Abstract
Gut microbiota alterations have been described in several diseases with altered gastrointestinal (GI) motility, and awareness is increasing regarding the role of the gut microbiome in modulating GI function. Serotonin [5-hydroxytryptamine (5-HT)] is a key regulator of GI motility and secretion. To determine the relationship among gut microbes, colonic contractility, and host serotonergic gene expression, we evaluated mice that were germ-free (GF) or humanized (HM; ex-GF colonized with human gut microbiota). 5-HT reduced contractile duration in both GF and HM colons. Microbiota from HM and conventionally raised (CR) mice significantly increased colonic mRNAs Tph1 [(tryptophan hydroxylase) 1, rate limiting for mucosal 5-HT synthesis; P < 0.01] and chromogranin A (neuroendocrine secretion; P < 0.01), with no effect on monoamine oxidase A (serotonin catabolism), serotonin receptor 5-HT4, or mouse serotonin transporter. HM and CR mice also had increased colonic Tph1 protein (P < 0.05) and 5-HT concentrations (GF, 17 ± 3 ng/mg; HM, 25 ± 2 ng/mg; and CR, 35 ± 3 ng/mg; P < 0.05). Enterochromaffin (EC) cell numbers (cells producing 5-HT) were unchanged. Short-chain fatty acids (SCFAs) promoted TPH1 transcription in BON cells (human EC cell model). Thus, gut microbiota acting through SCFAs are important determinants of enteric 5-HT production and homeostasis.—Reigstad, C. S., Salmonson, C. E., Rainey, III, J. F., Szurszewski, J. H., Linden, D. R., Sonnenburg, J. L., Farrugia, G., Kashyap, P. C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells.
Keywords: 5-hydroxytryptamine, gnotobiotic, gut motility, host-microbe interaction, microbiome
Serotonin [5-hydroxytryptamine (5-HT)] is a neurotransmitter and hormone with diverse biologic functions, which include modulation of intestinal secretion and motility (1, 2). The vast majority of 5-HT (>90%) in the human body is produced by enterochromaffin (EC) cells of the gut, where it is synthesized by the rate-limiting enzyme tryptophan hydroxylase (Tph/TPH) 1, and stored in secretory granules prior to release (3). 5-HT is also produced by a second isoform, TPH2, in the myenteric plexus; however, this represents a much smaller fraction of total 5-HT (4, 5). EC cells function as luminal sensors, and increasing pressure in the colonic lumen leads to a change in the level of endogenous, luminal 5-HT (6). The role of EC cell-derived serotonin in modulating gastrointestinal (GI) motility has been well described (1). Although in the absence of EC cell-derived 5-HT, Tph1−/− mice do not exhibit significantly different whole-gut transit times (7) from wild-type mice, mucosal 5-HT synthesized by Tph1 is required for normal colonic morphology, pellet formation, and propulsive motility integrating stretch and mucosal reflexes (8). Exogenous, luminal 5-HT stimulates colonic transit in rats (6), and luminal administration of serotonin receptor 5-HT4 (Htr4) agonists has been shown to accelerate propulsive motility in guinea pigs (9).
EC cells are exposed to the vast diversity of microbial products in the human gut. Despite the increased awareness of the role of the gut microbiome in GI function such as GI motility, there are few data on interactions of gut microbiota with EC cells and the resultant functional consequences. Colonization of germ-free (GF) mice with normal human- or mouse-derived fecal microbiota significantly accelerates whole-gut transit, an effect that can be partially blocked by systemic pharmacologic antagonism of 5-HT3/4 receptors (10). Human gut microbial constituents do not appear to make 5-HT, suggesting that alterations in 5-HT signaling in response to bacteria are likely due to changes in host serotonergic expression (11).
Here, in an effort to move toward gnotobiotic models that may be more relevant to translational medicine, we evaluated mice that were GF or humanized (HM; ex-GF mice colonized with human fecal microbiota) to study effects of human-derived gut microbes on host serotonergic pathways and colonic contractility. We report that human- and mouse-derived complex microbial communities increased serotonin synthesis in the host gut, and we identified likely key mediators of the interaction of gut microbiota with the host serotonergic system.
MATERIALS AND METHODS
Animal models
All protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee. All animal experiments used 8- to 12-week-old Swiss-Webster mice. Schematics of experimental timelines and tissue preparation are shown in Supplemental Fig. 1. Molecular studies of host response to microbiota were confined to the proximal colon, which has one of the highest densities of microbes and is a prime site for bacterial fermentation. Midcolonic segments, where individual pellets begin to form, were employed in the assessment of intraluminal pressure ex vivo to ensure that sufficient colonic tissue was available for physiologic study from the same mice used to study host transcriptional responses. Conventionally raised (CR) mice, associated with normal mouse microbiota from birth, and GF breeders, used to produce the GF and HM mice in this study, were obtained from Taconic Farms (Germantown, NY, USA). HM mice were generated (colonized) at 4–6 weeks of age by gavage with 300 µl of a 1:1 suspension of prereduced PBS and fecal microbiota from a healthy human donor (a 49-yr-old man) or at age 8 weeks using material from a second donor (a 37-year-old man), as described previously (10). There were 2 independent human donors employed to ensure that identified responses in HM were not unique to a single human fecal microbiota; however, future studies focused on interindividual variation in effects mediated by human-derived gut microbiota in mice will require additional donor samples. All HM mice were colonized with human microbiota for 4–5 weeks before killing by CO2 asphyxiation. Selected mouse tissues then were immediately resected, frozen in liquid nitrogen, and stored at −80°C [for quantitative RT-PCR (qRT-PCR), Western blot, and ELISA], subjected to fixation (for immunohistochemistry), or immediately prepared for physiologic analysis (organ bath; Supplemental Fig. 1B).
Gnotobiotic mouse husbandry and screening
GF mice were maintained and bred in sterile isolators (Class Biologically Clean, Limited, Madison, WI, USA) until killing or humanization; HM mice remained in gnotobiotic isolators, in accordance with Biosafety Level 2 guidelines from the Centers for Disease Control and Prevention. GF and HM mice were fed an autoclaved standard diet (Purina LabDiet 5K67; St. Louis, MO, USA), and isolator sterility was confirmed with negative cultures (absence of growth) from swabs of GF isolators and GF fecal pellets in Sabouraud dextrose media, brain-heart infusion media, and nutrient broth media at 37°C for 7 days under aerobic and anaerobic conditions, as well as by conducting PCR assays using universal (12, 13) and genus-specific primers (14) to screen for bacterial DNA encoding 16S rRNAs (Supplemental Table 1).
Ex vivo measurement of intraluminal pressure in colonic segments
To measure intraluminal pressure, full-thickness, midcolonic segments (∼1.5 cm) were placed in a Krebs buffer-jacketed organ bath and tied with silk sutures to barbed tube fittings upstream of a calibrated transducer (model 6069; Utah Medical, Midvale, UT, USA) and bridge amplifier (Transbridge 4M; World Precision Instruments, Sarasota, FL, USA). Ex vivo segments were infused luminally with vehicle (lactated Ringer solution) consisting of 102 mM NaCl, 4 mM KCl, 1.5 mM CaCl2, 28 mM C3H5NaO3, and 0.05% DMSO (pH 6.8). After infusion, preparations were closed at the proximal end, and intraluminal pressure was measured distally. To provide basal pressure to the lumen, the preparation remained open (distal to the transducer) to a 5 cm column of vehicle approximately equal in diameter to that of the colonic lumen. The outer surface (serosa) was continuously bathed in 37°C oxygenated, normal Krebs solution: 137.4 mM Na+, 5.9 mM K+, 2.5 mM Ca2+, 1.2 mM Mg2+, 124 mM Cl−, 15.5 mM HCO3−, 1.2 mM H2PO4−, and 11.5 mM dextrose (pH 7.4). All preparations were allowed to equilibrate for at least 30 minutes before analysis, and all recordings were analyzed using LabScribe 2 software (iWorx Systems, Inc., Dover, NH, USA).
qRT-PCR
Total RNA was extracted from emptied colonic segments using a tissue homogenizer and the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) with on-column DNase I (Qiagen) treatment. Total RNA (500 ng) was used to synthesize random hexamer-primed cDNA template by reverse transcription with the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). qRT-PCR assays were performed in 25 µl reactions containing 1× LightCycler 480 SYBR Green I Master (Roche, Basel, Switzerland) and 900 nM gene-specific primers [300 nM primer concentrations were used to assess Rpl32 (60S ribosomal protein L32; L32) and ACTB (β-actin) transcripts]. A melting curve was performed with each primer pair to identify a temperature at which only the amplicon (and not primer dimers) accounted for SYBR Green-bound fluorescence. Each sample was assayed in analytic triplicate using a LightCycler 480 Instrument II (Roche), and mRNA expression was calculated after normalization to L32 mRNA (mouse colon) or ACTB mRNA (BON cells) expression using the ΔΔCT analysis method (15). Relative mRNA expression (fold difference) was based on the sample or group with the lowest detected expression after normalization. All primer sequences used in this study were published previously and are described in Supplemental Table 1: mouse L32 (16, 17), Tph1 (18), Chga (chromogranin A) (19), and Maoa (monoamine oxidase A) (20), as well as human ACTB (21) and TPH1 (22); primer sequences for mouse Slc6a4 [serotonin transporter; PrimerBank identification (ID) 7110639a1] and Htr4 (PrimerBank ID 6680325a1) were obtained from the PrimerBank website (23).
Western blot
Proximal colonic segments emptied of luminal contents were snap-frozen before homogenization in freshly prepared lysis buffer: 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, 1% NP-40, 0.25% Na-deoxycholate, and 1 mM cOmplete, Mini, EDTA-free Protease Inhibitor Cocktail Tablet (Roche) per 7 ml. Total protein concentration of each tissue lysate was determined using the Bio-Rad Protein Assay (Hercules, CA, USA). Protein extracts were loaded onto 12% Mini-PROTEAN TGX gels [40 µg/lane for Tph1 detection; 4 µg/lane for the housekeeping protein Gapdh (glyceraldehyde 3-phosphate dehydrogenase)] and subjected to electrophoresis. Size-separated proteins were blotted onto Immun-Blot PVDF membranes (Bio-Rad). After blocking for 1 hour in PBS with 0.1% Tween 20 (PBS-Tween) and supplemented with 5% fat-free milk powder, membranes were incubated overnight at 4°C with primary antibodies diluted in blocking buffer, e.g., α-Tph1 antibody (1:1000, #12339; Cell Signaling Technology, Danvers, MA, USA) or α-Gapdh (1:40,000, G9545; Sigma-Aldrich, St. Louis, MO, USA). Antibody-bound membranes were washed in PBS-Tween, incubated for 1 hour at room temperature with secondary antibodies [goat α-rabbit IgG, horseradish peroxidase linked; #7074 (Cell Signaling Technology)] diluted 1:2000 in blocking buffer, and then washed with PBS-Tween. Proteins were detected with the ECL Advance kit (GE Healthcare, Pittsburgh, PA, USA) and quantified by histogram analysis using Adobe Photoshop CS6 (San Jose, CA, USA). Protein abundance (fold difference) was determined relative to the sample or the group with the lowest detected expression after normalization to Gapdh.
Determination of 5-HT concentration by ELISA
Segments of emptied proximal colon were weighed and homogenized in appropriate volumes of stabilization buffer (0.05 N HCl, 0.1% ascorbic acid; 25 µl buffer/mg of tissue). Lysates were assessed in analytic duplicate using the Serotonin Research ELISA kit (Rocky Mountain Diagnostics, Colorado Springs, CO, USA) according to the manufacturer’s instructions. Tissue 5-HT concentrations were determined by using a standard curve.
Immunohistochemistry
Proximal colonic segments were resected 2–4 cm from the cecum (Supplemental Fig. 1) and fixed overnight at 4°C in 4% paraformaldehyde, 0.2% picric acid in PBS. Fixed tissue was transferred to 30% sucrose in PBS and equilibrated overnight at 4°C before embedding in optimum cutting temperature compound (Sakura Finetek, Tokyo, Japan). Cryosections (8 μm thick) were dehydrated for 5 minutes on a slide warmer and enclosed with Drierite dessicant (W.A. Hammond Drierite Company, Ltd., Xenia, OH, USA) at room temperature for 30 minutes before rehydration with PBS (3 cycles, 5 min each). Sections were then incubated with blocking buffer (5% normal goat serum, 0.05% Triton X-100 in PBS) for 1 h before incubation with rabbit anti-serotonin primary antibody (1:8000 in blocking buffer; ImmunoStar, Hudson, WI, USA). To visualize antigen-antibody complexes, sections were washed 3 times with PBS and incubated for 1 hour with goat anti-rabbit Cy3-conjugated IgG (1:1600 in blocking buffer; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Unbound antibody was removed by washing 3 times with PBS, and nuclei were labeled with DAPI (SlowFade Gold Antifade Reagent with DAPI; Life Technologies, Carlsbad, CA, USA) before sealing with a coverslip. All steps were performed at room temperature (20–22°C). Sections were examined and photographed using a ×40 objective on an Olympus BX51WI microscope (Olympus Corp., Tokyo, Japan).
EC cell culture model and treatments
BON cells (human EC cell model derived from pancreatic carcinoid) (24, 25) were cultured in T75 flasks at 37°C before seeding in 12-well culture plates at a concentration of 105 cells/ml. After reaching 90% confluency, cells were treated in triplicate (3 wells) for 24 hours with various concentrations of either short-chain fatty acids (SCFAs) Na-butyrate or Na-acetate (Sigma-Aldrich) or bacterial LPS from Escherichia coli K12 or E. coli O111:B4 (InvivoGen, San Diego, CA, USA). Cells were isolated in RNA Protect (Qiagen) and stored at −20°C before RNA extraction. qRT-PCR assays of human-derived BON cells used primers against TPH1 and ACTB (normalization transcript).
Statistics
All values are represented as the mean ± sem. Statistical significance was determined by GraphPad Prism (La Jolla, CA, USA) using Student’s t test or ANOVA with appropriate posttest for comparison of means. The statistical test used for each comparison is indicated in the RESULTS. P values <0.05 were taken as a statistically significant difference.
RESULTS
GF and HM mouse colonic segments have a similar contractile response to exogenous luminal 5-HT treatment ex vivo
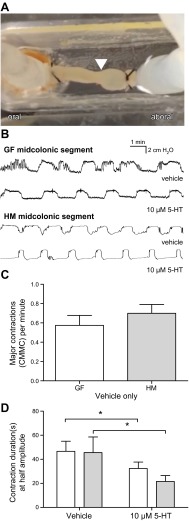
Colonization of GF mice with human-derived bacteria is associated with increased colonic contractility in vivo and increased concentrations of 5-hydroxyindoleacetic acid (a surrogate marker of serotonin) in stool (10). To determine whether luminal 5-HT had a differential effect on colonic contractility in HM and GF mice, we measured intraluminal pressure and quantified major colonic contractions [colonic migrating motor complexes (CMMCs)] by assessing the mean contractile duration of each CMMC (at half-maximal amplitude) over 10-minute intervals before and after luminal instillation of 5-HT (10 µM) in full-thickness, midcolonic segments ex vivo (Fig. 1A). Representative trace recordings are shown (Fig. 1B). We did not observe a significant difference in the basal contractile frequency of GF and HM colonic segments: 0.58 ± 0.10 versus 0.70 ± 0.09 CMMC per minute (4 mice per group); P > 0.05, Student’s t test (Fig. 1C). This result contrasts with a previous study (10) that reported greater colonic contractility in vivo in conscious HM mice compared to GF mice, likely reflecting differences in in vivo and ex vivo experimental design. Both GF and HM colonic segments had significantly reduced mean contractile duration after intracolonic administration of 10 µM 5-HT: GF, 47 ± 8 seconds in controls versus 32 ± 5 seconds with luminal 5-HT; HM, 46 ± 13 seconds in controls versus 22 ± 5 seconds with luminal 5-HT (4 mice per group); P < 0.05, 2-way ANOVA. The magnitude of response was not significantly different between the 2 groups (Fig. 1D).
Figure 1.
GF and HM colonic segments show similar contractile responses to exogenous, luminal 5-HT ex vivo. A) Intracolonic pressure was measured in midcolonic segments ex vivo (arrowhead indicates migrating contraction). B) Representative recordings from GF and HM segments, with and without addition of luminal 5-HT (10 μM). C) Basal contractile frequency of HM and GF colonic segments (4 mice per group). Student’s t test: P > 0.05. D) Mean contractile duration (seconds) measured at half-maximal amplitude over 10 minutes (4 mice per group). Two-way ANOVA: *P < 0.05.
These data suggest that the influence of gut microbes on colonic contractility, previously described to be through serotonergic pathways, may not be due to a reduced ability of the GF gut to respond to 5-HT. Thus, we focused next on alteration of gene expression leading to mucosal 5-HT biosynthesis.
HM mice have increased expression of colonic Tph1 mRNA and protein, without evidence of altered 5-HT catabolism or reuptake
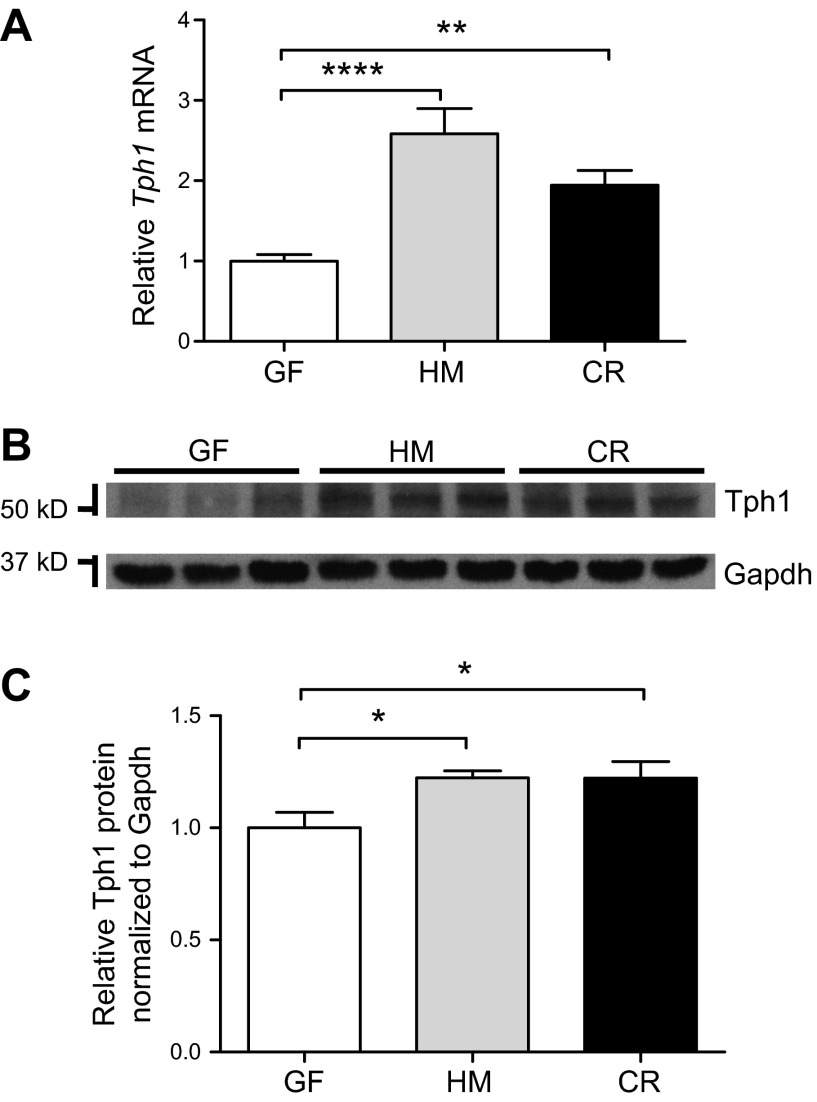
To investigate changes in Tph1 after colonization, we used qRT-PCR and Western blotting to compare Tph1 mRNA and Tph1 protein expression, respectively, in proximal colonic segments. We found that relative Tph1 mRNA expression was significantly higher in the proximal colon in HM mice compared with GF mice. To determine whether this was specific to human gut-adapted microbiota, we also assessed changes in CR mice and found a similar increase in Tph1 mRNA (Fig. 2A): GF, 1.00 ± 0.08; HM, 2.65 ± 0.31; and CR, 1.94 ± 0.18 (14–16 mice per group); P < 0.01. To further determine whether the observed results were sex dependent, we assessed GF, HM, and CR mice and found that sex had no effect on Tph1 expression in the proximal colon (2-way ANOVA; Supplemental Fig. 2A), suggesting that increased 5-HT synthesis after colonization with human- or mouse-derived gut bacteria was independent of sex. We also showed that this effect in HM mice was not unique to the donor microbiota from a single human; analysis of another set of GF and HM mice from a second, healthy donor also revealed significantly higher (∼2-fold) colonic Tph1 mRNA in the proximal colon of HM mice compared with GF mice (Supplemental Fig. 3A; 4–5 mice per group; P < 0.05).
Figure 2.
Tph1 mRNA and protein expression are increased in the proximal colon of HM and CR mice compared with GF mice, without alteration of 5-HT catabolic (Maoa) and transporter (Slc6a4) mRNAs. A) Relative expression of Tph1 mRNA was determined in the proximal colon by qRT-PCR (14–16 mice per group). One-way ANOVA: **P < 0.01; ****P < 0.0001. B) Western blot detection of Tph1 and Gapdh from proximal colonic lysates. C) Quantification of relative Tph1 protein in the proximal colon of GF, HM, and CR mice (9 mice per group). One-way ANOVA: *P < 0.05.
Relative Tph1 protein abundance in proximal colonic tissue lysates was detected by Western blot and assessed after normalization to Gapdh expression (Fig. 2B, C). As with Tph1 mRNA, relative Tph1 protein expression was also significantly higher in HM and CR mice compared with GF mice (fold difference relative to GF: HM, 1.2 ± 0.03; CR, 1.2 ± 0.07; 9 mice per group; P < 0.05). Therefore, the absence of microbiota was associated with decreased colonic Tph1 expression, suggesting that human and mouse gut microbiota can affect the 5-HT biosynthetic pathway. We next examined the effect of human and mouse gut microbiota on 5-HT catabolism and reuptake in GF, HM, and CR mice.
The main enzyme responsible for catabolic degradation of 5-HT in colonic tissues is Maoa, which is encoded by Maoa mRNA and compartmentalized in the mitochondria of numerous cell types. Using qRT-PCR, we saw no significant difference in colonic Maoa mRNA expression among GF, HM, or CR mice (14–16 mice per group; Supplemental Fig. 4A), and similar results were seen in both male and female mice (Supplemental Fig. 4B). Next, as a proxy for measuring differential reuptake of 5-HT in the proximal colon, we examined expression of Slc6a4 mRNA but observed no significant differences among GF, HM, and CR groups (14–16 mice per group; Supplemental Fig. 4C). Interestingly, female CR mice had significantly lower expression compared with male CR mice (P < 0.05, 2-way ANOVA with Bonferroni multiple comparison tests; Supplemental Fig. 4D), but a similar significant effect of sex was not seen in HM mice, reflecting a differential effect of gut microbiota, colonization duration, or both on expression of serotonin transporter. Similar to Tph1 expression, neither Maoa nor Slc6a4 expression significantly differed between GF and HM mice generated from the second donor (4–5 mice per group; Supplemental Fig. 3B, C). Given that Htr4s are widely expressed in the colonic epithelium, where they likely modulate gut motility (9), we measured expression of Htr4 in the proximal colon of GF, HM, and CR mice; however, we did not observe significant differences among the groups (Supplemental Fig. 4E), and similar results were seen in both male and female mice (Supplemental Fig. 4F).
Tissue 5-HT content and Chga mRNA expression in the colon are significantly lower in GF than in HM mice
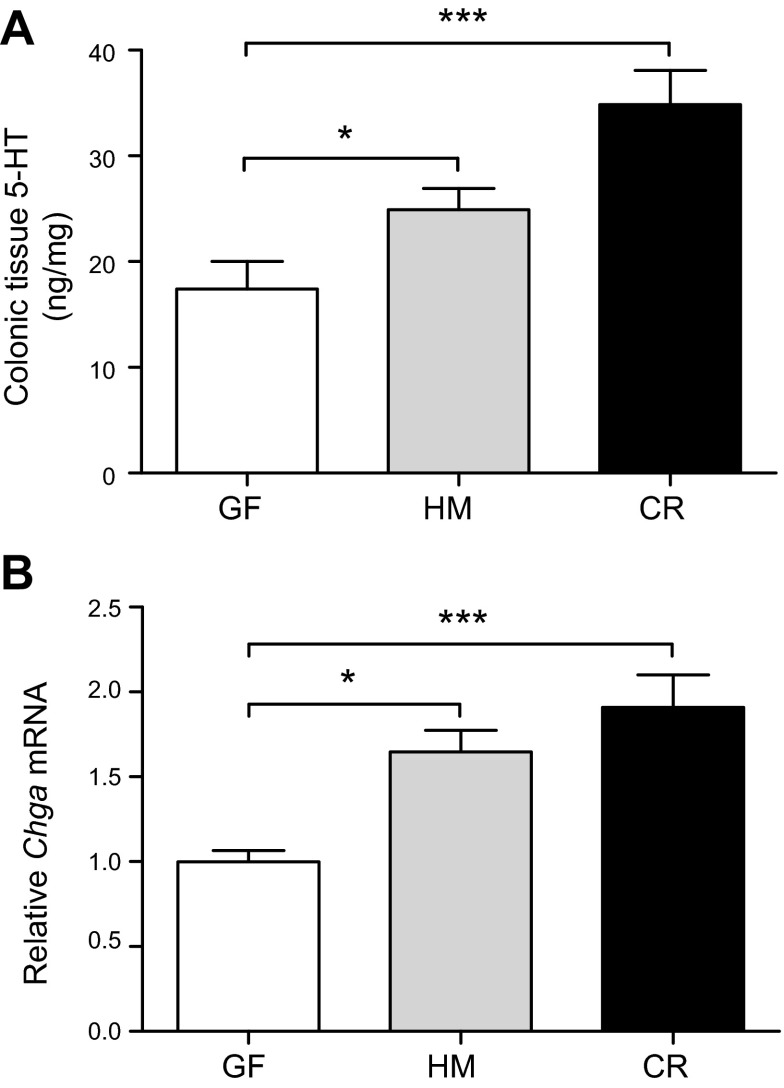
To test whether increased expression of Tph1 is associated with increased tissue 5-HT abundance, luminal contents were removed from the proximal colons of GF and HM mice, and ascorbic acid-stabilized tissue lysates were prepared and assessed with ELISA. 5-HT was detected in HM and CR lysates (24.9 ± 2.0 and 34.8 ± 3.2 ng/mg of tissue, respectively), which was significantly higher than that measured in the GF colon: 17.4 ± 2.6 ng/mg; 8 mice per group (4 males); P < 0.05 (Fig. 3A). This effect was independent of sex (Supplemental Fig. 5), though there was a trend toward significant increase in female CR mice but not female HM mice compared with male mice.
Figure 3.
Presence of human- or mouse-derived microbiota increases tissue concentration of 5-HT and Chga mRNA in the mouse proximal colon. A) 5-HT concentration was assessed in tissue lysates from the proximal colon by ELISA [8 mice per group (4 males)]. One-way ANOVA: *P < 0.05; ***P < 0.001. B) Relative expression of Chga mRNA (Chga, a neuroendocrine secretory marker) was determined in the proximal colon by qRT-PCR (14–16 mice per group). One-way ANOVA: *P < 0.05; ***P < 0.001.
To test whether a transcriptional biomarker involved in the formation and release of 5-HT-containing secretory granules was differentially expressed in the proximal colon of GF, HM, and CR mice, we used qRT-PCR to quantify Chga mRNA, which encodes Chga, a neuroendocrine secretory protein that is released with 5-HT. The relative expression of Chga mRNA was similar to that observed for total tissue 5-HT content; relative expression of Chga mRNA in the proximal colon was significantly higher in CR and HM mice compared with GF mice (fold differences: GF, 1.00; HM, 1.65 ± 0.13; CR, 1.91 ± 0.19; 14–16 mice per group; P < 0.01; Fig. 3B) independent of sex (Supplemental Fig. 2B), suggesting that gut microbiota increase colonic 5-HT production and possibly secretion by EC cells. Chga mRNA assays in HM mice colonized by the second donor showed similar results as Tph1 (Supplemental Fig. 3D).
EC cell density in the proximal colon is not different among GF, HM, and CR mice
Increased colonic Tph1 and 5-HT release in response to intestinal microbiota could potentially result from the increased abundance of EC cells in the colonic epithelium of colonized mice. We therefore enumerated 5-HT+ cells per high-power field (HPF; ×40 objective) by immunohistochemically staining fixed frozen sections of proximal colon from GF, HM, and CR mice. Rodent mast cells also produce 5-HT; however, compared with EC cells, mast cells are scarce in the colonic mucosa of normal mice [∼0.05 versus ∼1 per crypt section for mast and EC cells, respectively (26)].
We observed no significant difference in the abundance of proximal colonic EC cells per HPF among GF, HM, and CR mice. GF proximal colon mucosa had 4.8 ± 0.3 EC cells per HPF, whereas HM and CR had 5.0 ± 0.6 and 4.9 ± 0.2 cells per HPF, respectively [6–7 mice per group; 7 sections and 21 HPFs per mouse (i.e., 3 HPFs per section); Supplemental Fig. 6]. These results suggest that alteration of mucosal Tph1 expression and tissue 5-HT concentration were not due to an increased abundance of EC cells in microbe-associated mice; rather, they appear to be attributable to increased transcription of Tph1 stimulated by the gut microbiota. We also assessed differences in intestinal morphology after colonization by human-derived microbes. We noted significant reductions in small intestine length in HM and CR mice [53.47 ± 1.12 (GF) versus 45.28 ± 1.70 (HM) and 47.02 ± 0.76 (CR) cm; 14–21 mice per group] and cecal weight (2.04 ± 0.12 vs. 0.76 ± 0.04 and 0.69 ± 0.02 g; 14–21 mice per group), after colonization by either human- or mouse-derived microbiota, but we did not observe significant differences in overall colonic length (8.44 ± 0.24 versus 8.07 ± 0.17 and 8.66 ± 0.18 cm; 14–21 mice per group) (Supplemental Fig. 7).
SCFAs, but not LPSs, induce TPH1 expression in a model of human EC cells
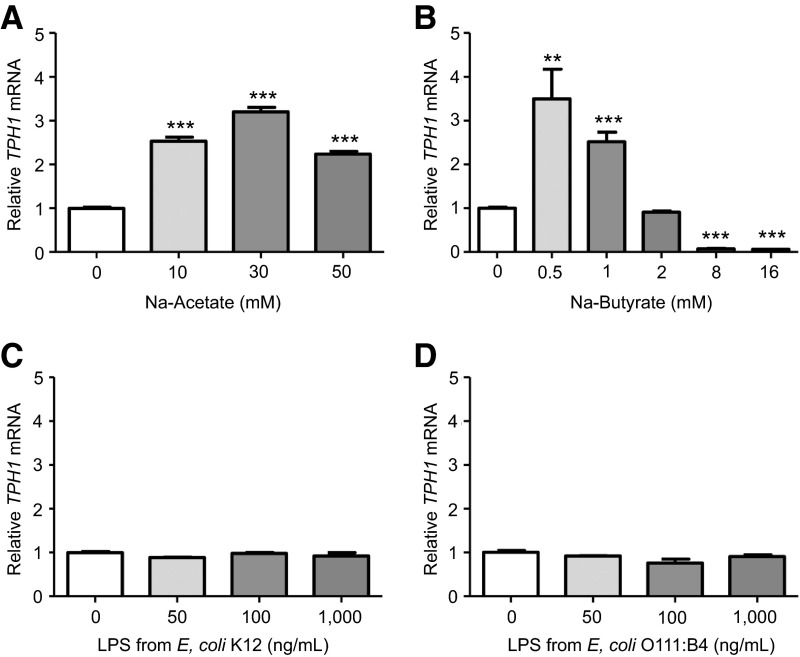
To assess which gut microbial products increase TPH1 expression, we treated a human-derived EC cell model (BON cells) with microbiota-derived SCFAs (acetate and butyrate) and bacterial LPSs. SCFAs are generated chiefly by gut microbes capable of fermenting dietary saccharides, whereas LPSs are a major component of the outer membrane of gram-negative bacteria. We hypothesized that the microbiota-induced 5-HT production in the colon is caused by luminal SCFAs and LPS (from gut microbes) stimulating EC cells to increase transcription of TPH1 mRNA.
Sodium acetate (10–50 mM) significantly increased TPH1 mRNA expression (>2-fold, with a maximal increase of 3.2-fold observed with the 30 mM treatment; P < 0.001; Fig. 4A). Treatment with 0.5 and 1 mM sodium butyrate significantly increased TPH1 mRNA expression (3.5- and 2.5-fold, respectively; P < 0.01), whereas 2 mM treatment did not significantly alter TPH1 expression, and 8 and 16 mM treatments significantly suppressed TPH1 transcription to levels below untreated controls (13.5- and 15.7-fold, respectively; Fig. 4B).
Figure 4.
SCFAs, but not LPS, modulate TPH1 expression in a human model of EC cells. Relative expression is shown of TPH1 mRNA in BON cells treated with different concentrations of SCFAs (A) acetate and (B) butyrate or LPSs from (C) commensal K12 E. coli and (D) pathogenic O111:B4 E. coli (3 replicate treatments per group). One-way ANOVA, Dunnett multiple comparison test: **P < 0.01; ***P < 0.001.
Despite reported expression of TLR 4 protein (host receptor for LPS) in BON cells (27), no significant differences in TPH1 expression were observed after 24-hour treatments with various concentrations of LPS from either commensal E. coli K12 (Fig. 4C) or pathogenic E. coli O111:B4 (Fig. 4D).
DISCUSSION
This study shows that human- and mouse-derived gut microbiota promote colonic Tph1 expression and 5-HT production through stimulatory activities of SCFAs on EC cells. Several observations support this conclusion. First, response to exogenous, luminal 5-HT was similar between GF and HM groups, suggesting that previously reported differences in whole-gut transit times and colonic contractility (10) are not due to a primary deficit in the ability of the GF neuromuscular apparatus to sense and respond to 5-HT. Second, mRNA transcript levels for Tph1 (the rate-limiting enzyme for serotonin biosynthesis in EC cells) and ChgA (a biomarker of neuroendocrine secretion) were significantly increased in the proximal colon of HM and CR mice compared with GF mice, without altering EC cell density among the 3 groups. Third, colonic Tph1 protein and tissue 5-HT concentration increased with colonization by gut microbiota. Finally, in an in vitro human model of EC cells, SCFAs butyrate and acetate, which are produced in abundance by distal gut microbes in vivo, significantly affected TPH1 expression in a concentration-dependent manner.
Dysfunction of serotonergic systems (28) and dysbiosis of the gut microbiota (29) have been implicated in subsets of irritable bowel syndrome (IBS), depression, and other systemic disorders. The effects of gut microbes on mucosal, neuronal, and systemic 5-HT homeostasis are important for various pathophysiologic conditions in humans. For example, gut microbes can produce compounds (e.g., SCFAs) that potentially affect serotonergic regulation (11, 30, 31), and pathogenic strains of E. coli can inhibit intestinal serotonin transporter function and expression (32). In a recent study, a subset of patients with IBS was shown to harbor gut microbiomes characterized by overrepresentation of Firmicutes-associated taxa and depletion of taxa within phylum Bacteroidetes (33); this study also identified provocative associations between gut microbial taxa and clinical characteristics of IBS, e.g., colonic transit time. The gut serotonergic system has an important role in modulating peripheral mechanisms implicated in IBS. Tph1 is important for normal 5-HT production by gut mucosal EC cells; however, optimal serotonergic signaling also requires coordination of 5-HT secretion, the actions of a large family of 5-HT receptors, and a high-affinity serotonin transporter (SERT in humans, Slc6a4 in mice). SERT polymorphisms have been associated with subsets of IBS in humans (34), and Slc6a4 is required for normal intestinal motility in mice (35). Although several 5-HT receptors are involved in gut motility, Htr4 is among the most likely to be influenced by luminal microbes, in part because of its expression in enterocytes and function in modulating gut motility (9). Interestingly, both Slc6a4 and Htr4 are expressed by intestinal epithelial cells and other cell types (9, 36). However, few data link gut microbes with the gut serotonergic system. In our study, we identified this link and determined whether differences in GI function in the absence of gut microbes were partly due to altered tissue responsiveness to neurotransmitters such as 5-HT or to the relative lack of these neurotransmitters.
To assess differences in the responsiveness of host tissue in the absence of microbes, we used an ex vivo system to study the effects of luminally introduced 5-HT. Serotonin administration did not affect the contractile response in the proximal colon of GF or HM mice, suggesting that the colonic neuromuscular apparatus is capable of responding to neurotransmitter stimuli and that the altered physiology in the GF state may be due to a relative lack of these stimuli. This is consistent with a recent study (37) that reported normal colonic secretomotor responses in GF mice. However, in contrast to previous reports of the intact colon (10), we did not observe a difference in basal colonic contractile frequency in HM and GF mice ex vivo, perhaps suggesting a role for central innervation in gut microbiota-mediated alterations in GI motility, although this possibility needs further rigorous investigation.
Next, we focused on the effects of gut microbiota on the host serotonergic system in the colon. Although the small intestine is also a major source of serotonin (38), we focused our studies on the colon given the higher densities of microbes in this region. Female GF mice have significantly lower colonic expression of Tph1 mRNA compared with CR C57Bl6/J mice (39); however, the specific impact of human-relevant gut microbiota on regulation of host 5-HT synthesis, catabolism, reuptake, and receptor binding, and whether this regulation was sex dependent, was largely unknown. We showed that microbiota from 2 human donors increased serotonin biosynthesis in the gut, with no significant impact on reuptake or catabolism. Moreover, this effect was independent of sex. The effect appeared to be conserved among different gut-adapted microbial communities, given that a similar effect was seen in CR mice harboring mouse-adapted gut microbiota.
To identify potential mechanisms through which microbial products such as SCFAs could augment Tph1 and 5-HT synthesis, we used BON cells, a human EC cell model. BON cells (40) are a human carcinoid cell line that produces and secretes 5-HT (41) and expresses TPH1; they are the most widely studied EC cell model, despite their pancreatic origin (25). Cecal concentrations of SCFAs in GF mice have been reported to be ∼1 mmol/kg, whereas average concentrations in CR mouse ceca are ∼125 mmol/kg; thus, nearly all SCFAs in the distal gut are produced through bacterial fermentation (42). Human fecal SCFA concentrations can vary widely among individuals (e.g., butyrate concentration ranges from 3.5 to 32.6 mmol/kg) and are usually increased by diets high in resistant starch (43). Although EC cells appear to lack SCFA receptors GPR41 (44) and GPR43 (45, 46), butyrate can augment Tph1 transcription in mice through an inducible zinc finger transcription factor ZBP-89, which functions in the secretion of antimicrobial peptides (47). Furthermore, we showed that elevated concentrations of butyrate (8 and 16 mM) significantly suppressed TPH1 expression in BON cells; in fact, butyrate concentrations from 0.5 to 16 mM modulated a ≥50-fold range of relative TPH1 in vitro. These data suggest that investigating the effects of local concentrations and proportions of different colonic SCFAs in vivo may be beneficial because they could be important determinants of mucosal 5-HT homeostasis in humans.
In this study, we showed a mechanistic link between complex microbial communities and host gene expression affecting host function. Although significant work is still necessary to elucidate the downstream pathways affecting host function and the environmental factors affecting this interaction, our study represents an important first step. As mechanistic aspects of the human microbiome and its effects on the host are further clarified, we will continue to build a deeper understanding of the relationship between microbial symbionts and human physiology. This process will enable elucidation of microbe-dependent mechanisms that alter host physiology and facilitate the development of novel, microbiome-targeted therapeutic approaches to improve human health.
Supplementary Material
Acknowledgments
The authors thank Dr. Courtney M. Townsend, Jr., for the generous gift of the BON cell line, Kristy Zodrow for administrative assistance, and Mr. Peter R. Strege for preparing the figures. This work was made possible by funding from U.S. National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grants K08 DK100638 and P30DK84567 (to P.C.K.), and R01 DK085025 (to J.L.S.), as well as funding from the Global Probiotic Council (to P.C.K.), Minnesota Partnership for Biotechnology and Genomics (to P.C.K.), and the Center for Individualized Medicine, Mayo Clinic (to P.C.K.). C.S.R. and P.C.K. designed the research study. C.S.R., J.F.R., and C.E.S. performed the research. C.S.R., D.R.L., J.H.S., J.L.S., G.F., and P.C.K. analyzed the data and contributed to interpretation of data. C.S.R. and P.C.K. wrote the manuscript. Each author has read the final manuscript and approved it. The authors declare no conflicts of interest.
Glossary
- 5-HT
serotonin, 5-hydroxytryptamine
- ACTB
β-actin
- Chga
chromogranin A
- CMMC
colonic migrating motor complex
- CR
conventionally raised
- EC
enterochromaffin
- Gapdh
glyceraldehyde 3-phosphate dehydrogenase
- GF
germ-free
- GI
gastrointestinal
- HM
humanized
- HPF
high-power field
- Htr4
serotonin receptor 5-HT4
- IBS
irritable bowel syndrome
- ID
identification
- L32
60S ribosomal protein L32
- Maoa
monoamine oxidase A
- PBS-Tween
PBS with 0.1% Tween 20
- qRT-PCR
quantitative RT-PCR
- SCFA
short-chain fatty acid
- SERT
serotonin transporter (human)
- Slc6a4
serotonin transporter (mouse)
- Tph/TPH
tryptophan hydroxylase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Gershon M. D., Tack J. (2007) The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 [DOI] [PubMed] [Google Scholar]
- 2.Mawe G. M., Hoffman J. M. (2013) Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershon M. D. (2013) 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 20, 14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Côté F., Thévenot E., Fligny C., Fromes Y., Darmon M., Ripoche M. A., Bayard E., Hanoun N., Saurini F., Lechat P., Dandolo L., Hamon M., Mallet J., Vodjdani G. (2003) Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl. Acad. Sci. USA 100, 13525–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walther D. J., Peter J. U., Bashammakh S., Hörtnagl H., Voits M., Fink H., Bader M. (2003) Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76. [DOI] [PubMed] [Google Scholar]
- 6.Tsukamoto K., Ariga H., Mantyh C., Pappas T. N., Yanagi H., Yamamura T., Takahashi T. (2007) Luminally released serotonin stimulates colonic motility and accelerates colonic transit in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R64–R69 [DOI] [PubMed] [Google Scholar]
- 7.Li Z., Chalazonitis A., Huang Y. Y., Mann J. J., Margolis K. G., Yang Q. M., Kim D. O., Côté F., Mallet J., Gershon M. D. (2011) Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci. 31, 8998–9009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heredia D. J., Gershon M. D., Koh S. D., Corrigan R. D., Okamoto T., Smith T. K. (2013) Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J. Physiol. 591, 5939–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman J. M., Tyler K., MacEachern S. J., Balemba O. B., Johnson A. C., Brooks E. M., Zhao H., Swain G. M., Moses P. L., Galligan J. J., Sharkey K. A., Greenwood-Van Meerveld B., Mawe G. M. (2012) Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142, 844–854.e844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashyap P. C., Marcobal A., Ursell L. K., Larauche M., Duboc H., Earle K. A., Sonnenburg E. D., Ferreyra J. A., Higginbottom S. K., Million M., Tache Y., Pasricha P. J., Knight R., Farrugia G., Sonnenburg J. L. (2013) Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 144, 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wikoff W. R., Anfora A. T., Liu J., Schultz P. G., Lesley S. A., Peters E. C., Siuzdak G. (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 106, 3698–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley R. E., Harris J. K., Wilcox J., Spear J. R., Miller S. R., Bebout B. M., Maresca J. A., Bryant D. A., Sogin M. L., Pace N. R. (2006) Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat. Appl. Environ. Microbiol. 72, 3685–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stackebrandt E., Goodfellow M. (1991) Nucleic Acid Techniques in Bacterial Systematics, Wiley, Chichester [Google Scholar]
- 14.Taylor N. S., Xu S., Nambiar P., Dewhirst F. E., Fox J. G. (2007) Enterohepatic Helicobacter species are prevalent in mice from commercial and academic institutions in Asia, Europe, and North America. J. Clin. Microbiol. 45, 2166–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 16.Sonnenburg J. L., Chen C. T., Gordon J. I. (2006) Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 4, e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stappenbeck T. S., Hooper L. V., Manchester J. K., Wong M. H., Gordon J. I. (2002) Laser capture microdissection of mouse intestine: characterizing mRNA and protein expression, and profiling intermediary metabolism in specified cell populations. Methods Enzymol. 356, 167–196 [DOI] [PubMed] [Google Scholar]
- 18.Liu C., Maejima T., Wyler S. C., Casadesus G., Herlitze S., Deneris E. S. (2010) Pet-1 is required across different stages of life to regulate serotonergic function. Nat. Neurosci. 13, 1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y., Ippolito J. E., Garabedian E. M., Humphrey P. A., Gordon J. I. (2002) Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J. Biol. Chem. 277, 44462–44474 [DOI] [PubMed] [Google Scholar]
- 20.Lee A. K., Mojtahed-Jaberi M., Kyriakou T., Astarloa E. A., Arno M., Marshall N. J., Brain S. D., O’Dell S. D. (2010) Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition 26, 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gahete M. D., Córdoba-Chacón J., Hergueta-Redondo M., Martínez-Fuentes A. J., Kineman R. D., Moreno-Bueno G., Luque R. M., Castaño J. P. (2011) A novel human ghrelin variant (In1-ghrelin) and ghrelin-O-acyltransferase are overexpressed in breast cancer: potential pathophysiological relevance. PLoS One 6, e23302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minderhoud I. M., Oldenburg B., Schipper M. E., ter Linde J. J., Samsom M. (2007) Serotonin synthesis and uptake in symptomatic patients with Crohn’s disease in remission. Clin. Gastroenterol. Hepatol. 5, 714–720 [DOI] [PubMed] [Google Scholar]
- 23.Spandidos A., Wang X., Wang H., Seed B. (2010) PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 38, D792–D799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parekh D., Ishizuka J., Townsend C. M. Jr, Haber B., Beauchamp R. D., Karp G., Kim S. W., Rajaraman S., Greeley G. Jr., Thompson J. C. (1994) Characterization of a human pancreatic carcinoid in vitro: morphology, amine and peptide storage, and secretion. Pancreas 9, 83–90 [DOI] [PubMed] [Google Scholar]
- 25.Siddique Z. L., Drozdov I., Floch J., Gustafsson B. I., Stunes K., Pfragner R., Kidd M., Modlin I. M. (2009) KRJ-I and BON cell lines: defining an appropriate enterochromaffin cell neuroendocrine tumor model. Neuroendocrinology 89, 458–470 [DOI] [PubMed] [Google Scholar]
- 26.Reichardt F., Baudry C., Gruber L., Mazzuoli G., Moriez R., Scherling C., Kollmann P., Daniel H., Kisling S., Haller D., Neunlist M., Schemann M. (2013) Properties of myenteric neurones and mucosal functions in the distal colon of diet-induced obese mice. J. Physiol. 591, 5125–5139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogunovic M., Davé S. H., Tilstra J. S., Chang D. T., Harpaz N., Xiong H., Mayer L. F., Plevy S. E. (2007) Enteroendocrine cells express functional Toll-like receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1770–G1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowell M. D. (2004) Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br. J. Pharmacol. 141, 1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohman L., Simrén M. (2013) Intestinal microbiota and its role in irritable bowel syndrome (IBS). Curr. Gastroenterol. Rep. 15, 323. [DOI] [PubMed] [Google Scholar]
- 30.Barcenilla A., Pryde S. E., Martin J. C., Duncan S. H., Stewart C. S., Henderson C., Flint H. J. (2000) Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66, 1654–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett E., Ross R. P., O’Toole P. W., Fitzgerald G. F., Stanton C. (2012) γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417 [DOI] [PubMed] [Google Scholar]
- 32.Esmaili A., Nazir S. F., Borthakur A., Yu D., Turner J. R., Saksena S., Singla A., Hecht G. A., Alrefai W. A., Gill R. K. (2009) Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 137, 2074–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeffery I. B., O’Toole P. W., Öhman L., Claesson M. J., Deane J., Quigley E. M., Simrén M. (2012) An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61, 997–1006 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z. F., Duan Z. J., Wang L. X., Yang D., Zhao G., Zhang L. (2014) The serotonin transporter gene polymorphism (5-HTTLPR) and irritable bowel syndrome: a meta-analysis of 25 studies. BMC Gastroenterol. 14, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J. J., Li Z., Pan H., Murphy D. L., Tamir H., Koepsell H., Gershon M. D. (2001) Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J. Neurosci. 21, 6348–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres G. E., Gainetdinov R. R., Caron M. G. (2003) Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci. 4, 13–25 [DOI] [PubMed] [Google Scholar]
- 37.Lomasney K. W., Houston A., Shanahan F., Dinan T. G., Cryan J. F., Hyland N. P. (2014) Selective influence of host microbiota on cAMP-mediated ion transport in mouse colon. Neurogastroenterol. Motil. 26, 887–890 [DOI] [PubMed] [Google Scholar]
- 38.Ebert-Zavos E., Horvat-Gordon M., Taylor A., Bartell P. A. (2013) Biological clocks in the duodenum and the diurnal regulation of duodenal and plasma serotonin. PLoS One 8, e58477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjögren K., Engdahl C., Henning P., Lerner U. H., Tremaroli V., Lagerquist M. K., Bäckhed F., Ohlsson C. (2012) The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 27, 1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evers B. M., Townsend C. M. Jr, Upp J. R., Allen E., Hurlbut S. C., Kim S. W., Rajaraman S., Singh P., Reubi J. C., Thompson J. C. (1991) Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology 101, 303–311 [DOI] [PubMed] [Google Scholar]
- 41.Tran V. S., Marion-Audibert A. M., Karatekin E., Huet S., Cribier S., Guillaumie K., Chapuis C., Desnos C., Darchen F., Henry J. P. (2004) Serotonin secretion by human carcinoid BON cells. Ann. N. Y. Acad. Sci. 1014, 179–188 [DOI] [PubMed] [Google Scholar]
- 42.Høverstad T., Midtvedt T. (1986) Short-chain fatty acids in germfree mice and rats. J. Nutr. 116, 1772–1776 [DOI] [PubMed] [Google Scholar]
- 43.McOrist A. L., Miller R. B., Bird A. R., Keogh J. B., Noakes M., Topping D. L., Conlon M. A. (2011) Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J. Nutr. 141, 883–889 [DOI] [PubMed] [Google Scholar]
- 44.Tazoe H., Otomo Y., Karaki S., Kato I., Fukami Y., Terasaki M., Kuwahara A. (2009) Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 30, 149–156 [DOI] [PubMed] [Google Scholar]
- 45.Karaki S., Mitsui R., Hayashi H., Kato I., Sugiya H., Iwanaga T., Furness J. B., Kuwahara A. (2006) Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 324, 353–360 [DOI] [PubMed] [Google Scholar]
- 46.Tazoe H., Otomo Y., Kaji I., Tanaka R., Karaki S. I., Kuwahara A. (2008) Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 59(Suppl 2), 251–262 [PubMed] [Google Scholar]
- 47.Essien B. E., Grasberger H., Romain R. D., Law D. J., Veniaminova N. A., Saqui-Salces M., El-Zaatari M., Tessier A., Hayes M. M., Yang A. C., Merchant J. L. (2013) ZBP-89 regulates expression of tryptophan hydroxylase I and mucosal defense against Salmonella typhimurium in mice. Gastroenterology 144, 1466–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.