, McCluskey J, Rossjohn J. MAITs, MR1 and vitamin B metabolites. Curr Opin Immunol. 2014;26:7–13. doi: 10.1016/j.coi.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Corbett AJ, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509(7500):361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 14.Matafome P, Sena C, Seica R. Methylglyoxal, obesity, and diabetes. Endocrine. 2013;43(3):472–484. doi: 10.1007/s12020-012-9795-8. [DOI] [PubMed] [Google Scholar]
- 15.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 16.Cotillard A, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 17.Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson FH, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 19.Furet JP, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong LC, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 22.Lee OJ, et al. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp Gerontol. 2014;49:47–54. doi: 10.1016/j.exger.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Grimaldi D, et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2013;40(2):192–201. doi: 10.1007/s00134-013-3163-x. [DOI] [PubMed] [Google Scholar]
- 24.Leeansyah E, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013;121(7):1124–1135. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novak J, Dobrovolny J, Novakova L, Kozak T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in males and females of reproductive age. Scand J Immunol. 2014;80(4):271–275. doi: 10.1111/sji.12193. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez CS, et al. MAIT cells are depleted early but retain functional cytokine expression in HIV infection [published online ahead of print October 28, 2014]. doi: 10.1038/icb.2014.91. doi: 10.1038/icb.2014.91. [DOI] [PubMed] [Google Scholar]
- 27.Billerbeck E, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A. 2010;107(7):3006–3011. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosgrove C, et al. Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood. 2013;121(6):951–961. doi: 10.1182/blood-2012-06-436436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serriari NE, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176(2):266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong EB, et al. Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co-infection. PLoS One. 2013;8(12):e83474. doi: 10.1371/journal.pone.0083474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Waart AB, et al. Decreased levels of circulating IL17-producing CD161+CCR6+ T cells are associated with graft-versus-host disease after allogeneic stem cell transplantation. PLoS One. 2012;7(12):e50896. doi: 10.1371/journal.pone.0050896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ussher JE, et al. CD161 CD8 T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2013;44(1):195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maury E, Ehala-Aleksejev K, Guiot Y, Detry R, Vandenhooft A, Brichard SM. Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2007;293(3):E656–E665. doi: 10.1152/ajpendo.00127.2007. [DOI] [PubMed] [Google Scholar]
- 34.Burcelin R, et al. Metagenome and metabolism: the tissue microbiota hypothesis. Diabetes Obes Metab. 2013;15(suppl 3):61–70. doi: 10.1111/dom.12157. [DOI] [PubMed] [Google Scholar]
- 35.Jia X, Chang T, Wilson TW, Wu L. Methylglyoxal mediates adipocyte proliferation by increasing phosphorylation of Akt1. PLoS One. 2012;7(5):e36610. doi: 10.1371/journal.pone.0036610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 38.Dalmas E, et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr. 2011;94(2):450–458. doi: 10.3945/ajcn.111.013771. [DOI] [PubMed] [Google Scholar]
- 39.Abdennour M, et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab. 2014;99(3):898–907. doi: 10.1210/jc.2013-3253. [DOI] [PubMed] [Google Scholar]
- 40.Dalmas E, et al. T cell-derived IL-22 amplifies IL-1beta-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014;63(6):1966–1977. doi: 10.2337/db13-1511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.