Abstract
Inhalation of agricultural occupational dusts from swine confinement facilities can result in lung inflammation. The innate immune response to organic barn dusts results in production of a number of pro-inflammatory factors in the lungs of barn workers such as cytokines, chemokines, and an influx of neutrophils. Many of these inflammatory factors are influenced by the chemokine CXCL8/IL-8 (KC or MIP-2 in mice). Previously, we have demonstrated that an endotoxinin-dependent component of swine barn dust extract (SBE) elevates lung chemokines in a protein kinase C (PKC)-dependent manner resulting in the significant formation of lung inflammatory cell infiltrates in a mouse model of SBE injury. In this study we test the ability of a CXCR1/CXCR2 antagonist, CXCL8(3-74)K11R/G31P (G31P) to block many of the features of lung-inflammation in response to challenge with SBE in an established mouse exposure system. Injection of G31P concurrent with SBE nasal instillation over a course of 3 weeks significantly reduced neutrophil accumulation in the lungs of barn dust exposed animals compared to those given SBE alone. There was a similar reduction in pro-inflammatory cytokines and chemokines IL-6, KC, and MIP-2 in SBE plus G31P-treated mice. In addition to excreted products, the receptors ICAM-1, CXCR1, and CXCR2, which all were elevated with SBE exposure, were also decreased with G31P treatment. SBE activation of PKCα and PKCε was reduced as well with G31P treatment. Thus, G31P was found to be highly effective at reducing several features of lung inflammation in mice exposed to barn dust extracts.
Introduction
Workers in animal confinement facilities are at a greater risk for contracting a variety of lung disorders such as chronic bronchitis, acute respiratory distress syndrome (ARDS), hypersensitivity pneumonitis, as well as developing asthma or COPD (Iversen et al. 2000, 283–288; Radon et al. 2001, 405–410). Those even acutely exposed to these environments show clear signs of airway inflammation (Cormier et al. 1997, 1516–1522; Wang et al. 1997, 381–387). Work by several groups has shown that organic dust inhalation from these facilities is associated with these symptoms (Cormier et al. 1997, 1516–1522; Dosman et al. 2006, 761–766). The inflammation seen in response to these dusts is the result of innate immune responses to bacterial components from these facilities such as proteoglycans and endotoxin. In particular the toll-like receptors (TLRs) TLR2 and TLR4 are crucial to organic dust responses (Poole and Romberger 2012, 126–132). Signaling through these receptors results in the expression of an array of cytokines and chemokines, in particular the potent pro-inflammatory chemokine IL-8 (CXCL8).
PKCα and PKCε play a critical role in the inflammatory response to barn dust (Wyatt et al. 2010, 706–715). Barn dust stimulates a sequential activation of PKC isoforms and cytokines in isolated airway epithelial cells. PKCα is activated within an hour and is required for TNFα and IL-6 production. TNFα precedes IL-6 production and subsequently activates PKCε, which is required for eventual IL-8 production several hours later. The effects of PKCs extend beyond just cytokines as cell surface adhesion markers responsible for neutrophil migration such as ICAM-1 are also up-regulated in response to PKCα activation (Mathisen et al. 2004, 1738–1744). Thus the activity of both protein kinases is critical to inflammation in response to barn dust.
The receptors for IL-8 in humans are the chemokine receptors CXCR1 and CXCR2. Both are high-affinity receptors for IL-8, but are capable of binding other chemokines (Ahuja, Lee, and Murphy 1996, 225–232; Fan et al. 2007, 11658–11666). In the mouse, IL-8 is functionally replaced by keratinocyte factor (KC) and MIP-2 (Lee et al. 1995, 2158–2164; Fan et al. 2007, 11658–11666) that bind these same CXCR1 and CXCR2 receptors (Fan et al. 2007, 11658–11666). A wide variety of cells are known to express either CXCR1 or CXCR2 and thus be signaled by these chemokines. Binding of IL-8 to CXCR1 or CXCR2 can produce a host of chemotactic and antimicrobial responses resulting in increased neutrophil recruitment into the lung (Murphy 1997, 311–318; Tateda et al. 2001, 2017–2024; Feniger-Barish et al. 1999, 996–1009). Therefore, successful blocking of CXCR1 and or CXCR2 is of great therapeutic interest for the control of inflammation. Indeed, there are currently CXCR2 agonists under development for diseases such as cystic fibrosis, neutrophilic asthma and COPD that have been studied in human models of LPS (Leaker, Barnes, and O’Connor 2013, 137-9921-14-137) and ozone exposure (Lazaar et al. 2011, 282–293; Holz et al. 2010, 564–570).
The IL-8 analog, G31P, has been shown to antagonize neutrophil migration and can bind CXCR1 and CXCR2 with a higher affinity than even IL-8 (Li, Zhang, and Gordon 2002, 939–944). As IL-8 has a higher affinity for CXCR1 and CXCR2 than other ELR-CXC chemokines, it has been suggested that G31P may block most chemokine binding to these receptors (Li, Zhang, and Gordon 2002, 939–944). In a bovine system G31P has been shown to be effective at reducing cytokine production and neutrophil migration in response to LPS (Gordon et al. 2005, 1265–1272).
As exposure to organic dust results in significant IL-6 and KC production and neutrophilia in the lung (Poole et al. 2009, L1085–95), we hypothesized that G31P could effectively inhibit chemokine responses in a mouse model of organic swine barn dust extract (SBE) exposure. We also looked at commonly induced features of barn dust exposure such as activation of signaling molecules (PKCα and PKCε) and increased cell migration (lavage cell counts and ICAM-1 expression). We show here that G31P was useful in reducing neutrophilia in mice exposed to SBE. Lung responses also showed reductions in the cytokines, chemokines, and activation of PKC isoforms measured. Finally, the expression of ICAM-1, CXCR1, and CXCR2 appeared elevated by SBE exposure but were reduced in a number of cell types in the lungs of mice receiving G31P. Taken together we show G31P is a potent inhibitor of the innate immune response to barn dust.
Materials and Methods
Hog confinement dust extract preparation
Extracts were created as described previously (Mathisen et al. 2004, 1738–1744) from settled dust collected from hog confinement buildings. Briefly, SBE was made by mixing 10 g of collected dust in 10 ml PBS (Dulbecco’s phosphate buffered saline, pH 7.4, Gibco, Frederick, MD) without calcium at room temperature for 1 hr. The mixture was then centrifuged 10 min, and supernatant saved and centrifuged a second time for 10 min before sterile filtering the supernatant. The barn dust used has been previously characterized (Poole et al. 2010, 684–700) as containing protein (1–2 mg/ml), endotoxin (22.5–48.75 EU/ml), and muramic acid (400 pmol/mg) in a 5% extract. A variety of bacterial sources (Clostridium ssp., Lactobacillus ssp., Ruminococcus ssp., and Eubacterium ssp.) contribute these toxins as recently characterized (Boissy et al. 2014, e95578).
Mouse exposure to SBE
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. Female 6–8 weeks old C57BL/6 mice (Charles River, Wilmingtom, MA), were acclimated to facilities for one week after arrival. The animals were group-housed, and their diet consisted of commercial rodent chow and water ad libitum. Mice were weighed weekly and no significant changes in body weight were observed under any experimental condition (data not shown). Mice were assigned randomly to each of the treatment groups: control, control + G31P administration, SBE instillation (12.5%), or SBE instillation + G31P. All mice (4 per group) were instilled nasally (Bailey et al. 2008, L1049–L1054) with 50 μl of treatment once every day for 5 days a week (Monday to Friday) for 3 weeks. Mice given G31P were injected peritoneally with 250 μg/kg every 2 days. At end of study, mice were sacrificed by injection with sodium pentobarbital (100 μl, 75mg/ml). The experiment was conducted twice, one week apart, and data pooled.
Bronchiolar alveolar lavage (BALF) collection
Lungs were lavaged as described previously (Poole et al. 2009, L1085–95). Briefly, lungs were washed three times with 1 ml sterile saline each time. Lavage fluid was centrifuged 1750g for 10 min and supernatant stored at −80°C prior to ELISA analysis. Cells were resuspended in 1 ml PBS, counted, and 1.5 × 103 cells were adhered to glass slides via cytospin onto glass slides. Cells were stained using a Diff-Quik kit (Siemens Healthcare Diagnostics, Newark, DE) and cover slips mounted. A differential count of at least 300 cells was made based on morphometric criteria and expressed as absolute cell numbers (mean +/− SEM).
Histology and immunohistochemical staining
After bronchoalveolar lavage, lungs were inflated with 10% buffered formalin and hung under 17 cm H2O pressure for 24 hr, after which they were placed in formalin for an additional 48 hr. Sections were cut at 5 mm thickness for staining. Sections were deparaffinized in Protocol Safeclear II (Fisher Scientific, Kalamazoo, MI) and rehydrated through an ethanol gradient (100%, 95%, 80%, 50%) and rinsed. One set of slides was stained with hematoxylin and eosin for histological examination while others were used for immunohistochemical examination.
For immunohistochemistry, antigen unmasking was accomplished by incubating slides in Diva Decloaker solution (Biocare Medical, Concord, CA) at 98°C for 20 min, followed by an additional 20 min where the solution was allowed to slowly cool to room temperature. After further rinsing in PBS, slides were blocked 30 min at room temperature in 5% skim milk in PBS. Antibodies to CXCR1 (Bioss, Woburn MA) or CXCR2 (Abcam, Cambridge MA) were subsequently added in 5% skim milk at 1:50 concentration and slides incubated overnight at 4° C. After washing the next day with PBST (PBS + 0.05% Tween-20 pH 7.4), slides were incubated with biotinylated rabbit anti-goat antibody (goat anti-rabbit-HRP, Jackson Immunoresearch, West Grove, PA) at 1:50 concentration overnight at 4° C. Slides were washed in PBS then developed with ImmPACT DAB kit (Vector, Burlingame, CA) for 3 min and counterstained with Harris-modified hematoxylin (Fisher Scientific, FairLawn, NJ) before being dehydrated through an ethanol gradient and fixed with Safeclear II. Samples were mounted using Cytoseal XYL (Thermo Scientific, Kalamazoo MI). For CD54/ICAM-1, staining was completed as mentioned previously (Poole et al. 2009, L1085–95). Briefly, blocking was done overnight at 4° C, with primary antibody added at 1:75 (rat anti-mouse ICAM-1; Rockland Immunochemicals, Gilbertsville, PA) and incubated for 1 hr at room temperature, and secondary (rat anti-CD54, 1:300; Biolegend, San Diego, CA) for 2 hr.
PKC activity
Lung trachea was taken from mice after lavage was completed. These trachea were flash frozen in cell lysis buffer as described (Wyatt et al. 2000, 91–97). Bronchial epithelial cells were sonicated and centrifuged 10000 × g for 30 min at 4° C and supernatant and pellet separated and resuspended in cell lysis buffer with 0.01% Triton X-100. Assay for isoforms α and ε of PKC in each fraction were carried out as previously described (Wyatt et al. 2007, L1163–L1170) and read by scintillation counter.
Cytokine and chemokine quantitation
Cytokine and chemokine quantitation of BALF fluid was done by enzyme linked immunoabsorbant assay kits to IL-6, KC, and MIP-2 (R&D Systems, Minneapolis, MN), according to manufacturer’s instructions.
Statistical analysis
All data was analyzed using GraphPad Prism (GraphPad Software, San Diego, CA). Graph bars represent the mean +/− SE of three replicate experiments performed in triplicate (n=3). Statistical significance was determined using ANOVA, with 95% confidence interval being considered significant.
Results
BALF cell population
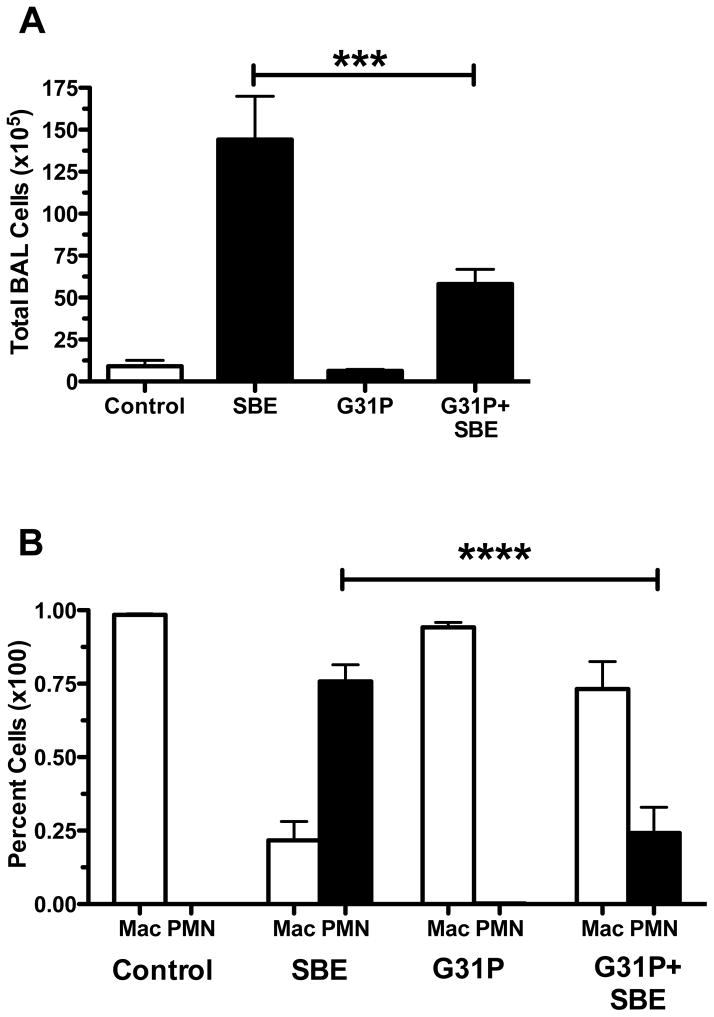
Cells in BALF were counted and identified to determine migration of both number and type of cells into the alveoli. SBE significantly increased the overall number of cells in BALF (Fig. 1A). However, pre-treatment of SBE-treated animals with G31P significantly (p<0.001) reduced the number of these cells, though the number of cells was still higher than non-SBE treated control groups. When the type of cells present was determined (Fig. 1B), control and G31P-only treated animals had BALF cellular populations that were almost entirely macrophages. With SBE treatment, this population however became dominated by neutrophils, accounting for a large proportion of the overall increase in BALF total cell numbers. Pre-treatment of SBE-treated animals with G31P significantly (p<0.001) reduced the percentage of neutrophils within the BALF cell population compared to SBE-only treated mice. G31P pretreatment did not completely abrogate the number of neutrophils present compared to control groups.
Figure 1.
Mean total of lung lavage fluid cells (A) and percent composition of macrophage (mac) and neutrophils (PMN) in lavage cells (B) after repeated nasal instillation with saline, SBE, G31P, or SBE+G31P. Error bars are SE (n=8 mice/group). ***P < 0.001, ****P < 0.0001
Cytokine and chemokine expression to SBE is decreased with G31P
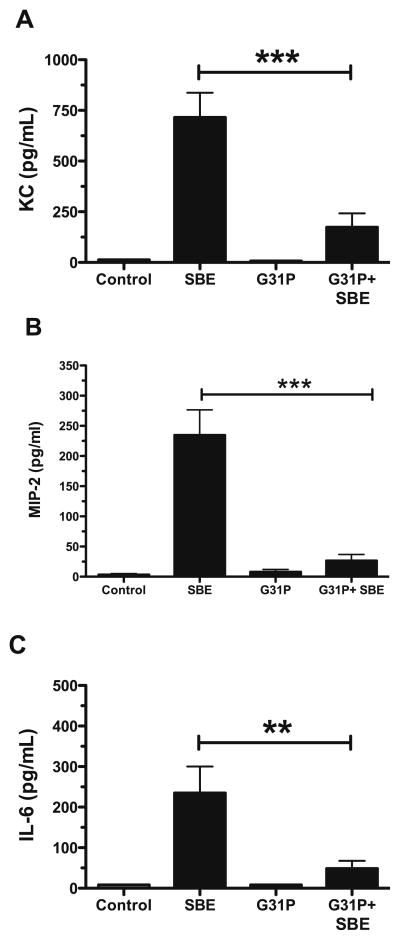
Because G31P significantly reduced the numbers of neutrophils in the BALF in response to SBE, we determined whether G31P caused changes in SBE-stimulated chemotactic cytokines. Control and Control + G31P treated animals produced little to near undetectable amounts of either KC or MIP-2 (Fig. 2A and 2B) in their BALF, whereas treatment with SBE caused a significant increase in both. SBE-instilled mice treated with G31P showed a significant (p<0.001) drop in the expression of each chemokine, though not a total elimination of either. Another indicator of inflammation, IL-6 (Fig. 2C), followed the same pattern (p<0.01) of expression as KC and MIP-2.
Figure 2.
Mean lung lavage fluid cytokine expression of KC (A), MIP-2 (B), and IL-6 (C). Error bars are SE (n=8 mice/group). **P < 0.01, ***P < 0.001
G31P blocks stimulation of PKCα and PKCε activity in lung tissue
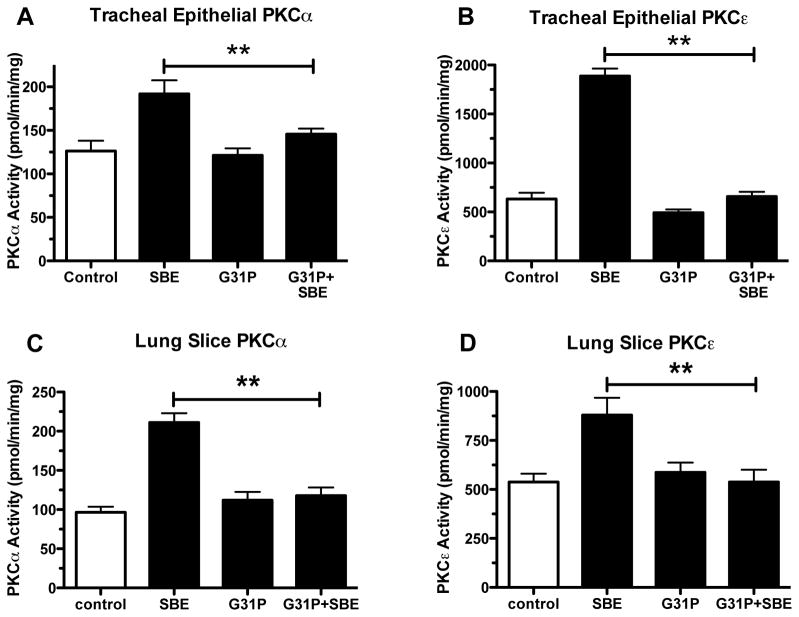
Airway epithelial cells harvested from tracheal tissue of mice and precisioncut lung slices were tested for catalytic PKC activity. Past work has shown that PKC activation is also a feature of SBE exposure (Wyatt et al. 2007, L1163–L1170; Romberger et al. 2002, 289–296). Mice exposed to SBE showed clearly significant (p<0.01) PKCα and PKCε activation (Fig. 3A and 3B respectively) in both tracheal epithelial cells and precision-cut lung slices (Fig. 3C and 3D respectively). In contrast those exposed to SBE that were given G31P showed minimal activity of either kinase.
Figure 3.
PKC activity. Lung trachea (A and B) and lung slices (C and D) were measured for PKCa and PKCε activity from saline, SBE, G31P and SBE+G31P treated mice. **P < 0.01
Histological Examination
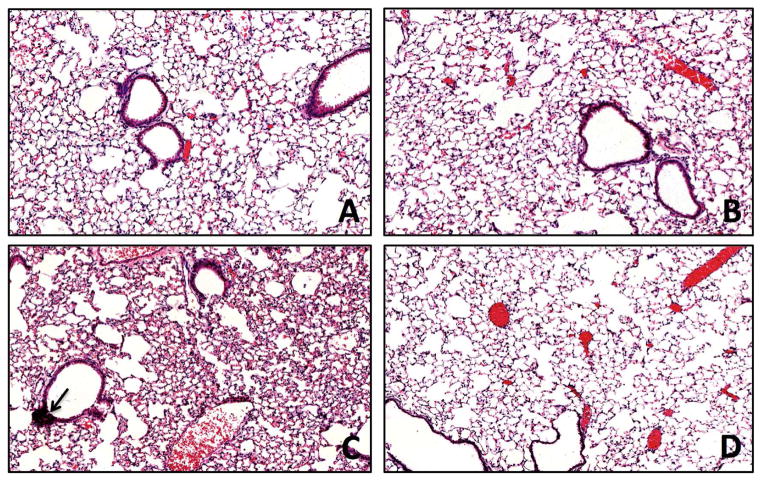
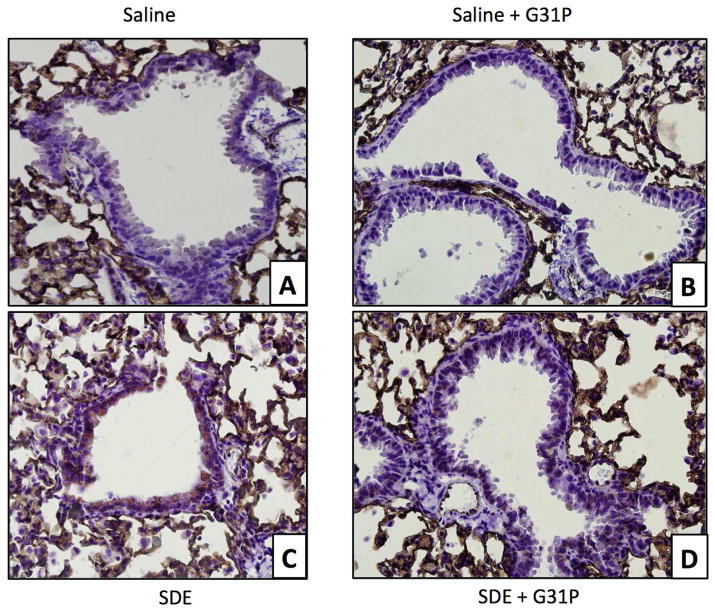
Examination of lung sections that were stained with hematoxylin and eosin revealed no apparent increases in lung infiltration in the control (Fig 4A) or control + G31P (Fig. 4B) treated animals. Animals that were given SBE however showed clear indications of increased cellularity in the alveoli as well as formation of foci of peribronchial mononuclear cells (Fig 4C, arrow), typical of such dust instillations into these animals. SBE + G31P treated animals showed some increase in cellular infiltration over controls similar to the SBE treated mice, but few if any mononuclear foci were apparent (Fig. 4D). This demonstrates that G31P was effective at blocking formation of these dust-induced mononuclear cell aggregates.
Figure 4.
Hematoxylin and eosin staining of mouse lung from mice treated with saline (A), G31P (B), SBE (C), and SBE+G31P (D). No change was apparent in G31P treatment compared to Saline. SDE induced. SBE induced increased cellularity and peribronchial foci of mononuclear cells (arrow), both of which were reduced with G31P co-administration.
Expression of CD54/ICAM-1 is reduced by G31P
Work by others has shown that ICAM-1 is increased in the bronchial epithelium after exposure to barn dust (Mathisen et al. 2004, 1738–1744), and as an important neutrophil chemotactic factor may play a role in neutrophil migration (Reviewed in Springer 1994, 301–314). Staining of the bronchial epithelium of mouse lungs using an ICAM-1 specific antibody clearly showed an increase in the expression of ICAM-1 in the bronchial epithelium of SBE treated mice (Fig. 5B) compared to saline treated animals (Fig. 5A). The administration of G31P did not induce ICAM-1 (Fig. 5C), but in SBE-treated animals (Fig 5D) it was able to inhibit the increased ICAM-expression, eliminating most if not all ICAM-1 staining of the bronchial epithelium.
Figure 5.
ICAM-1 staining of mouse lung from mice treated with saline (A), G31P (B), SBE (C), and SBE+G31P (D). ICAM-1 (brown stain) was expressed on bronchial epithelium of SDE treated mouse lung, but eliminated with co-administration of G31P.
Expression of CXCR1 and CXCR2 is reduced by G31P
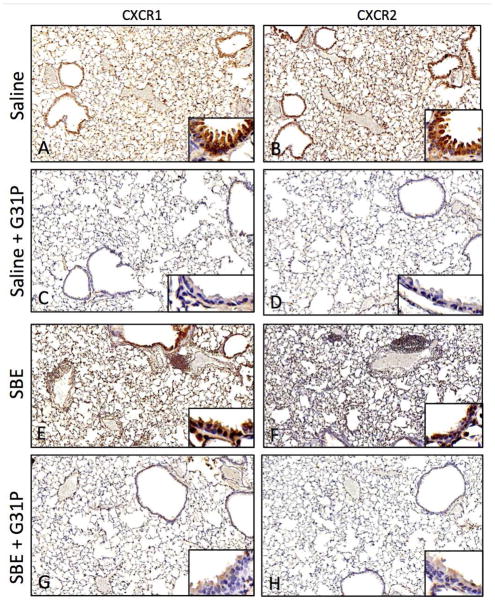
As no previous work exists on the effect of G31P on its receptors CXCR1 and CXCR2, we examined their expression in our model. Expression of both CXCR1 and CXCR2 was readily apparent in the lung (Fig 6). Control saline-treated animals showed strong expression of both receptors on macrophages within the alveolar space (Fig. 6A, 6B). There was also staining within the alveolar walls, though this was not present through all cells. There was however clear staining of the bronchial epithelium. Administration of G31P (Fig. 6C, 6D) eliminated staining of both receptors in the alveolar walls and the bronchial epithelium, however alveolar macrophages still stained clearly for both receptors.
Figure 6.
Mouse lung staining for CXCR1 (A,C,E,G) and CXCR2 (B,D,F,H). Both receptors were clearly expressed in alveolar epithelium, bronchial epithelium, and alveolar macrophages. Levels of both receptors in Saline (A,B) appeared reduced in G31P treated animals (C,D), particularly in the bronchial epithelium (inset images). SDE treated animals (E,F) showed increased expression of both receptors, and G31P treatment (G,H) was able to reduce this, particularly in bronchial epithelium (inset images).
With SBE treated animals (Fig. 6C), there was a general increase of both receptors through all locations described above, with the exception of weaker (but still apparent) staining of the bronchial epithelium for CXCR2. SBE-treated animals given G31P (Fig. 6D) showed decreases in staining of both these receptors, particularly in the alveolar walls and the bronchial epithelium, the latter of which showed only very faint staining for either receptor. As with the control + G31P animals, expression was still strong in cells within the alveolar space, however, we noted that unlike the SBE group not all of the cells in these alveolar spaces were stained.
Discussion
Lung inflammation and innate immune responses in the lung are a critical part of the immune system. These rapid and often vigorous responses are vital to the rapid containment and elimination of potential pathogens we are exposed to on a daily basis. Excessive inflammation in the lung however can rapidly lead to pathology and a loss of gas exchange function. Organic barn dusts represent an environmental exposure where such inflammatory responses may often be mal-adaptive. Immune responses to these dusts can be rapid (Gamage et al. 2007, 18; Dosman et al. 2004, 698–702) or prolonged and chronic (Senthilselvan et al. 1997, 1733–1741; Kirychuk et al. 2003, 375–380). Much of these responses appears directed to proteoglycans and lipopolysaccharides present in these dusts, signaling through toll-like receptors such as TLR2 and TLR4 (Reviewed in Poole and Romberger 2012, 126–132). In this case, no live organisms need be present to induce these vigorous innate responses.
A feature that has been shown to be critical in limiting lung inflammation is inhibiting the migration of neutrophils into the lung (Grommes and Soehnlein 2011, 293–307). As such, we examined the BALF of mice to determine if G31P had any effect on neutrophil migration in response to SBE. Total cell number in the BALF of SBE exposed animals was significantly elevated compared to control animals as others have shown (Poole et al. 2009, L1085–95), with the predominance of cells shifting from macrophages to neutrophils, indicating a large influx of these cells into the lungs, and presumably a more neutrophil-driven response. Treatment with G31P prevented much of this cellular influx into the lung. While some of this influx may still be neutrophils, the predominant lavage cell by numbers is again the alveolar macrophage. This lowering of neutrophil migration, and presumed activation in the lung will also contribute to limiting lung inflammation and damage.
Cytokine and chemokine expression, particularly IL-8 and IL-6 are good indicators of lung inflammation. Increased production of IL-6 and KC are common with SBE exposure (Poole et al. 2009, L1085–95; Bailey et al. 2008, L1049–L1054; Wyatt et al. 2007, L1163–L1170)and important in inducing cell migration and activation (Tsai et al. 1998, 2435–2440; Biffl et al. 1996, 575–8; discussion 578–9). In the mouse, the structurally similar KC, and the closely related chemokine MIP-2 cover the same functions as IL-8 in humans. KC is shown to bind CXCR1 while MIP-2 binds to CXCR2 (Ahuja, Lee, and Murphy 1996, 225–232; Fan et al. 2007, 11658–11666), though other chemokines may bind each receptor. Both chemokines may act in an autocrine fashion (Vanderbilt et al. 2003, 661–668)so blocking cytokine/chemokine receptor binding may also reduce native chemokine production. Therefore, we wished to see if the G31P inhibitor could block the expression of new KC and MIP-2 expression, as both are strongly induced with SBE exposure. We showed that indeed G31P is effective at reducing the expression of both chemokines induced to SBE exposure as well as IL-6, the last of which is capable of activating a number of neutrophil antimicrobial functions (Biffl et al. 1996, 575–8; discussion 578–9). Therefore, in considering G31P effectiveness we must also consider reduced KC, MIP-2, and IL-6 expression due to reduced intracellular activation in the lungs as another possible reason for the reduced inflammation observed.
Previous work from our lab has established that exposure to SBE can induce the activation of PKC in cells, in particular PKCα and PKCε (Romberger et al. 2002, 289–296; Poole et al. 2009, L1085–95; Poole et al. 2007, 366–373; Wyatt et al. 2007, L1163–L1170). As others have shown, PKCα may interact with TLR receptor via adaptor protein MyD88, and is critical in their signaling to increase downstream migration of transcription factors MAPK, NF-κB, and AP-1 to the nucleus (Langlet et al. 2010, 505–515). Similarly, PKCε is phosphorylated by all MyD88-associated TLRs and failure to do so results in a lack of NF-κB induction (Faisal et al. 2008, 18591–18600). Therefore, both PKCs are good indicators of TLR activation and signaling and potentially have critical effects on TLR-induced immune responses. Our results show that G31P can significantly reduce activation of both PKCs in response to SBE. As a role has been established for MyD88 in the induction of both KC and MIP-2 (Langlet et al. 2010, 505–515; De Filippo et al. 2008, 4308–4315; Orlichenko et al. 2010, G867-G876) this may also explain the similarly reduced expression of both chemokines.
Expression of the receptor CXCR2 can be found in airway epithelium (Schulz et al. 2012, 108–116; Farkas et al. 2005, 3724–3734), vascular endothelium (Schraufstatter, Chung, and Burger 2001, L1094–L1103), type-II cells (Vanderbilt et al. 2003, 661–668), and fibroblasts (Dunlevy and Couchman 1995, 311–321), many of which may be vital to neutrophil migration such as in the endothelium (Reutershan et al. 2006, 695–702). The research on CXCR1 in mice is more limited than in humans, due in part to a much later discovery of analogous function (Fan et al. 2007, 11658–11666). Binding CXCR1 or CXCR2 can induce internalization of these receptors that requires replacement of said receptors by protein synthesis (Cummings et al. 1999, 2341–2346). CXCR1 and CXCR2 mRNA expression was found to be induced by inflammatory stimuli coincident with increase of KC, GCP-2, and MIP-2 (Fan et al. 2007, 11658–11666). Because we showed that G31P can reduce expression of two of these chemokines, we postulated G31P could have an impact on expression of CXCR1 and CXCR2 proteins. We show that both receptors were expressed on the bronchial epithelium, some cells within the alveolar walls/septa, and cells within the alveolar space. Given our BALF results, these cells of the alveolar space should be macrophage as these are all we find in untreated animals. The positive cells within the alveoli septa are harder to clearly determine. The most likely candidates are type-II alveolar cells and/or fibroblasts (Vanderbilt et al. 2003, 661–668; Dunlevy and Couchman 1995, 311–321). With G31P treatment, CXCR1 and CXCR2 expression appears to be eliminated or greatly reduced in all cells except the alveolar macrophages. These patterns of expression appeared to be similar but with much higher overall expression of CXCR1 and CXCR2 in SBE exposed animals. To rule out the possibility of G31P interfering with antibody binding to target proteins we tested antibody binding to purified CXCR1 and CXCR2 proteins via dot blot with and without G31P, noting no change in staining efficiency (data not shown). In SBE-treated animals, additional cells in the alveolar spaces were present and also stained positively for CXCR1 and CXCR2. Because our lavage data does not support an increase in macrophages, these additional cells are likely neutrophils. As with the control animals, G31P treatment eliminated much of the staining for either receptor in the alveolar septa of SBE-treated animals, however this was not complete and some staining was still present. Bronchial epithelial cells, while clearly showing reduced CXCR1 and CXCR2 expression with G31P treatment also showed what appears to be a slight reduction in CXCR2 in SBE-treated animals. While we know of no mention of this in the literature, CXCR2 reduction in sepsis as a result of slow receptor turnover has been demonstrated in neutrophils (Cummings et al. 1999, 2341–2346). While we did not see an apparent reduction of CXCR2 staining in the alveolar macrophages with G31P treatment, staining was so intense in these cells with all treatments that a more sensitive method may need to be employed to determine if such changes do take place.
Taken together we propose that the ability of G31P to inhibit inflammation and neutrophil migration into the lung may be mediated at several levels. Treatment prevents or greatly inhibits chemokine signaling to a number of cell types such as epithelial and type-II cells (Zhao et al. 2009, 3213–3222). This could then result in reduction in PKCα and PKCε activation and production of cytokines, chemokines, or receptor expression, such as ICAM-1, and CXCR1 and CXCR2. Reduced CXCR1 and CXCR2 expression would further limit potential stimulatory signaling. Reductions of both chemokines and ICAM-1, the latter of which is vital to neutrophil migration (Tosi et al. 1992, 214–221)and upregulated in response to organic dusts (Mathisen et al. 2004, 1738–1744)will reduce neutrophil migration. Finally, neutrophils reaching the lung will be deprived of important signals for cytokine production, degranulation, formation of reactive oxygen species, or survival through reduced chemokine signaling (Biffl et al. 1996, 575–8; discussion 578–9).
Whatever the precise mechanism, we show that the IL-8 analog G31P is effective at blocking several key indicators of lung inflammation in response to organic dusts, and may be a viable therapy to consider for chronic organic dust exposure-induced lung injury.
Acknowledgments
This work was supported by NIH-NIOSH (R01OH008539) to DJR, NIH-NIAAA (R01AA017993) to TAW, and the Central States Center for Agricultural Safety and Health (NIOSH U54OH010162).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahuja SK, Lee JC, Murphy PM. CXC Chemokines Bind to Unique Sets of Selectivity Determinants that can Function Independently and are Broadly Distributed on Multiple Domains of Human Interleukin-8 Receptor B: Determinants of High Affinity Binding and Receptor Activation are Distinct. Journal of Biological Chemistry. 1996;271(1):225–232. doi: 10.1074/jbc.271.1.225. [DOI] [PubMed] [Google Scholar]
- Bailey KL, Poole JA, Mathisen TL, Wyatt TA, Von Essen SG, Romberger DJ. Toll-Like Receptor 2 is Upregulated by Hog Confinement Dust in an IL-6-Dependent Manner in the Airway Epithelium. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2008;294(6):L1049–L1054. doi: 10.1152/ajplung.00526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffl WL, Moore EE, Moore FA, Barnett CC., Jr Interleukin-6 Delays Neutrophil Apoptosis Via a Mechanism Involving Platelet-Activating Factor. The Journal of Trauma. 1996;40(4):575–8. doi: 10.1097/00005373-199604000-00009. discussion 578–9. [DOI] [PubMed] [Google Scholar]
- Boissy RJ, Romberger DJ, Roughead WA, Weissenburger-Moser L, Poole JA, LeVan TD. Shotgun Pyrosequencing Metagenomic Analyses of Dusts from Swine Confinement and Grain Facilities. PloS One. 2014;9(4):e95578. doi: 10.1371/journal.pone.0095578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier Y, Duchaine C, Israel-Assayag E, Bedard G, Laviolette M, Dosman J. Effects of Repeated Swine Building Exposures on Normal Naive Subjects. European Respiratory Journal. 1997;10(7):1516–1522. doi: 10.1183/09031936.97.10071516. [DOI] [PubMed] [Google Scholar]
- Cummings CJ, Martin TR, Frevert CW, Quan JM, Wong VA, Mongovin SM, Hagen TR, Steinberg KP, Goodman RB. Expression and Function of the Chemokine Receptors CXCR1 and CXCR2 in Sepsis. Journal of Immunology (Baltimore, Md: 1950) 1999;162(4):2341–2346. [PubMed] [Google Scholar]
- De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil Chemokines KC and Macrophage-Inflammatory Protein-2 are Newly Synthesized by Tissue Macrophages using Distinct TLR Signaling Pathways. Journal of Immunology. 2008;180(6):4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- Dosman JA, Fukushima Y, Senthilselvan A, Kirychuk SP, Lawson JA, Pahwa P, Cormier Y, Hurst T, Barber EM, Rhodes CS. Respiratory Response to Endotoxin and Dust Predicts Evidence of Inflammatory Response in Volunteers in a Swine Barn. American Journal of Industrial Medicine. 2006;49(9):761–766. doi: 10.1002/ajim.20339. [DOI] [PubMed] [Google Scholar]
- Dosman JA, Lawson JA, Kirychuk SP, Cormier Y, Biem J, Koehncke N. Occupational Asthma in Newly Employed Workers in Intensive Swine Confinement Facilities. The European Respiratory Journal. 2004;24(4):698–702. doi: 10.1183/09031936.04.00112102. [DOI] [PubMed] [Google Scholar]
- Dunlevy JR, Couchman JR. Interleukin-8 Induces Motile Behavior and Loss of Focal Adhesions in Primary Fibroblasts. Journal of Cell Science. 1995;108(1):311–321. doi: 10.1242/jcs.108.1.311. [DOI] [PubMed] [Google Scholar]
- Faisal A, Saurin A, Gregory B, Foxwell B, Parker PJ. The Scaffold MyD88 Acts to Couple Protein Kinase Ce to Toll-Like Receptors. Journal of Biological Chemistry. 2008;283(27):18591–18600. doi: 10.1074/jbc.M710330200. [DOI] [PubMed] [Google Scholar]
- Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, et al. Murine CXCR1 is a Functional Receptor for GCP-2/CXCL6 and Interleukin-8/CXCL8. Journal of Biological Chemistry. 2007;282(16):11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- Farkas L, Hahn M-C, Schmoczer M, Jentsch N, Kratzel K, Pfeifer M, Schulz C. Expression of CXC Chemokine Receptors 1 and 2 in Human Bronchial Epithelial Cells. Chest. 2005;128(5):3724–3734. doi: 10.1378/chest.128.5.3724. [DOI] [PubMed] [Google Scholar]
- Feniger-Barish R, Ran M, Zaslaver A, Ben-Baruch A. Differential Modes of Regulation of CXC Chemokine-Induced Internalization and Recycling of Human CXCR1 and CXCR2. Cytokine. 1999;11(12):996–1009. doi: 10.1006/cyto.1999.0510. [DOI] [PubMed] [Google Scholar]
- Gamage LN, Charavaryamath C, Swift TL, Singh B. Lung Inflammation Following a Single Exposure to Swine Barn Air. Journal of Occupational Medicine and Toxicology (London, England) 2007;2:18. doi: 10.1186/1745-6673-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JR, Li F, Zhang X, Wang W, Zhao X, Nayyar A. The Combined CXCR1/CXCR2 Antagonist CXCL8(3_74)K11R/G31P Blocks Neutrophil Infiltration, Pyrexia, and Pulmonary Vascular Pathology in Endotoxemic Animals. Journal of Leukocyte Biology. 2005;78(6):1265–1272. doi: 10.1189/jlb.0805458. [DOI] [PubMed] [Google Scholar]
- Grommes J, Soehnlein O. Contribution of Neutrophils to Acute Lung Injury. Molecular Medicine. 2011;17(3–4):293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz O, Khalilieh S, Ludwig-Sengpiel A, Watz H, Stryszak P, Soni P, Tsai M, Sadeh J, Magnussen H. SCH527123, a Novel CXCR2 Antagonist, Inhibits Ozone-Induced Neutrophilia in Healthy Subjects. The European Respiratory Journal. 2010;35(3):564–570. doi: 10.1183/09031936.00048509. [DOI] [PubMed] [Google Scholar]
- Iversen M, Kirychuk S, Drost H, Jacobson L. Human Health Effects of Dust Exposure in Animal Confinement Buildings. Journal of Agricultural Safety and Health. 2000;6(4):283–288. doi: 10.13031/2013.1911. [DOI] [PubMed] [Google Scholar]
- Kirychuk SP, Senthilselvan A, Dosman JA, Juorio V, Feddes JJ, Willson P, Classen H, Reynolds SJ, Guenter W, Hurst TS. Respiratory Symptoms and Lung Function in Poultry Confinement Workers in Western Canada. Canadian Respiratory Journal : Journal of the Canadian Thoracic Society. 2003;10(7):375–380. doi: 10.1155/2003/109679. [DOI] [PubMed] [Google Scholar]
- Langlet C, Springael C, Johnson J, Thomas S, Flamand V, Leitges M, Goldman M, Aksoy E, Willems F. PKC-a Controls MYD88-Dependent TLR/IL-1R Signaling and Cytokine Production in Mouse and Human Dendritic Cells. European Journal of Immunology. 2010;40(2):505–515. doi: 10.1002/eji.200939391. [DOI] [PubMed] [Google Scholar]
- Lazaar AL, Sweeney LE, MacDonald AJ, Alexis NE, Chen C, Tal-Singer R. SB-656933, a Novel CXCR2 Selective Antagonist, Inhibits Ex Vivo Neutrophil Activation and Ozone-Induced Airway Inflammation in Humans. British Journal of Clinical Pharmacology. 2011;72(2):282–293. doi: 10.1111/j.1365-2125.2011.03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaker BR, Barnes PJ, O’Connor B. Inhibition of LPS-Induced Airway Neutrophilic Inflammation in Healthy Volunteers with an Oral CXCR2 Antagonist. Respiratory Research. 2013;14 doi: 10.1186/1465-9921-14-137. 137-9921-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine Binding and Activities Mediated by the Mouse IL-8 Receptor. Journal of Immunology (Baltimore, Md: 1950) 1995;155(4):2158–2164. [PubMed] [Google Scholar]
- Li F, Zhang X, Gordon JR. CXCL8(3-73)K11R/G31P Antagonizes Ligand Binding to the Neutrophil CXCR1 and CXCR2 Receptors and Cellular Responses to CXCL8/IL-8. Biochemical and Biophysical Research Communications. 2002;293(3):939–944. doi: 10.1016/S0006-291X(02)00318-2. [DOI] [PubMed] [Google Scholar]
- Mathisen T, Von Essen SG, Wyatt TA, Romberger DJ. Hog Barn Dust Extract Augments Lymphocyte Adhesion to Human Airway Epithelial Cells. Journal of Applied Physiology (Bethesda, Md: 1985) 2004;96(5):1738–1744. doi: 10.1152/japplphysiol.00384.2003. [DOI] [PubMed] [Google Scholar]
- Murphy PM. Neutrophil Receptors for Interleukin-8 and Related CXC Chemokines. Seminars in Hematology. 1997;34(4):311–318. [PubMed] [Google Scholar]
- Orlichenko LS, Behari J, Yeh T-H, Liu S, Stolz DB, Saluja AK, Singh VP. Transcriptional Regulation of CXC-ELR Chemokines KC and MIP-2 in Mouse Pancreatic Acini. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2010;299(4):G867–G876. doi: 10.1152/ajpgi.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, Mehaffy J, Reynolds SJ. Muramic Acid, Endotoxin, 3-Hydroxy Fatty Acids, and Ergosterol Content Explain Monocyte and Epithelial Cell Inflammatory Responses to Agricultural Dusts. Journal of Toxicology and Environmental Health. Part A. 2010;73(10):684–700. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Romberger DJ. Immunological and Inflammatory Responses to Organic Dust in Agriculture. Current Opinion in Allergy and Clinical Immunology. 2012;12(2):126–132. doi: 10.1097/ACI.0b013e3283511d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal Organic Dust Exposure-Induced Airway Adaptation Response Marked by Persistent Lung Inflammation and Pathology in Mice. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2009;296(6):L1085–95. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, Romberger DJ. Repeat Organic Dust Exposure-Induced Monocyte Inflammation is Associated with Protein Kinase C Activity. The Journal of Allergy and Clinical Immunology. 2007;120(2):366–373. doi: 10.1016/j.jaci.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Radon K, Weber C, Iversen M, Danuser B, Pedersen S, Nowak D. Exposure Assessment and Lung Function in Pig and Poultry Farmers. Occupational and Environmental Medicine. 2001;58(6):405–410. doi: 10.1136/oem.58.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. Critical Role of Endothelial CXCR2 in LPS-Induced Neutrophil Migration into the Lung. Journal of Clinical Investigation. 2006;116(3):695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter IU, Chung J, Burger M. IL-8 Activates Endothelial Cell CXCR1 and CXCR2 through Rho and Rac Signaling Pathways. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2001;280(6 24–6):L1094–L1103. doi: 10.1152/ajplung.2001.280.6.L1094. [DOI] [PubMed] [Google Scholar]
- Schulz C, Stoelcker B, Ruhland B, Jentsch N, Steege A. Bronchoepithelial Expression of CXCR1 and CXCR2 does Not Facilitate Transepithelial Migration of Neutrophils. Respiration. 2012;84(2):108–116. doi: 10.1159/000332826. [DOI] [PubMed] [Google Scholar]
- Senthilselvan A, Dosman JA, Kirychuk SP, Barber EM, Rhodes CS, Zhang Y, Hurst TS. Accelerated Lung Function Decline in Swine Confinement Workers. Chest. 1997;111(6):1733–1741. doi: 10.1378/chest.111.6.1733. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic Signals for Lymphocyte Recirculation and Leukocyte Emigration: The Multistep Paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-Dependent Neutrophil Recruitment in a Murine Model of Legionella Pneumonia: Potential Role of Neutrophils as Immunoregulatory Cells. Infection and Immunity. 2001;69(4):2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi MF, Stark JM, Smith CW, Hamedani A, Gruenert DC, Infeld MD. Induction of ICAM-1 Expression on Human Airway Epithelial Cells by Inflammatory Cytokines: Effects on Neutrophil-Epithelial Cell Adhesion. American Journal of Respiratory Cell and Molecular Biology. 1992;7(2):214–221. doi: 10.1165/ajrcmb/7.2.214. [DOI] [PubMed] [Google Scholar]
- Tsai WC, Strieter RM, Wilkowski JM, Bucknell KA, Burdick MD, Lira SA, Standiford TJ. Lung-Specific Transgenic Expression of KC Enhances Resistance to Klebsiella Pneumoniae in Mice. Journal of Immunology (Baltimore, Md: 1950) 1998;161(5):2435–2440. [PubMed] [Google Scholar]
- Vanderbilt JN, Mager EM, Allen L, Sawa T, Wiener-Kronish J, Gonzalez R, Dobbs LG. CXC Chemokines and their Receptors are Expressed in Type II Cells and Upregulated Following Lung Injury. American Journal of Respiratory Cell and Molecular Biology. 2003;29(6):661–668. doi: 10.1165/rcmb.2002-0227OC. [DOI] [PubMed] [Google Scholar]
- Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of Swine Dust Induces Cytokine Release in the Upper and Lower Airways. European Respiratory Journal. 1997;10(2):381–387. doi: 10.1183/09031936.97.10020381. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Schmidt SC, Rennard SI, Tuma DJ, Sisson JH. Acetaldehyde-Stimulated PKC Activity in Airway Epithelial Cells Treated with Smoke Extract from Normal and Smokeless Cigarettes (44556) Experimental Biology and Medicine. 2000;225(1):91–97. doi: 10.1046/j.1525-1373.2000.22511.x. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Slager RE, DeVasure J, Auvermann BW, Mulhern ML, Von Essen S, Mathisen T, Floreani AA, Romberger DJ. Feedlot Dust Stimulation of Interleukin-6 and -8 Requires Protein Kinase Ce in Human Bronchial Epithelial Cells. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2007;293(5):L1163–L1170. doi: 10.1152/ajplung.00103.2007. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Slager RE, Heires AJ, DeVasure JM, VonEssen SG, Poole JA, Romberger DJ. Sequential Activation of Protein Kinase C Isoforms by Organic Dust is Mediated by Tumor Necrosis Factor. American Journal of Respiratory Cell and Molecular Biology. 2010;42(6):706–715. doi: 10.1165/rcmb.2009-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Town JR, Li F, Zhang X, Cockcroft DW, Gordon JR. ELR-CXC Chemokine Receptor Antagonism Targets Inflammatory Responses at Multiple Levels. Journal of Immunology (Baltimore, Md: 1950) 2009;182(5):3213–3222. doi: 10.4049/jimmunol.0800551. [DOI] [PubMed] [Google Scholar]