Abstract
Promoting bone regeneration and repair of bone defects is a need that has not been well met to date. We have previously found that adenosine, acting via A2A receptors (A2AR) promotes wound healing and inhibits inflammatory osteolysis and hypothesized that A2AR might be a novel target to promote bone regeneration. Therefore, we determined whether direct A2AR stimulation or increasing endogenous adenosine concentrations via purine transport blockade with dipyridamole regulates bone formation. We determined whether coverage of a 3 mm trephine defect in a mouse skull with a collagen scaffold soaked in saline, bone morphogenetic protein-2 (BMP-2; 200 ng), 1 μM CGS21680 (A2AR agonist, EC50 = 160 nM), or 1 μM dipyridamole (EC50 = 32 nM) promoted bone regeneration. Microcomputed tomography examination demonstrated that CGS21680 and dipyridamole markedly enhanced bone regeneration as well as BMP-2 8 wk after surgery (60 ± 2%, 79 ± 2%, and 75 ± 1% bone regeneration, respectively, vs. 32 ± 2% in control, P < 0.001). Blockade by a selective A2AR antagonist (ZM241385, 1 μM) or deletion of A2AR abrogated the effect of CGS21680 and dipyridamole on bone regeneration. Both CGS21680 and dipyridamole treatment increased alkaline phosphatase-positive osteoblasts and diminished tartrate resistance acid phosphatase-positive osteoclasts in the defects. In vivo imaging with a fluorescent dye for new bone formation revealed a strong fluorescent signal in treated animals that was equivalent to BMP-2. In conclusion, stimulation of A2AR by specific agonists or by increasing endogenous adenosine levels stimulates new bone formation as well as BMP-2 and represents a novel approach to stimulating bone regeneration.—Mediero, A., Wilder, T., Perez-Aso, M., Cronstein, B. N. Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2.
Keywords: CGS21680, dipyridamole, bone formation, osteoclast, osteoblast
There is a pressing need for new medical and surgical approaches that can capitalize on the intrinsic regenerative capacity of mineralized tissues to provide bone repair following trauma or after surgical repair of degenerative conditions. Indeed, following such procedures as spinal fusion or after repair of bone defects caused by trauma, infection, or metastatic disease, new bone formation is required as part of the healing process. Bone healing can be divided into 4 overlapping stages: inflammation, soft callus formation, hard callus formation, and bone remodeling (1). Each stage of bone healing is characterized by specific cellular and molecular processes, and the coordinated action of signaling molecules, growth factors, osteoprogenitor cells, extracellular matrix scaffold, and preservation of mechanical stability of the repair site is required to restore osseous integrity (1–3).
At present, in addition to autologous or cadaveric bone grafts, recombinant human bone morphogenetic proteins (rhBMP-2 and rhBMP-7) are in clinical use for promotion of bone growth following reparative procedures (spinal fusion), and the use of rhBMP-2 and rhBMP-7 is controversial. The BMPs are members of the TGF-β superfamily and are involved in committing multipotent stromal cells toward an osteogenic lineage and induction of new bone formation (4). Both in vitro and in vivo, BMP-2 has shown a strong osteogenic activity. rhBMP-2 in combination with a collagen sponge has been approved for the treatment of open long bone fractures and combined with a metal cage for spinal fusions (5). BMP-7 [osteogenic protein 1 (OP-1)] acts through receptors on local connective tissue stem and progenitor cells, either as a soluble factor or a matrix-bound protein (6). The capacity of exogenous recombinant human OP-1 to improve the healing of bone defects has been widely investigated in animal models (7, 8) and provides supportive evidence for the use of OP-1 in the treatment of fracture nonunions (9). Nonetheless, clinical studies show that rhBMP-2 and BMP-7 have critical side effects, such as vertebral osteolysis, ectopic bone formation, radiculitis, and even stimulation of cancer growth (10–12). Thus, it is necessary to develop new approaches to enhance bone regeneration.
Adenosine, generated from the catabolism of adenine nucleotides, modulates cell function by interacting with specific cell surface receptors [adenosine A1 receptor (A1R), adenosine A2A receptor (A2AR), adenosine A2B receptor (A2BR), and adenosine A3 receptor (A3R)], each of which has a unique pharmacologic profile (13). The adenosine A1R is critical for osteoclast differentiation and function; A1R blockade or deletion suppresses receptor activated by NF-κB ligand (RANKL)-induced NF-κB activation in vitro, increases bone density, and prevents ovariectomy-induced bone loss in vivo (14–16). Stimulation of the A2AR inhibits M-CSF/RANKL-stimulated osteoclast differentiation and function in vitro, in part by decreasing IL-1β and TNF-α secretion (17), and the selective A2AR agonist CGS21680 reduces wear particle-induced bone pitting and porosity in vivo, increasing cortical bone and bone volume compared with control mice (18). Strong evidence implicates a role for A2BR in osteoblast differentiation and function (19). Interestingly, the effects of A2AR activation are more ambiguous; A2AR stimulation promotes osteoblast proliferation but either has no effect on or inhibits osteoblast differentiation (20, 21) or promotes differentiation of mesenchymal stem cells to adipocytes (22) but does alter osteoblast function by changing the ratio of RANKL/osteoprotegerin (OPG) expression both in vivo and in vitro (23). Here we determined whether A2AR stimulation or enhancing adenosine concentrations by blockade of purine transport into cells via ent1 with dipyridamole regulates bone formation in a critical size defect in murine calvaria (24).
MATERIALS AND METHODS
Animals
C57Bl/6 [wild-type (WT)] mice (n = 70) and adenosine A2AR knockout (A2AKO) mice (n = 50), 6–8 weeks of age, were used. A2AKO mice were a gift of Dr. J. F. Chen (Boston University School of Medicine, Boston, MA, USA). Female A2AKO mice were bred onto a C57BL/6 background (≥10 backcrosses) in the New York University School of Medicine Animal Facility. A2AKO animals were derived from 4 original heterozygous breeding pairs for each mouse strain. Mice described as WT were all maintained on the C57Bl/6 background by the breeder (Taconic Laboratories, Hudson, NY, USA). Genotyping was performed by PCR, as reported previously (25). All protocols were approved by the New York University School of Medicine Animal Facility Institutional Animal Care and Use Committee.
Surgical procedure
WT (n = 70) and A2AKO (n = 50) mice were anesthetized by intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. The hair over the skull was shaved, and the underlying skin was aseptically prepared. A full-thickness midline incision, extending from the nasofrontal to occipital region, was made under sterile conditions. The subcutaneous tissue was sharply dissected along the same line as the skin. The underlying periosteum was sharply incised on the midline and subsequently elevated off the skull to obtain sufficient exposure for the trephine. In the single defect model, a 3 mm defect to remove bone from the middle of the dorsal calvarium was done using the trephine-irrigating saline to avoid bone overheating with caution to prevent damage to the underlying sagittal sinus and dura matter. In some mice, the defect was covered with a collagen sponge (DuraGen Plus; Integra LifeScienes Corp., Plainsboro, NJ, USA) soaked in 20 µl of 0.9% saline (control) (n = 10 WT and n = 10 A2AKO mice), and others were treated with a collagen sponge soaked in 1 µM CGS21680 or 1 µM dipyridamole both alone or in the presence of 1 µM ZM241385 (n = 10 WT and n = 10 A2AKO mice per treatment group). As control for bone formation, some animals were treated with 200 ng BMP-2. Treatment was done by daily local injection for CGS21680 and dipyridamole vs. a single dose for BMP-2, beginning immediately after incision closure and continuing every day until death. Water and food were given ad libitum until death. Animals were euthanized after 2, 4, 6, and 8 weeks of defect formation in a CO2 chamber, and the calvaria were removed, fixed, and prepared for microcomputed tomography (microCT) and histologic staining.
In vivo bone formation quantification
Two days after surgery, and then once per week up to 8 weeks, Xenolight Rediject Bone Probe 680 conjugate (Caliper; PerkinElmer, Waltham, MA, USA) was injected intravenously in a dose of 2 nmol/150 µl. This fluorescence reagent targets hydroxyapatite, and in combination with the IVIS imaging system (Caliper), allows in vivo detection and measurement of skeletal changes (26–28). Fluorescence images were taken with an excitation peak of 681 nm and an emission peak of 696 nm. Images were analyzed following manufacturer’s instructions. Total flux in photons per second were normalized and expressed as a percentage of control to avoid intrinsic changes among animals.
MicroCT
After death, WT and A2AKO (n = 5 calvaria per treatment group) were fixed in 70% ethanol and prepared for high-resolution microCT. This 3-dimensional (3D) imaging technology was used to perform qualitative and quantitative analyses of new bone formation areas in murine calvarial bone. Analyses were performed in the microCT core at the New York University College of Dentistry using the Scanco Medical MicroCT 40 Scanner (Scanco Medical, Wayne, PA, USA) with 25 µm resolution (KVp: 5 T µA/45). Every field of view chosen for analysis was scanned by increments using a CCD detector, with an integration time of 300 milliseconds with a 30.7 holder size and average data of 5. For qualitative analysis, 3D images of the mice heads were then reconstructed from the cross-sectional slices using the software provided by Scanco Medical MicroCT 40 and processing was done to get direct morphometric measurements in 3D. For quantitative analysis of new bone formation, area of interest was segmented manually by marking the volume of interest—a round-shaped region across the defected bone of ∼3 mm. Data were calculated in percentages to avoid intrinsic differences among animals. Percentage of bone regenerated was calculated by subtracting the remaining defect area to the total defect area.
Histologic studies
WT and A2AKO (n = 5 calvaria per treatment group) were removed and fixed in 4% paraformaldehyde for 48 hours, followed by decalcifying in 10% EDTA for 4 weeks and paraffin embedding. Sections (5 µm) were cut, and hematoxylin and eosin staining was performed. Photomicrographs were taken at an original magnification of ×800.
Tartrate resistance acid phosphatase (TRAP) staining was carried out with a homemade TRAP buffer (0.1 M acetate buffer, 0.3 M sodium tartrate, 10 mg/ml Naphtol AS-MX phosphate, 0.1% Triton X-100, and 0.3 mg/ml Fast Red Violet LB; Sigma-Aldrich, St. Louis, MO, USA). After deparaffinized and acetate buffer washing processes, samples were incubated in TRAP buffer for 30 minutes and counterstained with Fast Green.
Immunohistochemistry analysis of markers for osteoclasts (cathepsin K, RANK; Santa Cruz Biotechnology, Santa Cruz, CA, USA), osteoblasts (alkaline phosphatase; Abcam, Incorporated, Cambridge, MA, USA), RANKL (Abcam, Incorporated), and bone remodeling (osteopontin, osteocalcin, and osteoprotegerin; Abcam, Incorporated) were carried out in WT and A2AKO calvaria in paraffin-embedded sections. In brief, deparaffinized and hydrated sections were incubated with proteinase K solution (20 μg/ml in Tris-EDTA (TE) buffer, pH 8.0) for 15 minutes in a water bath at 37°C for antigen retrieval. After cooling, the sections were rinsed twice with PBS, and the internal peroxidase was removed with 3% H2O2 in methanol by incubating sections for 15 minutes at room temperature. After rinsing sections in PBS-bovine serum albumin (BSA) 3%, 1 hour blocking at room temperature (PBS-BSA 3%, 1% Triton X-100, and 5% fetal bovine serum) was performed. Primary antibody diluted in PBS with 3 wt% BSA (cathepsin K, 1:25; RANK, 1:100; RANKL, 1:200; osteopontin, 1:100; osteocalcin, 1:200; and osteoprotegerin, 0.5 µg/ml) was incubated overnight at 4°C in a humidifying chamber. After secondary goat anti-rabbit-FITC antibody (1:200; Santa Cruz Biotechnology) incubation, sections were mounted with Fluoroshield mounting media with DAPI (Sigma-Aldrich). Images were observed under fluorescence microscope (Nikon, Tokyo, Japan) equipped with Nis Elements F3.0 SP7 software and under a Leica microscope equipped with SlidePath Digital Image Hub Version 3.0 software (Leica Microsystems, Buffalo Grove, IL, USA).
Quantitative real-time RT–PCR
To confirm that activation of A2AR is a key modulator in promotion of bone formation, different markers for osteoclast (cathepsin K, nuclear factor of activated T cells (NFATc1), and osteopontin) and osteoblast (RANKL, OPG, and osteonectin) mRNA expression were analyzed. Bone marrow cells from 8-week-old C57BL/6 female mice were isolated by flushing out the bone marrow cavity; 5 × 106 cells/cm2 nonadherent cells were collected and seeded in α-MEM with 30 ng/ml M-CSF (R&D Systems, Minneapolis, MN, USA) for 2 days. At day 3 (day 0 of differentiation), 30 ng/ml RANKL (R&D Systems) was added to cultures in the presence or absence of 1 µM CGS21680 or 1 µM dipyridamole alone or in the presence of 1 µM ZM241385 for 24 hours. Adherent bone marrow cells were seeded at a density of 5 × 104 cells/cm2 with osteogenic media (1 µM α-MEM containing dexamethasone, 50 µg/ml ascorbic acid, and 10 mM β-glycerophosphate) in the presence or absence of 1 µM CGS21680 or 1 µM dipyridamole alone or in the presence of 1 µM ZM241385 for 24 hours. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). RNA was retrotranscribed using the MuLV Reverse transcriptase PCR kit (Applied Biosystems, Foster City, CA, USA). Real-time RT-PCR was performed using the Brilliant Fast SYBR Green Kit QPCR Master Mix (Agilent Technologies, Santa Clara, CA, USA). The following primers were used—cathepsin K: forward, 5′-GCTGAACTCAGGACCTCTGG-3′; reverse, 5′-GAAAAGGGAGGCATGAATGA-3′; NFATc1: forward, 5′-TCATCCTGTCCAACACCAAA-3′; reverse, 5′-TCACCCTGGTGTTCTTCCTC-3′; osteopontin: forward, 5′-TCTGATGAGACCGTCACTGC-3′; reverse, 5′-TCTCCTGGCTCTCTTTGGAA-3′; mouse RANKL: forward, 5′-AGCCGAGACTACGGCAAGTA-3′; reverse, 5′-GCGCTCGAAAGTACAGGAAC-3′; mouse OPG: forward, 5′-CTGCCTGGGAAGAAGATCAG-3′; reverse, 5′-TTGTGAAGCTGTGCAGGAAC-3′; mouse osteonectin: forward, 5′-TGGGAGAATTTGAGGACGGTG-3′; reverse, 5′-GAGTCGAAGGTCTTGTTGTCAT-3′; mouse GAPDH: forward, 5′-CTACACTGAGGACCAGGTTGTCT-3′; reverse, 5′-GGTCTGGGATGGAAATTGTG-3′. The Pfaffl method (29) was used for relative quantification.
Western blot
To further confirm key bone remodeling proteins modulation by A2AR activation, different markers for osteoclast (cathepsin K, NFATc1, osteopontin, and RANK) and osteoblast (osteocalcin, RANKL, OPG, and osteonectin) were analyzed by Western blot. Bone marrow cells from 8-week-old C57BL/6 female mice were isolated by flushing out the bone marrow cavity; 5 × 106 cells/cm2 nonadherent cells were collected and seeded in α-MEM with 30 ng/ml M-CSF (R&D Systems) for 2 days. At day 3 (day 0 of differentiation), 30 ng/ml RANKL (R&D Systems) was added to cultures in the presence or absence of 1 µM CGS21680 or 1 µM dipyridamole alone or in the presence of 1 µM ZM241385 for 24 hours. Adherent bone marrow cells were seeded at a density of 5 × 104 cells/cm2 with osteogenic media (α-MEM containing 1 µM dexamethasone, 50 µg/ml ascorbic acid, and 10 mM β-glycerophosphate) in the presence or absence of 1 µM CGS21680 or 1 µM dipyridamole alone or in the presence of 1 µM ZM241385 for 24 hours. Cells were lysed with RIPA buffer, and 4 µg protein was subjected to 7.5% or 10% SDS-PAGE and transferred to a nitrocellulose membrane. Nonspecific binding was blocked with Tris-buffered saline/Tween-20, 0.05–5% BSA. Membranes where incubated overnight (4°C) with primary antibodies against cathepsin K, NFATc1, osteopontin, RANK, osteocalcin, RANKL, OPG, osteonectin, and actin (1:1000 each). Membranes were incubated with goat anti-rabbit IRDye 800CW 1:10,000 and goat anti-mouse IRDye 680 RD 1:10,000 (LI-COR Biosciences, Lincoln, NE, USA) in the dark. Proteins were visualized by LI-COR Odyssey equipment, which detects near-infrared fluorescence. As each secondary antibody emits a signal in a different spectrum, reprobing with actin (to check that all lanes were loaded with the same amount of protein) was performed simultaneously with primary antibody incubation. Coomassie Brilliant Blue was used as loading marker for the supernatant proteins. Intensities of the respective band were quantitated by densitometric analysis using Image Studio 2.0.38 software (LI-COR Biosciences). Variations in band intensity were expressed as percentage of unstimulated controls to minimize disparities among different experiments.
Statistical analysis
Statistical significance for differences between groups was determined using 1-way ANOVA and Bonferroni post hoc testing, 2-way ANOVA, or Student t test, as appropriate. All statistics were calculated using GraphPad software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Activation of A2AR and blockade of adenosine uptake by dipyridamole increase bone regeneration in vivo
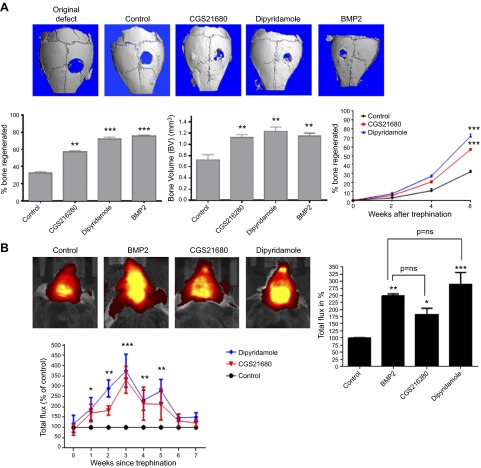
Eight weeks after surgery, microCT examination of mouse calvaria demonstrated that both the A2AR agonist CGS21680 and the inhibitor of ent1-mediated adenosine uptake, dipyridamole, markedly enhanced bone regeneration as well as BMP-2 (60 ± 2%, 79 ± 2%, and 75 ± 1% bone regeneration, respectively, vs. 32 ± 2% in control, P < 0.001, n = 5 mice per condition; Fig. 1A). The bone volume (BV) was also increased in CGS21680- and dipyridamole-treated defects as well as in the BMP-2–treated defects (1.12 ± 0.05, 1.23 ± 0.07, and 1.15 ± 0.05 mm3 BV, respectively, vs. 0.72 ± 0.09 mm3 in control, P < 0.01, n = 5 mice per condition; Fig. 1A). CGS21680- and dipyridamole-enhanced bone formation was detected as early as 4 weeks after surgery (Fig. 1A).
Figure 1.
CGS21680 (A2AR agonist) and dipyridamole (adenosine uptake inhibitor) promote bone growth in a similar fashion as BMP-2. C57Bl/6 mice were anesthetized; a 3 mm trephine defect was formed and covered with a collagen scaffold soaked in saline, CGS21680 1 μM, dipyridamole 1 μM, or BMP-2 200 ng. A) The figures show representative microCT images of calvaria. Graphs indicate morphometric quantitation of microCT analysis. Percentage of bone formation was calculated by subtracting the remaining defect area to the total defect area. BV units in mm3. B) One week after surgery, XenoLight RediJect Bone Probe 680 conjugate was injected intravenously, and the fluorescence image was captured weekly. Total flux in photons per second were normalized and expressed as a percentage of control to avoid intrinsic changes among animals. Red indicates low signal intensity and low rates of new bone formation, whereas yellow indicates high signal intensity and high rates of new bone formation. Data were expressed as mean ± sem (n = 5 per group). **P < 0.01 and ***P < 0.001 compared with control (ANOVA).
To detect new bone formation in a dynamic fashion XenoLight RediJect Bone Probe 680, a fluorescent conjugate that binds to hydroxyapatite in new bone, was injected intravenously as described under Materials and Methods (26–28). As shown in Fig. 1B, in vivo imaging reveals a strong fluorescent signal in CGS21680- and dipyridamole-treated animals, equivalent to the signal in BMP-2–treated mice, compared with control as soon as 1 week after the bone defect formation and lasting for ≥7 weeks.
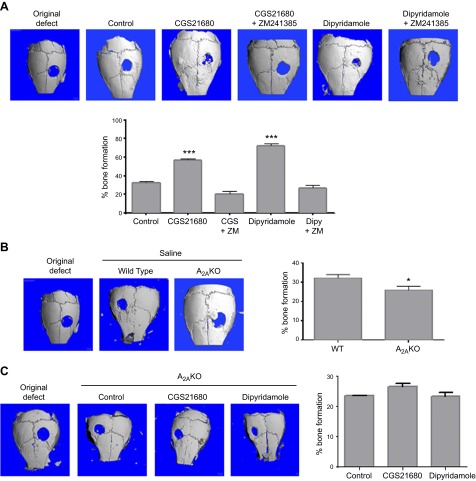
Bone regeneration induced by both CGS21680 and dipyridamole was abrogated when the A2AR was blocked with ZM241385 (20 ± 3% and 26 ± 4% bone regeneration, respectively, vs. 32 ± 2% in control, P = not significant, n = 5 mice per condition; Fig. 2A). Moreover, neither agent stimulated bone regeneration in mice lacking A2AR. Eight weeks after surgery, the absence of the A2AR dramatically reduced the percentage of new bone formation compared with WT mice (25 ± 1% bone regeneration vs. 32 ± 2% in control, P < 0.05, n = 5 mice per condition; Fig. 2B). Neither CGS21680 nor dipyridamole enhanced bone regeneration when the A2AR was deleted (Fig. 2C).
Figure 2.
The presence of A2A receptors is required to increase bone formation. C57Bl/6 or A2AKO mice were anesthetized; a 3 mm trephine defect was formed and covered with a collagen scaffold soaked in CGS21680 1 μM or dipyridamole 1 μM alone or in combination with ZM241385 1 μM. A) The figures show representative microCT images of calvaria treated with CGS21680 or dipyridamole alone or in combination with ZM241385. Graphs indicate morphometric quantitation of microCT analysis. B) The figures show representative microCT images of saline-treated WT calvaria compared with saline-treated A2AKO calvaria. Graphs indicate morphometric quantitation of microCT analysis. C) The figures show representative microCT images of A2AKO mice calvaria treated with CGS21680 or dipyridamole. Graphs indicate morphometric quantitation of microCT analysis. Data were expressed as mean ± sem (n = 5 per group). * P < 0.05 and ***P < 0.001 compared with control (ANOVA).
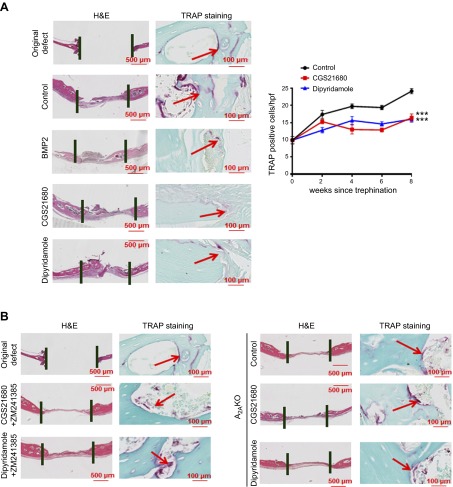
Activation of adenosine A2AR and blockade of adenosine uptake by dipyridamole increased osteoblast number and decreased osteoclast number in vivo
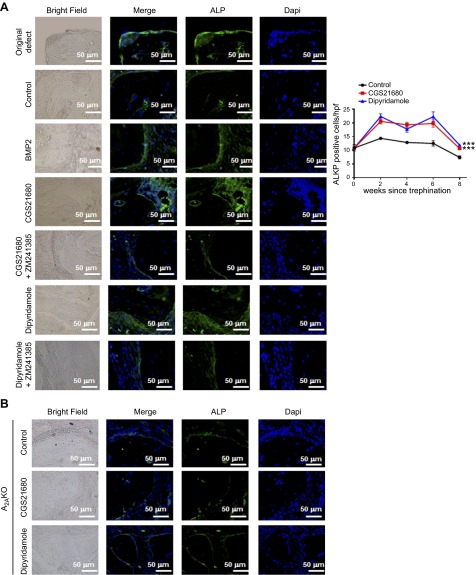
Histochemical staining of bone defects provided further evidence that A2AR stimulation increased bone regeneration and supported the results obtained from microCT. TRAP staining revealed fewer osteoclasts in CGS21680- and dipyridamole-treated defects (17 ± 1 and 16 ± 1 osteoclasts per high power field (hpf), respectively, vs. 24 ± 1 osteoclasts per hpf for saline-treated mice, P < 0.001, n = 5; Fig. 3A). These results mirrored those for BMP-2–treated mice (Fig. 3A). Moreover, when studied 8 weeks after defect formation, in CGS21680- and dipyridamole-treated mice, there was increased immunostaining for bone formation markers in the bony defects (alkaline phosphatase-positive cells per hpf increased from 15 ± 1 for saline-treated to 21 ± 1 for CGS21680-treated and 24 ± 1 for dipyridamole-treated, P < 0.001, n = 5; Fig. 4A). When studied over time, treatment with CGS21680 and dipyridamole induced a persistent increase in alkaline phosphatase-positive cells, whereas the number of TRAP-positive cells remained constant over time but was lower than that observed in saline-treated animals at all time points after 2 weeks (Figs. 3A and 4A).
Figure 3.
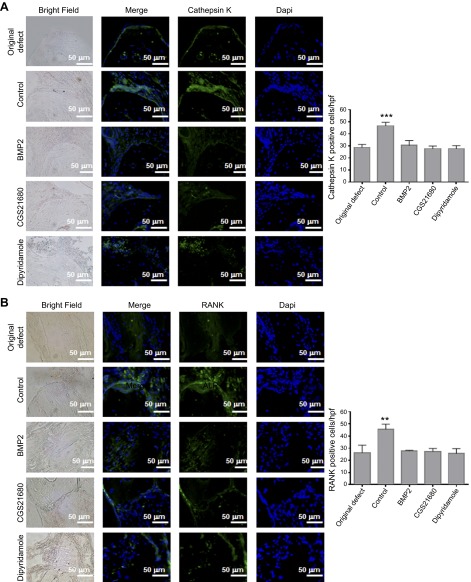
CGS21680 and dipyridamole decrease TRAP-positive cells 8 weeks after trephination in an A2AR-dependent manner. A) Five micrometer sections of WT mice calvaria were stained with hematoxylin and eosin to determine new bone formation. Figures indicate representative images for TRAP staining for osteoclast in calvaria treated with saline, BMP-2, CGS21680, and dipyridamole. Graph show quantification of the number of osteoclast per hpf (5 fields per slide). Results shown are means ± sem (n = 5 mice per group). B) Five micrometer sections of WT mice calvaria were stained with hematoxylin and eosin to determine new bone formation. Figures indicate representative images for TRAP staining for osteoclast in calvaria treated with CGS21680 and dipyridamole in combination with ZM241385. C) Five micrometer sections from A2AKO mice calvaria were stained with hematoxylin and eosin to determine new bone formation. Figures indicate representative images for alkaline phosphatase and TRAP staining for osteoblasts and osteoclasts in calvaria treated with saline, CGS21680, and dipyridamole. Images were taken at the same magnification: ×100 and ×40. Scale bar indicates 500 and 100 μm. ***P < 0.001 (ANOVA).
Figure 4.
CGS21680 and dipyridamole increase bone formation 8 weeks after trephination. Calvaria were processed and immunohistologic staining carried out. A) Representative sections of WT mice calvaria treated with saline, BMP-2, CGS21680, dipyridamole, CGS21680 + ZM241385, and dipyridamole + ZM241385 (from n = 5 mice per group) stained for alkaline phosphatase (green). Graph show quantification of the number of osteoclast per hpf (5 fields per slide). Results shown are means ± sem (n = 5 mice per group). B) Representative sections of A2AKO mice calvaria (from n = 5 mice per group) stained for alkaline phosphatase (green). Nucleus is shown in blue (DAPI). All images were taken at the same magnification (×400). Scale bar indicates 50 μm.
In addition, as observed by microCT, treatment of the animals with CGS21680 or dipyridamole in the presence of the A2AR antagonist ZM241385 did not increase bone regeneration or alkaline phosphatase-positive osteoblasts compared with controls (Figs. 2A and 4A). In the ZM241385-treated mice, the alkaline phosphatase-positive cell number was similar to saline-treated mice (15 ± 1 for control, 17 ± 1 for CGS21680 + ZM241385, and 14 ± 2 for dipyridamole + ZM241385, P = not significant, n = 5), and similar results were obtained for TRAP-positive cells (24 ± 1 for control, 21 ± 2 for CGS21680 + ZM241385, and 23 ± 1 for dipyridamole + ZM241385, P = not significant, n = 5; Fig. 3A). In the absence of the A2AR, neither CGS21680 nor dipyridamole altered the number of alkaline phosphatase- or TRAP-positive cells compared with saline-treated A2AKO mice (Figs. 3C and 4B).
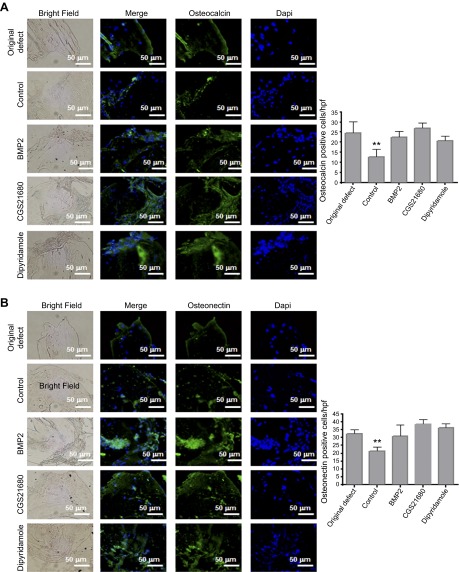
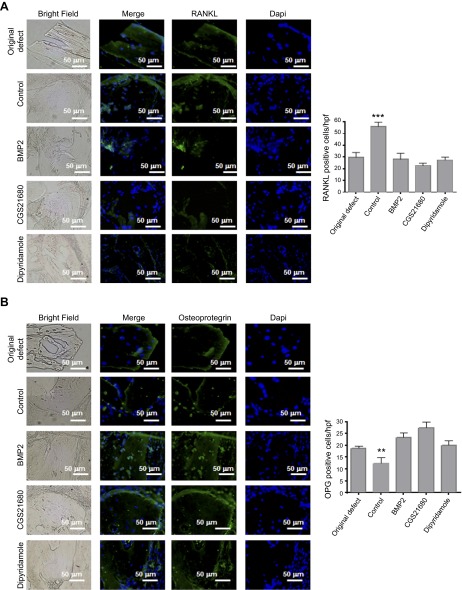
To further explore the underlying mechanisms by which A2AR activation and dipyridamole treatment promote bone regeneration, we studied the expression of other markers of bone resorption and formation (Figs. 5–7). In CGS21680- and dipyridamole-treated mice, there was a decrease in cells staining positive for cathepsin K, a lysosomal cysteine protease that plays a role in bone resorption that is commonly used as a marker for osteoclasts (28 ± 1 and 27 ± 1 cells per hpf, respectively, vs. 46 ± 2 cells per hpf for controls, P < 0.001, n = 5; Fig. 5A). Similar results were observed for RANK (27 ± 1 and 25 ± 2 cells per hpf, respectively, vs. 45 ± 2 cells per hpf for controls, P < 0.01, n = 5) and RANKL (22 ± 1 and 27 ± 1 cells per hpf, respectively, vs. 50 ± 2 cells per hpf for controls, P < 0.001, n = 5; Figs. 5B and 6A). The number of cells expressing OPG, a decoy receptor for RANKL, was increased in CGS21680-and dipyridamole-treated mice compared with saline-treated animals (27 ± 1 and 22 ± 1 cells per hpf, respectively, vs. 13 ± 2 cells per hpf for controls, P < 0.001, n = 5; Fig. 6B). Osteocalcin, a protein secreted by osteoblasts, was localized to cells adherent to bone in all treatment groups of mice (Fig. 7A), consistent with the increased osteoblast number in CGS21680- and dipyridamole-treated mice (27 ± 1 and 20 ± 3 cells per hpf, respectively, vs. 12 ± 1 cells per hpf for controls, P < 0.001, n = 5; Fig. 7A). Finally, osteonectin, a protein secreted by osteoblasts that initiates mineralization, colocalizes with osteoblasts on the surface of bone and was overexpressed in CGS21680- and dipyridamole-treated mice (38 ± 2 and 36 ± 1 cells per hpf, respectively, vs. 21 ± 1 cells per hpf for controls, P < 0.001, n = 5; Fig. 7B). As shown in Figs. 5–7, in all cases, the number of positive cells was similar to those observed in BMP-2–treated mice.
Figure 5.
Immunohistochemistry for markers of osteoclasts and bone remodeling. WT calvaria treated with saline, BMP-2, CGS21680, and dipyridamole were processed and immunohistologic staining was carried out. A) Representative images for cathepsin K (green). B) Representative sections of calvaria (from n = 5 mice per group) stained for RANK (green). Nucleus is shown in blue (DAPI). Graphs show quantification of the number of cells per hpf (5 fields per slide). Data are means ± sem (n = 5 mice per group). **P < 0.01 and ***P < 0.001 (ANOVA). All images were taken at the same magnification (×400). Scale bar indicates 50 μm.
Figure 7.
Immunohistochemistry for markers of bone remodeling. WT calvaria treated with saline, BMP-2, CGS21680, and dipyridamole were processed, and immunohistologic staining was carried out. A) Representative images for osteocalcin (green). B) Representative sections of calvaria (from n = 5 mice per group) stained for osteonectin (green). Nucleus is shown in blue (DAPI). Graphs show quantification of the number of cells per hpf (5 fields per slide). Data are means ± sem (n = 5 mice per group). **P < 0.01 and ***P < 0.001 (ANOVA). All images were taken at the same magnification (×400). Scale bar indicates 50 μm.
Figure 6.
Immunohistochemistry for markers of bone remodeling. WT calvaria treated with saline, BMP-2, CGS21680, and dipyridamole were processed, and immunohistologic staining was carried out. A) Representative images for RANKL (green). B) Representative sections of calvaria (from n = 5 mice per group) stained for OPG (green). Nucleus is shown in blue (DAPI). Graphs show quantification of the number of cells per hpf (5 fields per slide). Data are means ± sem (n = 5 mice per group). **P < 0.01 and ***P < 0.001 (ANOVA). All images were taken at the same magnification (×400). Scale bar indicates 50 μm.
Activation of adenosine A2AR and blockade of adenosine uptake by dipyridamole decreases osteoclast marker expression and increases osteoblast markers in vitro
To confirm that both CGS21680 and dipyridamole treatments promoted bone formation by modifying the expression of key cellular markers, expression of these markers was analyzed in vitro by RT-PCR (Supplemental Fig. S1) and Western blot (Supplemental Fig. S2). In primary bone marrow–derived osteoclasts, mRNA levels for cathepsin K were increased 24 hours after RANKL stimulation, and this increase was abrogated when either CGS21680 and dipyridamole was present, an effect abrogated by the A2AR antagonist ZM241385 (Supplemental Fig. S1A). Message levels for NFATc1 and osteopontin were increased by RANKL, and neither CGS21680 nor dipyridamole alone or in the presence of ZM241385 modified this increase (Supplemental Fig. S1A), as we have previously shown (30). In primary bone marrow-derived osteoblasts, mRNA for osteocalcin, Runx2, and osteonectin were increased after 24 hours incubation in osteogenic media, and both osteocalcin and osteonectin expression, but not Runx2 expression, were enhanced by CGS21680 and dipyridamole (Supplemental Fig. S1B); these effects were reversed by the A2AR antagonist ZM241385.
Similar results were observed at the protein level after 24 h stimulation (Supplemental Fig. S2). Both CGS21680 and dipyridamole decreased protein expression for cathepsin K, osteopontin, and RANK without changing NFATc1 expression (Supplemental Fig. S2A). Moreover, these treatments decreased RANKL expression in osteoblast precursors while increasing osteocalcin, osteoprotegerin, and osteonectin expression (Supplemental Fig. S2B).
DISCUSSION
Appropriate healing after many surgical procedures, such as spinal fusion and repair of bone defects caused by trauma, infection, or metastatic disease, require the formation of new bone. The critical size defect was originally defined as the smallest-sized intraosseous wound in a particular bone and species of animal that will not heal spontaneously during the lifetime of the animal and was developed as a model of craniofacial fibrous nonunion intended to standardize the testing of bone repair materials that could be used as alternatives to bone allo- or autografting (31). Using a collagen sponge as a carrier to fill the defect, we found that treatment with an A2AR agonist or enhancement of endogenous levels by dipyridamole, an agent that diminishes cellular purine uptake, promotes bone regeneration in a critical size defect.
To date, strong evidence has implicated A2BR in osteoblast differentiation and function (20, 22, 32–34), but little evidence supports a role for either A1R or A2AR in the regulation of osteoblast differentiation or function. Thus, Gharibi et al. (35) reported that, although A1R was expressed in osteoblast precursors, it was involved in induction of adipocyte differentiation rather than osteoblast differentiation. Nonetheless, Katebi et al. (36) reported that adenosine, acting via A2AR, plays a critical role in promoting the proliferation of mouse bone marrow-derived fibroblast-like mesenchymal stem cells, and Gharibi et al. (22) reported that A2AR is up-regulated in later osteoblast differentiation stages, but stimulation of A2AR more strongly promotes adipocyte differentiation. Conversely, a recent report from He et al. (21) reported that stimulation or blockade of A1R, A2AR, and A3R had no effect on human osteoblast differentiation and mineralization. Moreover, a recent report showed that pulsed electromagnetic field stimulation activates adenosine A2AR and stimulates osteoblast proliferation; pulsed electromagnetic field stimulation has been reported to have a positive effect on fracture healing (37). Here we describe, for the first time, a direct effect of A2AR activation on bone regeneration in vivo, either via direct activation of the receptor or by enhancing extracellular adenosine levels by blockade of Ent1 transporter.
Previously, we reported that activation of the A2AR inhibits osteoclast differentiation (17–19) both in vitro and in vivo. Moreover, application of an A2AR agonist reduces osteoclast-mediated bone resorption in a murine calvaria model of wear particle-induced bone resorption (18). A2AR activation reduces particle-induced bone pitting and porosity, diminishes inflammation, and reduces osteoclast number in this model. Ex vivo calvaria cultures exposed to wear particles secreted more M-CSF, IL-1β, and TNF-α, and treatment with CGS21680 decreased levels to normal, indicating that activation of A2AR inhibits the release of proinflammatory cytokines from macrophages and osteoclasts (18). Interestingly, IL-10 levels were markedly increased in the supernatants of the particle-exposed CGS21680-treated calvaria (18).
Here we report that A2AR activation promotes bone formation by 2 mechanisms. On one hand, A2AR activation inhibits osteoclast differentiation and function both in vitro and in vivo. A2AR stimulation suppresses osteoclast differentiation and function in vitro and in vivo (30, 38), confirmed here by altered expression of osteoclast markers in vitro and a marked reduction of osteoclasts in lesional bone in vivo. On the other hand, A2AR activation promotes osteoblast expression of key markers for bone formation, such as osteoclacin and osteonectin (Supplemental Figs. S1 and S2), and an increase in osteoblasts in lesional bone in vivo. These findings correlate with our prior demonstration that A2AR stimulation suppresses inflammatory osteolysis and increases osteoblast numbers in inflamed bone in a model wear particle-induced osteolysis (18).
Moreover, we recently demonstrated that methotrexate, an agent that regulates inflammation by increasing extracellular adenosine concentrations (39), diminishes bone pitting and porosity caused by wear particles and suppresses RANKL expression by osteoblasts in addition to directly inhibiting osteoclast differentiation (23). This has been confirmed in vitro in both murine and human primary osteoblast precursors; activation of A2AR decreases expression of message for RANKL and increases OPG mRNA expression (23). The methotrexate-induced change in osteoblast and osteoclast number and function correlates directly with the changes observed here. Once a bone defect occurs, our results indicate that A2AR stimulation promotes bone regeneration by increasing osteoblast number and activity and decreasing osteoclast number and activity until the defect is filled, after which normal bone homeostasis is restored.
He et al. (21) reported that dipyridamole significantly increased osteoblast differentiation of multipotent stromal cells derived from patients with myeloma and healthy subjects by activation of the A2BR, as the effect of dipyridamole was abrogated by the A2BR antagonist, MRS1754, but not the A2AR antagonist, ZM241385 (21). Although it is possible that dipyridamole has different effects in vitro and in vivo, a more likely explanation is that A2AR stimulation does not promote osteoblast differentiation, as demonstrated for the A2BR, but increases the function of osteoblasts that have already differentiated. Alternatively, the standard assays for osteoblast differentiation do not reflect the effects of other factors present in vivo so that A2AR stimulation enhances osteoblast differentiation and function in response to stimuli present in vivo but absent in the in vitro studies.
The effect of agents that inhibit ent1, similar to dipyridamole, on extracellular adenosine levels may vary depending on the expression of ent1 and other purine receptors, and the factors that regulate expression of these transporters may include a variety of stimuli. Thus, it has been reported that chronic hypoxia in cardiomyocytes down-regulates ent1 activity, thus modulating release and/or uptake of adenosine (40). Moreover, hypoxia increases the half-life of extracellular adenosine in part by HIF-1–regulated ent1 repression in vascular endothelial and mucosal epithelial cells (41), whereas HIF-1α–dependent repression of ent2 increases mucosal adenosine signaling and attenuates hypoxia-associated inflammation of the intestine (42). Solid tumors express low levels of nucleoside transporters, which lead to an increase in adenosine signaling in the cancer environment (43), and decreased nucleoside transporter expression in tumor tissue may contribute to reduced drug uptake and the development of resistance (43). Moreover, ent4 has been identified as a highly expressed transcript in desmoplastic small round cell tumors (44). As dipyridamole blocks purine transport into cells via ent1, it is possible that hypoxic transcriptional regulation of adenosine transporters may modulate adenosine regulated bone formation. It has been reported that osteoblast function and bone formation are strongly oxygen dependent (45), and HIF regulates osteoclast-mediated bone resorption and enhances osteoclast differentiation (46) and limits the anabolic effect of parathyroid hormone (47). A2AR expression and function is also regulated by, most notably, inflammatory stimuli (48, 49).
Currently autologous and cadaveric bone or rhBMP-2 or rhBMP-7 are used to promote bone regeneration following orthopedic surgeries. Because autologous bone must be harvested from the patient, there is the potential for complications such as pain at the harvest site, infection, bruising, and pelvic fracture. Donated bone carries a risk of infection, and the quality of the bone cannot be controlled. The U.S. Food and Drug Administration (FDA) has approved use of recombinant BMPs for promotion of bone growth at sites of spinal fusion. Nonetheless, early clinical studies, including those reviewed by the FDA, showed that rhBMPs has the potential to inflame nearby tissues and bone, cause vertebral osteolysis, ectopic bone formation, radiculitis, cervical soft tissue swelling, and urinary problems, and even stimulate cancer growth (10, 11). Indeed, BMP products are associated with a 43% higher overall complication rate. Therefore, there are continued efforts to develop new approaches to enhance bone regeneration. The clinical interest in A2AR agonists as modulators of inflammation and immune responses has increased strongly, especially for pathologic conditions such as heart, liver, kidney, or chronic inflammatory conditions (50, 51). Unfortunately, systemic treatment with specific agonists is associated with a number of on-target side effects (52). The topical application of adenosine receptor agonists or agents that increase endogenous adenosine levels has the advantage that the effects of the agents are concentrated locally and that fewer systemic toxicities develop because little of the agent is released systemically. Moreover, dipyridamole has been used systemically (both orally and intravascularly in patients) for decades with little systemic toxicity; the occurrence of severe drug-related adverse effects, including cardiac death, sustained ventricular arrhythmia, cerebral ischemic attack, stroke, or severe bronchospasm, is <0.02% of patients taking systemic doses of the agent (53, 54). Nonetheless, daily administration of either CGS21680 or dipyridamole to a collagen sponge is not the most efficient approach to promote bone regeneration; thus, a better carrier capable of sustained release of the compounds will be essential to mirror BMP-2 effects in bone regeneration and, more importantly, to be useful in the clinic.
In conclusion, inhibition of osteoclast formation via A2AR stimulation or increasing local adenosine concentration stimulates new bone formation as well as rhBMP-2 and represents a novel approach to stimulating bone regeneration.
Supplementary Material
Acknowledgments
The authors thank the New York University School of Medicine Histology Core for help with immunostainings. This work was supported by U.S. National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR56672, AR54897, AR046121 and NIH National Heart, Lung, and Blood Institute Grant RC1-HL100815; New York University Health and Hospitals Corporation (NYU-HHC) Clinical and Translational Science Institute (Grant UL1TR000038); New York University Caregiver Intervention (NYUCI) Center support grant from NIH National Cancer Institute Grant 5-P30CA16087-310; and grants from Takeda, Celgene, AstraZeneca, and Gilead Pharmaceuticals. A.M. and B.N.C. have filed a patent on the use of dipyridamole-coated matrices to promote bone growth and have received a patent for the use of adenosine A2AR agonists to prevent prosthesis loosening (patent number8183225). A.M. and B.N.C. have filed a patent on the use of anti–netrin-1 antibodies for the treatment of bone disease (pending). B.N.C. holds patents 5,932,558; 6,020,321; 6,555,545; and 7,795,427—adenosine A1R and A2BR antagonists to treat fatty liver (pending). B.N.C. is a consultant for Bristol-Myers Squibb, Novartis, Eli Lilly & Co., CanFite Biopharmaceuticals, Cypress Laboratories, Regeneron (Westat, DSMB), Endocyte, Protalex, Allos, Inc., Savient, Gismo Therapeutics, Antares Pharmaceutical, Medivector, King Pharmaceutical, Celizome, Tap Pharmaceuticals, Prometheus Laboratories, Sepracor, Amgen, Combinatorx, Kyowa Hakka, Hoffman-LaRoche, and Avidimer Therapeutics and has stock in CanFite Biopharmaceuticals. M.P.-A. and T.W. declare no conflicts of interest.
Glossary
- 3D
3-dimensional
- A2AR
adenosine A2A receptor
- BMP-2
bone morphogenetic protein-2
- BSA
bovine serum albumin
- BV
bone volume
- hpf
high power field
- KO
knockout
- microCT
microcomputed tomography
- OP-1
osteogenic protein 1
- OPG
osteoprotegerin
- RANK
receptor activated by NF-κB
- RANKL
RANK ligand
- rhBMP
recombinant human BMP
- TRAP
tartrate resistance acid phosphatase
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Schindeler A., McDonald M. M., Bokko P., Little D. G. (2008) Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 19, 459–466 [DOI] [PubMed] [Google Scholar]
- 2.Tsiridis E., Upadhyay N., Giannoudis P. (2007) Molecular aspects of fracture healing: which are the important molecules? Injury 38(Suppl 1), S11–S25 [DOI] [PubMed] [Google Scholar]
- 3.Pape H. C., Marcucio R., Humphrey C., Colnot C., Knobe M., Harvey E. J. (2010) Trauma-induced inflammation and fracture healing. J. Orthop. Trauma 24, 522–525 [DOI] [PubMed] [Google Scholar]
- 4.Riley E. H., Lane J. M., Urist M. R., Lyons K. M., Lieberman J. R. (1996) Bone morphogenetic protein-2: biology and applications. Clin. Orthop. Relat. Res. (324):39–46 [PubMed] [Google Scholar]
- 5.Boden S. D., Kang J., Sandhu H., Heller J. G. (2002) Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine 27, 2662–2673 [DOI] [PubMed] [Google Scholar]
- 6.Onishi T., Ishidou Y., Nagamine T., Yone K., Imamura T., Kato M., Sampath T. K., ten Dijke P., Sakou T. (1998) Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone 22, 605–612 [DOI] [PubMed] [Google Scholar]
- 7.den Boer, F. C., Wippermann, B. W., Blokhuis, T. J., Patka, P., Bakker, F. C., and Haarman, H. J. (2003) Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-1 or autologous bone marrow. J. Orthopaedic Res.21, 521–528 [DOI] [PubMed]
- 8.Cook, S. D., Salkeld, S. L., Patron, L. P., Sargent, M. C., and Rueger, D. C. (2002) Healing course of primate ulna segmental defects treated with osteogenic protein-1. J. Invest. Surg.5, 69–79 [DOI] [PubMed]
- 9.White A. P., Vaccaro A. R., Hall J. A., Whang P. G., Friel B. C., McKee M. D. (2007) Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int. Orthop. 31, 735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glassman S. D., Howard J., Dimar J., Sweet A., Wilson G., Carreon L. (2011) Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine 36, 1849–1854 [DOI] [PubMed] [Google Scholar]
- 11.Carragee, E. J., Hurwitz, E. L., and Weiner, B. K. (2011) A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J.11, 471–491 [DOI] [PubMed]
- 12.Spiro, A. S., Beil, F. T., Baranowsky, A., Barvencik, F., Schilling, A. F., Nguyen, K., Khadem, S., Seitz, S., Rueger, J. M., Schinke, T., and Amling, M. (2010) BMP-7-induced ectopic bone formation and fracture healing is impaired by systemic NSAID application in C57BL/6-mice. J. Orthopaedic Res.28, 785–791 [DOI] [PubMed]
- 13.Fredholm B. B., IJzerman A. P., Jacobson K. A., Linden J., Müller C. E. (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol. Rev. 63, 1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He W., Cronstein B. N. (2012) Adenosine A1 receptor regulates osteoclast formation by altering TRAF6/TAK1 signaling. Purinergic Signal. 8, 327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kara, F. M., Chitu, V., Sloane, J., Axelrod, M., Fredholm, B. B., Stanley, E. R., and Cronstein, B. N. (2010) Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J.24, 2325–2333 [DOI] [PMC free article] [PubMed]
- 16.Kara F. M., Doty S. B., Boskey A., Goldring S., Zaidi M., Fredholm B. B., Cronstein B. N. (2010) Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice. Arthritis Rheum. 62, 534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mediero, A., Kara, F. M., Wilder, T., and Cronstein, B. N. (2012) Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am J Pathol.180, 775–786 [DOI] [PMC free article] [PubMed]
- 18.Mediero, A., Frenkel, S. R., Wilder, T., He, W., Mazumder, A., and Cronstein, B. N. (2012) Adenosine A2A receptor activation prevents wear particle-induced osteolysis. Sci. Transl. Med. 4, 135ra165. [DOI] [PMC free article] [PubMed]
- 19.Mediero A., Cronstein B. N. (2013) Adenosine and bone metabolism. Trends Endocrinol. Metab. 24, 290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa M. A., Barbosa A., Neto E., Sá-e-Sousa A., Freitas R., Neves J. M., Magalhães-Cardoso T., Ferreirinha F., Correia-de-Sá P. (2011) On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. J. Cell. Physiol. 226, 1353–1366 [DOI] [PubMed] [Google Scholar]
- 21.He, W., Mazumder, A., Wilder, T., and Cronstein, B. N. (2013) Adenosine regulates bone metabolism via A1, A2A, and A2B receptors in bone marrow cells from normal humans and patients with multiple myeloma. FASEB J.27, 3446–3454 [DOI] [PMC free article] [PubMed]
- 22.Gharibi, B., Abraham, A. A., Ham, J., and Evans, B. A. (2011) Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J. Bone Mineral Res.26, 2112–2124 [DOI] [PubMed]
- 23.Mediero, A., Perez-Aso, M., Wilder, T., and Cronstein, B. N. (2014) Methotrexate prevents wear particle-induced inflammatory osteolysis via activation of the adenosine A2A receptor [Epub ahead of print]. Arthritis Rheumatol. 10.1002/art.38971 [DOI] [PMC free article] [PubMed]
- 24.Cooper G. M., Mooney M. P., Gosain A. K., Campbell P. G., Losee J. E., Huard J. (2010) Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast. Reconstr. Surg. 125, 1685–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montesinos M. C., Desai A., Cronstein B. N. (2006) Suppression of inflammation by low-dose methotrexate is mediated by adenosine A2A receptor but not A3 receptor activation in thioglycollate-induced peritonitis. Arthritis Res. Ther. 8, R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lassailly F., Griessinger E., Bonnet D. (2010) “Microenvironmental contaminations” induced by fluorescent lipophilic dyes used for noninvasive in vitro and in vivo cell tracking. Blood 115, 5347–5354 [DOI] [PubMed] [Google Scholar]
- 27.Van Gaalen S. M., Kruyt M. C., Geuze R. E., de Bruijn J. D., Alblas J., Dhert W. J. (2010) Use of fluorochrome labels in in vivo bone tissue engineering research. Tissue Eng. Part B Rev. 16, 209–217 [DOI] [PubMed] [Google Scholar]
- 28.Harmatys K. M., Cole E. L., Smith B. D. (2013) In vivo imaging of bone using a deep-red fluorescent molecular probe bearing multiple iminodiacetate groups. Mol. Pharm. 10, 4263–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mediero A., Kara F. M., Wilder T., Cronstein B. N. (2012) Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am. J. Pathol. 180, 775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitz J. P., Hollinger J. O. (1986) The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. (205):299–308 [PubMed] [Google Scholar]
- 32.Carroll S. H., Wigner N. A., Kulkarni N., Johnston-Cox H., Gerstenfeld L. C., Ravid K. (2012) A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J. Biol. Chem. 287, 15718–15727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans, B. A., Elford, C., Pexa, A., Francis, K., Hughes, A. C., Deussen, A., and Ham, J. (2006) Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J. Bone Mineral Res.21, 228–236 [DOI] [PubMed]
- 34.Takedachi M., Oohara H., Smith B. J., Iyama M., Kobashi M., Maeda K., Long C. L., Humphrey M. B., Stoecker B. J., Toyosawa S., Thompson L. F., Murakami S. (2012) CD73-generated adenosine promotes osteoblast differentiation. J. Cell. Physiol. 227, 2622–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gharibi B., Abraham A. A., Ham J., Evans B. A. (2012) Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int J Obes (Lond) 36, 397–406 [DOI] [PubMed] [Google Scholar]
- 36.Katebi M., Soleimani M., Cronstein B. N. (2009) Adenosine A2A receptors play an active role in mouse bone marrow-derived mesenchymal stem cell development. J. Leukoc. Biol. 85, 438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Mattei M., Caruso A., Traina G. C., Pezzetti F., Baroni T., Sollazzo V. (1999) Correlation between pulsed electromagnetic fields exposure time and cell proliferation increase in human osteosarcoma cell lines and human normal osteoblast cells in vitro. Bioelectromagnetics 20, 177–182 [DOI] [PubMed] [Google Scholar]
- 38.Mediero A., Perez-Aso M., Cronstein B. N. (2013) Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFκB nuclear translocation. Br. J. Pharmacol. 169, 1372–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan E. S., Cronstein B. N. (2010) Methotrexate—how does it really work? Nat Rev Rheumatol 6, 175–178 [DOI] [PubMed] [Google Scholar]
- 40.Chaudary N., Naydenova Z., Shuralyova I., Coe I. R. (2004) Hypoxia regulates the adenosine transporter, mENT1, in the murine cardiomyocyte cell line, HL-1. Cardiovasc. Res. 61, 780–788 [DOI] [PubMed] [Google Scholar]
- 41.Eltzschig H. K., Abdulla P., Hoffman E., Hamilton K. E., Daniels D., Schönfeld C., Löffler M., Reyes G., Duszenko M., Karhausen J., Robinson A., Westerman K. A., Coe I. R., Colgan S. P. (2005) HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 202, 1493–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morote-Garcia J. C., Rosenberger P., Nivillac N. M., Coe I. R., Eltzschig H. K. (2009) Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136, 607–618 [DOI] [PubMed] [Google Scholar]
- 43.Pennycooke M., Chaudary N., Shuralyova I., Zhang Y., Coe I. R. (2001) Differential expression of human nucleoside transporters in normal and tumor tissue. Biochem. Biophys. Res. Commun. 280, 951–959 [DOI] [PubMed] [Google Scholar]
- 44.Li H., Smolen G. A., Beers L. F., Xia L., Gerald W., Wang J., Haber D. A., Lee S. B. (2008) Adenosine transporter ENT4 is a direct target of EWS/WT1 translocation product and is highly expressed in desmoplastic small round cell tumor. PLoS ONE 3, e2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utting J. C., Robins S. P., Brandao-Burch A., Orriss I. R., Behar J., Arnett T. R. (2006) Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp. Cell Res. 312, 1693–1702 [DOI] [PubMed] [Google Scholar]
- 46.Knowles, H. J., Cleton-Jansen, A. M., Korsching, E., and Athanasou, N. A. (2010) Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4. FASEB J.24, 4648–4659 [DOI] [PMC free article] [PubMed]
- 47.Frey, J. L., Stonko, D. P., Faugere, M.-C., and Riddle, R. C. (2014) Hypoxia-inducible factor-1α restricts the anabolic actions of parathyroid hormone. Bone Res.2, 14005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khoa N. D., Postow M., Danielsson J., Cronstein B. N. (2006) Tumor necrosis factor-alpha prevents desensitization of Galphas-coupled receptors by regulating GRK2 association with the plasma membrane. Mol. Pharmacol. 69, 1311–1319 [DOI] [PubMed] [Google Scholar]
- 49.Khoa N. D., Montesinos M. C., Reiss A. B., Delano D., Awadallah N., Cronstein B. N. (2001) Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J. Immunol. 167, 4026–4032 [DOI] [PubMed] [Google Scholar]
- 50.Yang Z., Day Y. J., Toufektsian M. C., Ramos S. I., Marshall M., Wang X. Q., French B. A., Linden J. (2005) Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation 111, 2190–2197 [DOI] [PubMed] [Google Scholar]
- 51.Yang Z., Day Y. J., Toufektsian M. C., Xu Y., Ramos S. I., Marshall M. A., French B. A., Linden J. (2006) Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 114, 2056–2064 [DOI] [PubMed] [Google Scholar]
- 52.Cerqueira, M. D. (2004) The future of pharmacologic stress: selective A2A adenosine receptor agonists. Am. J. Cardiol.94, 33D–40D [DOI] [PubMed] [Google Scholar]
- 53.Lam J. Y., Chaitman B. R., Glaenzer M., Byers S., Fite J., Shah Y., Goodgold H., Samuels L. (1988) Safety and diagnostic accuracy of dipyridamole-thallium imaging in the elderly. J. Am. Coll. Cardiol. 11, 585–589 [DOI] [PubMed] [Google Scholar]
- 54.Lette, J., Tatum, J. L., Fraser, S., Miller, D. D., Waters, D. D., Heller, G., Stanton, E. B., Bom, H. S., Leppo, J., and Nattel, S. (1995) Safety of dipyridamole testing in 73,806 patients: the Multicenter Dipyridamole Safety Study. J. Nuclear Cardiol.2, 3–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.