Abstract
SETD1A is a member of trithorax-related histone methyltransferases that methylate lysine 4 at histone H3 (H3K4). We showed previously that Setd1a is required for mesoderm specification and hematopoietic lineage differentiation in vitro. However, it remains unknown whether or not Setd1a controls specific hematopoietic lineage commitment and differentiation during animal development. Here, we reported that homozygous Setd1a knockout (KO) mice are embryonic lethal. Loss of the Setd1a gene in the hematopoietic compartment resulted in a blockage of the progenitor B-cell-to-precursor B-cell development in bone marrow (BM) and B-cell maturation in spleen. The Setd1a-cKO (conditional knockout) mice exhibited an enlarged spleen with disrupted spleen architecture and leukocytopenia. Mechanistically, Setd1a deficiency in BM reduced the levels of H3K4me3 at critical B-cell gene loci, including Pax5 and Rag1/2, which are critical for the IgH (Ig heavy-chain) locus contractions and rearrangement. Subsequently, the differential long-range looped interactions of the enhancer Eμ with proximal 5′ DH region and 3′ regulatory regions as well as with Pax5-activated intergenic repeat elements and 5′ distal VH genes were compromised by the Setd1a-cKO. Together, our findings revealed a critical role of Setd1a and its mediated epigenetic modifications in regulating the IgH rearrangement and B-cell development.—Tusi, B. K., Deng, C., Salz, T., Zeumer, L., Li, Y., So, C. W. E., Morel, L. M., Qiu, Y., Huang, S. Setd1a regulates progenitor B-cell-to-precursor B-cell development through histone H3 lysine 4 trimethylation and Ig heavy-chain rearrangement.
Keywords: promoter H3K4me3, Setd1a KO mice, B-cell differentiation, long-range chromatin loops, transcriptional regulation
The trithorax (TrxG) group genes encode an evolutionarily conserved family of proteins that contains a highly conserved SET domain and acts to maintain specific patterns of gene expression throughout development. In mammals, this family of histone methyltransferases (HMTs) includes 6 complexes [mixed-lineage-leukemia (MLL) 1–4 and SETD1A/B], each containing a unique histone H3 lysine 4 (H3K4)-specific enzymatic subunit and other common subunits (1). Methylation of H3K4 by Set1/MLL represents a universal epigenetic mark for transcriptionally active euchromatin (1–3). Evidence supports the involvement of TrxG complexes, particularly MLLs, in hematopoiesis and hematologic malignancies. First, genetic alterations of MLLs have long been established in leukemia and more recently in lymphoma. MLL1 is frequently disrupted by chromosomal translocations in acute leukemia (4–6). Second, Mll-deficient embryos exhibited a defect in hematopoietic stem cells (HSCs) in the aorta-gonad-mesonephros region, and the Mll-deficient cells failed to contribute to fetal liver hematopoiesis (6–10), suggesting that MLL1 is required for definitive hematopoiesis. Finally, loss of Mll5 leads to pleiotropic hematopoietic defects and reduced neutrophil immune function (11, 12). These data suggest that TrxG-related complexes play an essential role in hematopoietic lineage development.
Although all mammalian TrxG-related Set1/MLL complexes have the ability to methylate H3K4, the recent biochemical characterization of Set1/MLL complexes suggests a distinct role of Setd1a/b and MLL1-4 in mono, di-, and trimethylation of H3K4 sites (13–16). Although MLL3/MLL4 act as major H3K4 monomethyltransferases at enhancers (16), Setd1a and Setd1b have been implicated in promoter-associated H3K4 trimethylation (14, 16, 17). In contrast, MLL1/2 complexes mediate H3K4 methylations in a highly gene-specific manner, e.g., regulating Hox gene clusters and other homeotic genes (18). Despite MLL1’s involvement in hematopoiesis, very little is known about the function of its paralog, Setd1a, in normal hematopoietic development. We recently demonstrated that knockdown of Setd1a in mouse embryonic stem cells blocks the endothelial-to-hematopoietic transition and decreases the expression of hematopoietic markers and transcription factors required for early onset of hematopoiesis (15). It has been shown that human SETD1A-mediated H3K4 methylations are required for transcription of TAL1 gene during normal hematopoiesis (19). Additionally, a recent large-scale study in zebrafish identified SET1 as one of the major regulators in hematopoiesis (20). However, the physiologic significance of mammalian SETD1A and its mediated H3K4me3 in hematopoietic lineage development is currently unknown.
During development, H3K4 methylations have been shown to play an important role in B-cell development by modulating chromatin structure and gene transcription (21, 22). MLL1 and tumor suppressor Menin are both required for B-cell differentiation (23). Furthermore, during progenitor B-cell (pro-B) to precursor B-cell (pre-B) differentiation, the H3K4me3 pattern is almost exclusively associated with J genes and nearby D genes in the rearranging IgH (Ig heavy-chain) locus (22), suggesting that the levels of H3K4me3 could well enforce V(D)J recombination in the IgH locus in the pro-B stage. During V(D)J recombination, the IgH locus forms rosette-like loop clusters that are important for recombination. The murine IgH locus spans around 2.8 Mb and contains 10–13 DH, 4 JH, and 195 VH gene segments. Recombination of these segments is tightly regulated by transcription factors and modulators, such as the Pax5 and Rag1/2 proteins, for proper IgH long-range looping and rearrangement (24). In addition, Pax5 is a B-cell-specific master regulator required for early B-cell differentiation (25). Yet, it remains elusive what enzymes methylate the IgH locus and how H3K4me3 and PAX5 cooperate to control B-cell development.
We investigated the role of Setd1a in hematopoiesis in vivo by utilizing Mx1-cre-mediated conditional Setd1a knockout (KO) in the hematopoietic system. Setd1a-deficient mice exhibited a perturbed B-cell development in the bone marrow (BM) and constricted/disrupted B-cell follicles in the spleen. Moreover, we demonstrated that Setd1a and its mediated H3K4me3 control pro-B-to-pre-B development by regulating B-cell master regulators: Pax5, Rag1, and Rag 2. Setd1a deficiency led to a decrease in H3K4me3 levels at rearranging Eμ enhancer and JH gene segments at the IgH locus and perturbed long-range chromatin interactions and locus contractions of the DHJH and VH to DHJH at the rearranging locus. Thus, our data suggest that Setd1a is required for pro-B to pre-B transition and IgH rearrangement during B-cell development.
MATERIALS AND METHODS
Setd1a KO mice
Setd1a+/− mice on a C57BL/6 background (MAYT; EPD0027_4_H10) were purchased from the Wellcome Trust Sanger Institute (Hinxton, Cambridge, United Kingdom) and intercrossed with B6 (C3)-Tg (PgK1-FLPo) (The Jackson Laboratory, Bar Harbor, ME, USA) to generate Setd1afl/fl mice. Then, Setd1afl/fl mice were intercrossed with Mx1-cre transgenic (The Jackson Laboratory) to generate Setd1a-conditional knockout (cKO) mice. Intraperitoneal injections of polyinosinic/polycytidylic acid (pI/pC) with the concentration of 20 µg/g of body weight at 2-day intervals were performed 3 times to induce deletion in Setd1afl/fl:Mx1-cre mice. Excision efficiency in the induced mice was assessed by genomic PCR. Animal protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
Antibodies and primers
The flow cytometry antibodies anti-IgM (11/41), anti-B220 (RA3-6B2), and anti-CD43 (eBioR2/60) were purchased from eBioscience (San Diego, CA, USA). The antibodies anti-CD23 (clone, B3B4), anti-AA4.1 (clone, AA4.1), anti-CD19 (clone, 1D3), and anti-CD24/HSA (clone, M1/69) were purchased from BD Pharmingen (San Diego, CA, USA). Antibodies used for chromatin immunoprecipitation (ChIP) assay were purchased from EMD Millipore (Billerica, MA, USA) for Anti-trimethyl-H3K4 (clone, MC315) and from Santa Cruz Biotechnology (Santa Cruz, CA, USA) for Pax5 (sc-1974). Normal goat IgG (sc-2028) was purchased from Santa Cruz Biotechnology, and normal rabbit IgG (P120-101) was purchased from Bethyl Laboratories (Montgomery, TX, USA). The genotyping/RT-PCR/ChIP/chromatin conformation capture (3C) primers are available upon request.
Flow cytometry
The expression of cell surface markers was examined using standard flow cytometric methods. BM and spleen single-cell suspensions were depleted for erythrocytes using BioWhittaker ACK lysing buffer solution (Lonza Group, Basel, Switzerland), and aliquots of 106 cells were stained with a combination of labeled antibodies on ice in PBS plus 4% fetal bovine serum for 30 min and analyzed by the LSR II flow cytometer (BD Biosciences, San Jose, CA, USA) using BD FACSDiva software (BD Biosciences). In the BM, pro-B cells were defined as B220+/IgM−/CD43+ and pre-B cells as B220+/IgM−/CD43−. The (T1/T2/T3) transitional B-cell populations in the spleen were defined with AA4.1, IgM, and CD23 cell surface markers.
Peripheral blood profile analysis
Blood samples were collected in EDTA-containing Microtainer tubes (BD Biosciences) and analyzed on a HemaTrue analyzer (Heska, Loveland, CO, USA).
Immunohistochemistry staining
Spleen tissue was fixed in 10% formalin and subsequently paraffin embedded. The hematoxylin and eosin (H&E)- or antibody-stained specimens were prepared by the University of Florida Molecular Pathology and Immunology Core.
Quantitative RT-PCR
Total RNA was isolated using the RNA isolation kit according to the manufacturer’s instructions (Qiagen, Germantown, MD, USA). RNA was reverse transcribed using the SuperScript II Reverse Transcriptase (Invitrogen, Life Technologies, Carlsbad, CA, USA). cDNA was analyzed using a quantitative PCR (qPCR) with a CFX Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The relative RNA levels were measured by normalizing with glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Colony-formation assay (colony-forming unit pre-B)
For colony-forming unit (CFU) pre-B assays, total BM cells isolated from control and Setd1a mutant mice were plated in triplicate in methylcellulose medium (MethoCult M3432; StemCell Technologies, Vancouver, BC, Canada) supplemented with 10 ng/ml IL-7 and 50 ng/ml stem cell factor for 2 weeks. CFU pre-B colonies were observed and counted following guidelines as described by StemCell Technologies.
ChIP and 3C assays
The ChIP and 3C assays were done using pro-B cells purified from BM and cultured in IL-7 (10 ng/ml) as described previously (19, 26). For 3C assay, 107 cells were fixed with 2% formaldehyde at room temperature for 10 minutes and lysed. Noncross-linked proteins were removed by SDS, and SDS was sequestered by Triton X-100. Nuclei were digested overnight at 37°C with 1600 U HindIII (New England Biolabs, Ipswich, MA, USA) with shaking. The digestion was terminated by adding SDS at 65°C. Digested nuclei (106) were ligated with T4 DNA ligase (New England Biolabs) for 5 hours at 16°C, and cross-linked chromatin was reversed. DNA was purified by phenol:chloroform extraction. Ligated DNA samples were analyzed by PCR. The quantitation of 3C interactions was calculated and plotted after normalization to loading controls using ImageJ 1.45n software (National Instiutes of Health, Bethesda, MD, USA).
RESULTS
Generation of a mouse model with Setd1a conditionally knocked out in the hematopoietic compartment
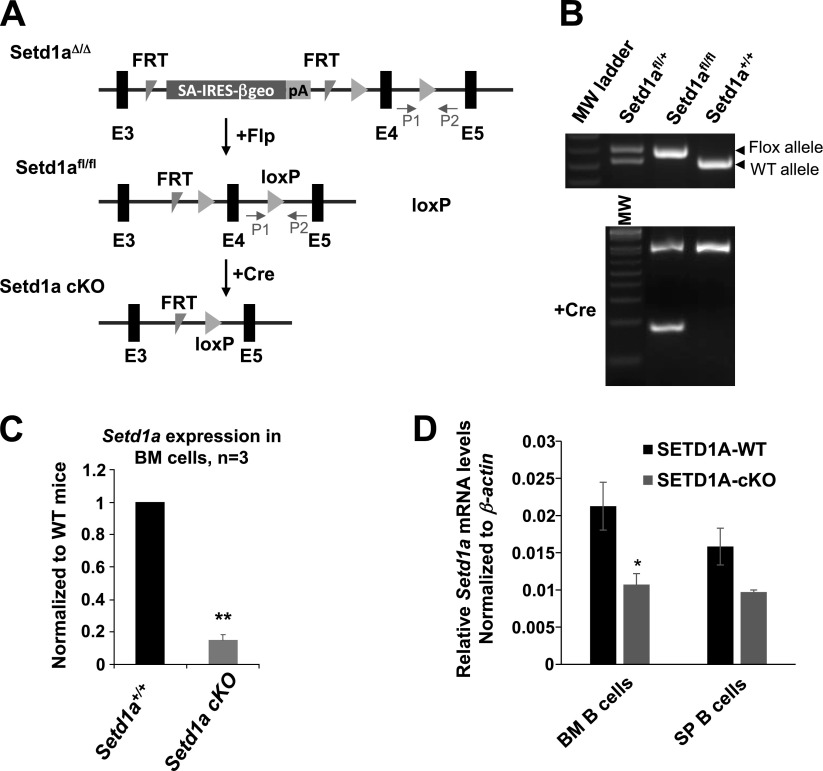
To investigate the biologic role of Setd1a in vivo, we generated a Setd1a KO mouse model utilizing a multipurpose targeting vector, in which germline KO can be converted to cKO using FLPo and/or Cre recombination (Fig. 1A) (27). Insertion of the FRT-flanked β-geo cassette upstream of the exon 4 of the Setd1a germline KO allele introduces a polyadenylation site that led to premature termination of Setd1a transcript, whereas the exon 4 is flanked by loxP sites allowing to remove exon 4 using a Cre recombinase-mediated cKO (Fig. 1A). The homozygous Setd1a KO resulted in early embryonic lethality, suggesting that Setd1a is required for early embryonic development (Table 1). Furthermore, the Vav1-cre-mediated homozygous deletion of the Setd1a allele in the hematopoietic compartment also resulted in embryonic lethality (Table 2), indicating that Setd1a plays a critical role in hematopoiesis. However, the Setd1a heterozygous (Setd1a+/Δ) mice survive (Table 1), and Setd1a mRNA levels are significantly decreased in the Setd1a+/Δ mice (Supplemental Fig. S1A). The Setd1a+/Δ mice exhibited a moderate reduction in B-cell populations in the BM and an inhibition of B-cell maturation in the spleen (Supplemental Fig. S1B, C). These data suggest that haploinsufficiency of Setd1a may impair B-cell development.
Figure 1.
Strategies for conditional inactivation of Setd1a alleles and mutant phenotypes. A) Schematic representation of a KO strategy and the construct used for generating cKO of Setd1a. E, exon; P, primer; SA-IRES, splice acceptor-internal ribosome entry site. The Setd1a-targeted deletion allele was converted to cKO allele upon Flp recombination and Cre-induced excision of exon 4. B) PCR-based genotyping of WT (+/+), heterozygous floxed (fl/+), and homozygous floxed (fl/fl) littermates (top). PCR-based genotyping of the polydI/dC-induced allele deletion in the hematopoietic compartment (bottom). MW, molecular weight. C) RT-qPCR assays of Setd1a expression in the BM cells derived from 3 Setd1a-cKO (Setd1afl/fl:Mx1-cre+) and 3 WT control mice (Setd1a+/+:Mx1-cre+). D) RT-qPCR assays of Setd1a expression in BM B220+ B cells and spleen (SP) IgM+/B220+ B cells.
TABLE 1.
Setd1a+/Δ × Setd1a+/Δ
| Genotype | +/+ | +/Δ | Δ/Δ |
|---|---|---|---|
| Expected no. | 10 | 21 | 10 |
| Observed no. | 27 | 14 | 0 |
| (66%) | (34%) | (0%) |
Genotypes of littermates from crossing Setd1a+/Δ mice. The homozygous Setd1a mutant allele (Setd1aΔ/Δ) exhibited embryonic lethality. n = 41.
TABLE 2.
Setd1a+/fl: Vav1-cre+ × Setd1a+/fl: Vav1-cre+
| Genotype | Vav1-cre+: | ||
|---|---|---|---|
| Setd1a+/+ | Setd1a+/fl | Setd1afl/fl | |
| Expected no. | 6 | 11 | 6 |
| Observed no. | 9 | 14 | 0 |
| (39%) | (61%) | (0%) | |
Genotypes of littermates from crossing Vav1cre:Setd1a+/fl mice. The homozygous deletion of the Setd1a allele in the hematopoietic compartment also exhibited embryonic lethality. n = 23.
To further address the functional role of Setd1a in hematopoiesis and B-cell development, the β-geo cassette was successfully removed and generated the Setd1afl/fl mice carrying LoxP sites flanking the critical exon 4 by crossing heterozygous Setd1a+/Δ mice with the B6(C3-Tg(Pgk1-FLPo) mice (The Jackson Laboratory) (Fig. 1B, top). Conditional Setd1a KO mice (Mx1-Cre:Setd1afl/fl, and hereafter termed Setd1a-cKO) were generated by crossing with the Mx1-cre knockin mice that express Cre recombinase under the control of the interferon-inducible Mx1 promoter (Fig. 1A). The deletion of the Setd1a allele was achieved upon 3 intraperitoneal pI/pC injections at 2-day intervals. The induced mice were killed 2 months after the last injection (Fig. 1B, bottom). The Mx1-cre:Setd1a+/+ mice induced with pI/pC were used as controls [wild-type (WT) controls] to exclude the possible effects of pI/pC induction. Although the Setd1a-cKO mice are viable and appear normal compared to the control mice, the BM hematopoietic cells from 3 WT controls and 3 Mx1-Cre:Setd1afl/fl mice induced with pI/pC exhibited an approximate 80% decrease in Setd1a transcripts (Fig. 1C). Interestingly, Setd1a mRNA levels were significantly reduced in BM B220+ B cells, which consist of more immature B cells, by comparing between the WT control and the Setd1a-cKO mice (Fig. 1D). Yet, Setd1a mRNA was moderately decreased in IgM+/B220+ more-matured B cells in the spleen (Fig. 1D). These results suggest that Setd1a may regulate early B-cell differentiation.
Loss of Setd1a resulted in impaired leukopoiesis and disrupted spleen architecture
Although Setd1a-cKO mice appear normal, biochemical analysis of peripheral blood at 2 mo post pI/pC induction revealed a significant reduction in the total number of white blood cells and lymphocytes in the mutants as compared with the control mice (Table 3), indicating that Setd1a loss resulted in a defective leukopoiesis. No significant changes were observed in the other blood parameters, such as the number of monocytes, granulocytes, red blood cells, as well as the amount of hemoglobin and hematocrit (Table 3). Fluorescence-activated cell sorting (FACS) analysis of B- and T-lymphocytes from the Setd1a+/Δ mice further revealed that CD4/CD8-positive T-cell populations are also affected in the spleen (Supplemental Fig. S2).
TABLE 3.
Peripheral blood analyses of control and Setd1a-cKO mice
| 2-mo-old mice | WBCs (K/μl) | LYM (K/μl) | MON (K/μl) | GRAN (K/μl) | RBCs (106/μl) | HGB (g/dl) | HCT (%) |
|---|---|---|---|---|---|---|---|
| Ctrl (n = 12) | 15.03 | 10.20 | 0.71 | 4.13 | 9.72 | 14.70 | 42.26 |
| Setd1a-cKO (n = 12) | 10.72 | 7.35 | 0.56 | 2.82 | 9.42 | 13.98 | 40.28 |
| P value | 0.05 | 0.02 | n.s. | n.s. | n.s. | n.s. | n.s. |
WBCs, white blood cells; LYM, lymphocyte; MON, monocyte; GRAN, granulocyte; RBCs, red blood cells; HGB, hemoglobin; HCT, hematocrit; Ctrl, control; n.s., not significant.
Furthermore, Mx1-cre-mediated deletion of Setd1a in BM caused splenomegaly (Fig. 2A). We did not observe significant changes in lymph nodes comparing the WT control and the Setd1a-cKO mice. Histologic examination of the spleen comparing Setd1a-cKO and WT control mice revealed that Setd1a-deficient mice exhibited a dramatic change in the spleen architecture because Setd1a-cKO spleens presented impaired lymphoid follicles (white pulp) and expanded the red pulp (Fig. 2B, top). Consistent with the increased spleen red pulp, the Gr-1+/CD11b+ myeloid cells and Ter119+/CD71+ erythroid cells in the spleen are increased in the Setd1a-cKO mice compared to the WT control (Supplemental Fig. S3). Immunohistochemistry staining with the B-cell-specific marker B220 antibody further revealed a decreased number of B-lymphocytes in the lymphoid follicles in the Setd1a-deficient spleens (Fig. 2B, bottom), suggesting that Setd1a is critical for B-cell development in vivo.
Figure 2.
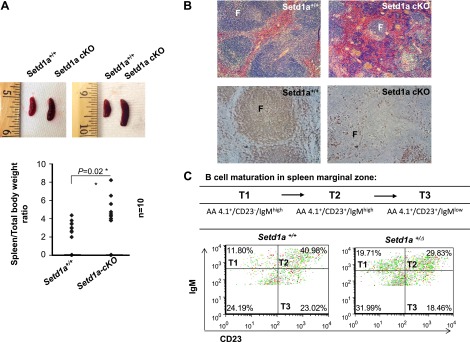
Loss of Setd1a in the hematopoietic compartment resulted in a blockage of B-cell development. A) Loss of Setd1a in the hematopoietic compartment resulted in splenomegaly. Whole spleens (top) and their weights (bottom; n = 10) are shown for Setd1a-cKO and WT control mice. Data are shown as the mean ± sd. *P ≤ 0.05. B) H&E staining (top) and immunohistochemical staining with anti- B220 antibody (bottom) for spleen sections in Setd1a mutant (Setd1afl/fl:Mx1-cre+; n = 3) revealed constricted and disrupted B-cell follicles compared with control (Setd1a+/+:Mx1-cre+; n = 3). The lymphoid follicles (F) are indicated. C) Representative FACS analysis of transitional B cells from T1 stage (AA4.1+/Cd23−/IgMhigh) to the more-matured T3 stage (AA4.1+/Cd23+/IgMlow) in the spleen. The Setd1a+/Δ mice (n = 2) exhibited a block in transition from T1 (AA4.1+/Cd23−/IgMhigh) to T2 (AA4.1+/Cd23+/IgMhigh) stages compared to the Setd1a+/+ control mice (n = 2).
In mammal, the marginal zone of the spleen is the site for B-cell maturation, and immature B cells mature through 3 stages, from T1 (AA4.1+/CD23−/IgMhigh), T2 (AA4.1+/CD23+/IgMhigh), and finally matured T3 (AA4.1+/CD23+/IgMlow). We further examined whether B-cell maturation is inhibited by the reduction of Setd1a using FACS with different B-cell surface markers expressed in different maturation stages. The heterozygous Setd1a+/Δ mice exhibited an increase in the T1 immature B-lymphocyte population and a decrease in both T2 and T3 B-cell populations in the spleen marginal zone (Fig. 2C). Thus, these data suggest that Setd1a deficiency also impairs B-cell maturation in the spleen. In addition, the heterozygous Setd1a+/Δ mice exhibited a decrease of mature B-lymphocytes in BM (Supplemental Fig. S1B, C).
Setd1a deficiency inhibits pro-B to pre-B cell development
B-cell development takes place in the BM where common lymphoid progenitors differentiate through different stages of B-cell development from preprogenitor B cells to pro-Bs, then pre-Bs, and finally immature B-lymphocytes, which then leave the BM and migrate to the spleen and lymph nodes for maturation (28). Given that the Setd1a-cKO mice exhibited a reduction in B-lymphocytes, we reasoned that Setd1a may regulate B-lineage differentiation in the BM. To test this possibility, we examined B-cell populations at different developmental stages within the BM using FACS with different B-cell surface markers expressed in different developmental stages. Examination of Setd1a-deficient BM-derived hematopoietic cells from the pI/pC-induced Mx1-Cre:Setd1afl/fl mice showed an increase in pro-B (IgM−B220+CD43+) frequencies and numbers but a decrease in the frequencies and numbers of pre-B cells (IgM−B220+CD43−) (Fig. 3A and Table 4). Analysis of the Setd1a+/Δ heterozygous mice confirmed a reduction in the frequencies of pre-Bs and increased levels of pro-Bs compared with WT mice (Supplemental Fig. S4A, B). Moreover, by using different combinations of cell markers, we confirmed that the B-cell differentiation in BM is blocked at the pro-B to pre-B stages (Supplemental Fig. S4C). Furthermore, we detected a significant increase in the frequency and number of B-lymphocytes in the thymus of the heterozygous Setd1a+/Δ mice (Supplemental Fig. S5). These results indicated that Setd1a loss inhibits differentiation of pro-Bs to pre-Bs in the BM. Thus, Setd1a acts as a regulator controlling the pro-B-to-pre-B transition of B-cell development.
Figure 3.

Setd1a conditional deletion blocks pro-B to pre-B development. A) Representative FACS analysis of BM pro-B population (IgM− B220+ CD43+) and pre-B population (IgM− B220+ CD43−) comparing between the control (n = 4) and Setd1a-cKO (n = 5) mice induced with polydI/dC. Numbers indicate the percentage of gated IgM− leukocytes (left) and IgM− B220+ total BM cells (right). B) Representative FACS analysis of mature B-lymphocytes in spleen comparing between the control (n = 5) and Setd1a-cKO (n = 6) mice induced with polydI/dC. Numbers indicate the percentage of gated total spleen leukocytes. C) CFC assay analysis of formation of definitive pre-B colonies comparing between the control (n = 3) and Setd1a-cKO (n = 3) mice induced with polydI/dC. Data are shown as the mean ± sd. ***P ≤ 0.001.
TABLE 4.
Setd1a deficiency blocked pro-B-to-pre-B development and B-cell maturation
| BM |
Spleen |
|||||
|---|---|---|---|---|---|---|
| Ctrl |
cKO |
Ctrl |
cKO |
|||
| ID | Pro-B (%) | Pre-B (%) | Pro-B (%) | Pre-B (%) | B220+ IgM+ (%) | B220+ IgM+ (%) |
| #324/#304979 | 5.4 | 7.2 | 6.2 | 2.3 | 44.5 | 29 |
| #324/#304980 | 5.4 | 7.2 | 5.4 | 1.9 | 44.5 | 30.1 |
| #383/#323956 | 4.8 | 10.8 | 4.8 | 6.2 | 60.7 | 48.5 |
| #227/#313265 | 3.3 | 5.3 | 3.2 | 2.1 | 50.7 | 45.2 |
| #2/#274 | 5.2 | 8.7 | 13.5 | 4.9 | 44.9 | 19.3 |
| #3/#275 | nd | nd | nd | nd | 55.9 | 28.3 |
| ID | Pro-B Ab. (no.) | Pre-B Ab. (no.) | Pro-B Ab. (no.) | Pre-B Ab. (no.) | B220+ IgM+ Ab. (no.) | B220+ IgM+ Ab. (no.) |
| #324/#304979 | 1,927,200 | 2,569,600 | 1,272,800 | 481,600 | 24,297,000 | 15,660,000 |
| #324/#304980 | 1,927,200 | 2,569,600 | 1,100,800 | 378,400 | 24,297,000 | 16,254,000 |
| #383/#323956 | 1,343,200 | 3,036,800 | 756,800 | 963,200 | 33,142,200 | 26,190,000 |
| #227/#313265 | 1,401,600 | 2,277,600 | 860,000 | 584,800 | 27,682,200 | 24,408,000 |
| #2/#274 | 1,635,200 | 2,744,800 | 2,683,200 | 997,600 | 24,515,400 | 10,422,000 |
| #3/#275 | nd | nd | nd | nd | 30,521,400 | 15,282,000 |
Total BM cells for control (Ctrl) are 58.4 × 106 and Setd1a-cKO are 34.4 × 106. Total spleen cells for Ctrl are 54.6 × 106 and Setd1a-cKO are 54.0 × 106. Percentage (%) of IgM− cells for BM samples is shown. ID, identification; nd, not determined; Ab., antibody; no., number.
Moreover, FACS analysis of the spleen cells also showed a significant reduction in the frequencies and numbers of mature B cells (IgM+/B220+) in the mutants as compared with the WT control mice in the spleen (Fig. 3B). To further assess the effect of Setd1a on B-lymphocyte differentiation, colony-forming cell (CFC) assays for pre-Bs were carried out using BM cells isolated from Setd1a-cKO and WT control mice in the presence of stem cell factor and lymphocyte-specific cytokine IL-7. Ablation of Setd1a resulted in significantly fewer and smaller pre-B colonies as compared with control cells (Fig. 3C). Our data revealed an important role for Setd1a in early B-cell differentiation by regulating pro-B to pre-B development.
Setd1a controls key regulators required for B-cell identity and differentiation
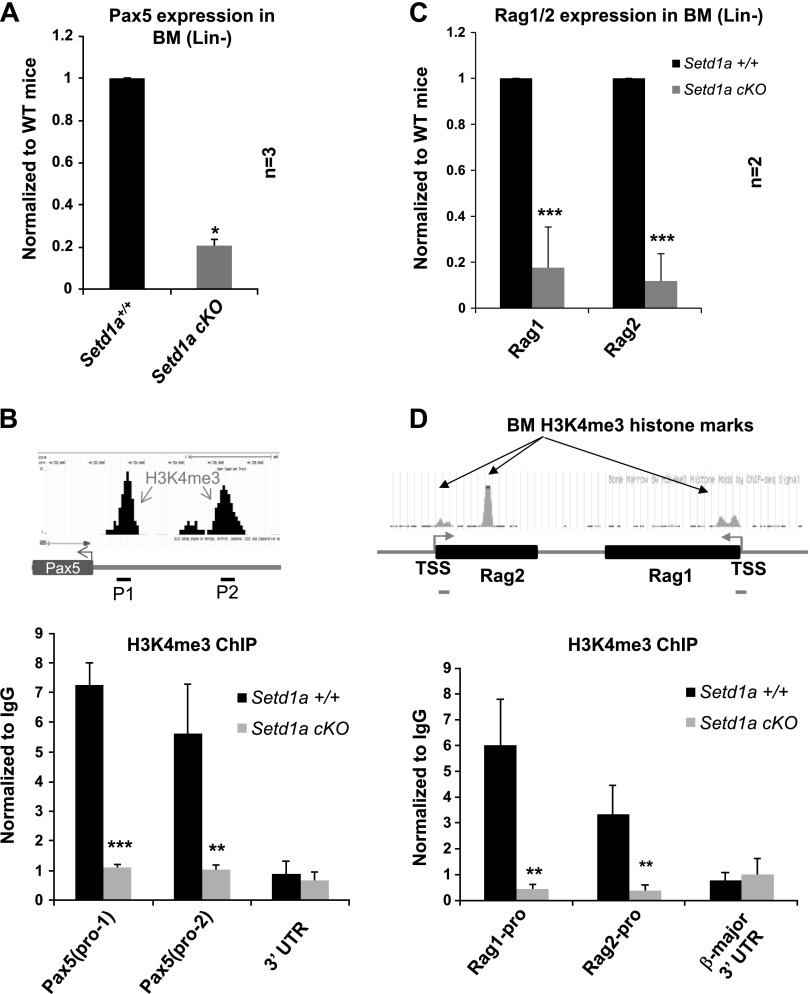
Pax5 is a master transcription factor controlling B-cell identity and differentiation. Pax5-deficient mice blocked B-cell development at the pro-B to pre-B transition stage in the BM (29), a phenotype similar to that of the Setd1a-cKO mice described above. Thus, we asked whether Setd1a-cKO affects Pax5 transcription. Quantitative RT-PCR (qRT-PCR) analysis of BM-derived hematopoietic cells from 3 controls and 3 Setd1a-cKO mice revealed that the levels of Pax5 transcript were reduced by approximately 80% in the Setd1a-cKO mice (Fig. 4A). Setd1a is an HMT, which specifically methylates H3K4me3 at the transcription start sites (TSSs) of active genes (13, 15). We then tested whether the loss of Setd1a decreases H3K4me3 marks in the promoter/enhancer regions of Pax5 where H3K4me3 is highly enriched in B cells (Fig. 4B). ChIP assay was performed using an antibody specifically against H3K4me3 and primers designed in the region enriched for H3K4me3 peaks in the Pax5 promoter/enhancer regions (Fig. 4B, top). We observed a significant decrease in H3K4me3 levels at the Pax5 TSS and regulatory region but not in the 3′ UTR in Setd1a-cKO BM hematopoietic cells as compared to the WT controls (Fig. 4B), which is consistent with the reduced Pax5 expression in these cells (Fig. 4A).
Figure 4.
Loss of Setd1a inhibits transcription of key regulators required for B-cell identity and development. A) qRT-PCR analysis of Pax5 transcripts in BM hematopoietic cells comparing the controls (Setd1a +/+:Mx1-cre+; n = 3) and Setd1a-cKO mutants (Setd1a fl/fl:Mx1-cre+; n = 3). Lin, lineage. B) Schematic representation of Pax5 locus and ChIP data from the Encyclopedia of DNA Elements (ENCODE) project showing H3K4me3 enrichment peaks at Pax5 promoter/enhancer regions. ChIP primers are indicated by black lines (top). ChIP analyses of H3K4me3 levels at the Pax5 promoter regions in BM-derived pro-B cells cultured in IL-7 (10ng/ml) comparing the Setd1a control and Setd1a-cKO mice (bottom). C) qRT-PCR analysis of Rag1 and Rag2 transcripts in BM hematopoietic cells comparing the controls (Setd1a +/+:Mx1-cre+; n = 2) and Setd1a-cKO mutants (Setd1a fl/fl:Mx1-cre+; n = 2). D) Schematic representation of Rag1 and Rag2 loci and ChIP data from the ENCODE project showing H3K4me3 enrichment peaks at their promoter regions. ChIP primers are indicated by gray lines (top). ChIP analyses of H3K4me3 levels at the Rag1/2 promoter regions in BM-derived pro-B cells cultured in IL-7 (10ng/ml) from Setd1a control and Setd1a-cKO mice (bottom). Data are shown as mean ± SD. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Differentiation of pro-Bs to pre-Bs depends on the proper rearrangement of the IgH locus (28, 30). Key regulatory factors required to initiate the V(D)J rearrangement at the pro-B stage are the Rag1/2 proteins. It has been shown that mice deficient in either Rag1 or Rag2 are unable to initiate V(D)J recombination and lack mature B cells (31). To investigate whether the defective transition from pro-Bs to pre-Bs in Setd1a-deficient mice is partly due to Rag1 and Rag2 deficiency, the expression levels of Rag1 and Rag2 were analyzed in the Setd1a-deficient BM cells. Significantly, the BM hematopoietic cells from Setd1a-cKO mice displayed a 4-fold decrease in the expression levels of Rag1 and Rag2 mRNA compared to that of the control mice (Fig. 4C).
These data again raised the question whether the loss of Setd1a reduces H3K4me3 enrichment at the promoter region of the Rag1/2 genes. To test this possibility, ChIP assay was performed using an antibody against H3K4me3. Primers were designed in the H3K4me3 peak regions in the Rag1 and Rag2 promoters (Fig. 4D, top). Consistent with the inhibition of Rag1/2 transcription by Setd1a-cKO (Fig. 4C), ChIP-qPCR analysis revealed a significant decrease in H3K4me3 enrichment at the Rag1/2 promoters in Setd1a-deficient BM cells as compared with cells from the control mice (Fig. 4D). Thus, Setd1a is important for regulating the expression of factors essential for B-cell development and proper recombination of the IgH locus.
Deficiency in Setd1a impairs the recruitment of Pax5 to the rearranging IgH locus
The pro-B to pre-B transition requires the rearrangement of the IgH locus (28, 30). Pax5 binds to the sequences that are interspersed in the distal VH gene region in the IgH locus called the Pax5-activated intergenic repeat (PAIR) elements (32), from which antisense noncoding RNAs (ncRNAs) are transcribed in pro-Bs (Fig. 5A). These ncRNAs generated within this region are required for IgH rearrangement at the pro-B stage (24, 32–34). To investigate whether the reduced Pax5 observed in the Setd1a-cKO mutants led to a reduction in its recruitment to the PAIR elements of the IgH locus, cross-linked chromatin from the Setd1a-cKO or control BM cells was immunoprecipitated with Pax5 antibody, and the Pax5-selected chromatin was purified and quantitated by qPCR. Consistent with the decrease in Pax5 transcription, Pax5 recruitment to the PAIR4/6/11 elements in the distal VH region was specifically reduced by 50% in Setd1a-cKO BM cells as compared with the control BM cells (Fig. 5A).
Figure 5.
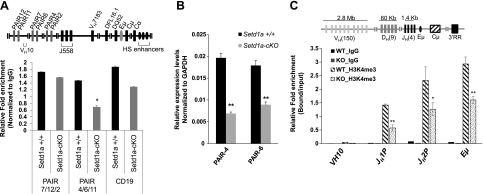
Setd1a deficiency impairs the binding of Pax5 and H3K4me3 enrichment in the rearranging IgH locus. A) Schematic representation of PAIR elements at the IgH locus (top). ChIP analyses of Pax5 recruitment at the PAIR element sites at IgH locus compared the control and Setd1a-cKO mice. B) Expression of PAIR4 and PAIR6 antisense transcripts is measured using RT-qPCR comparing the control and Setd1a-cKO mice. C) ChIP analysis in BM-derived pro-Bs cultured in IL-7 (10 ng/ml) revealed that loss of Setd1a disrupts H3K4me3 enrichment at the IgH locus. Data are shown as the mean ± sd. *P < 0.05; **P < 0.01.
Next, we asked whether the decreased Pax5 recruitment to the VH regions affected the expression levels of ncRNAs from the PAIR elements. qRT-PCR analysis of PAIR ncRNAs located in the VHJ558 region revealed a decrease in expression levels of PAIR4/6 ncRNAs in the Setd1a mutant compared with WT control (Fig. 5B). Thus, taken together, our results demonstrate that decreased Pax5 in Setd1a-cKO mice resulted in a disruption of Pax5 binding to the PAIR elements and a reduction in antisense noncoding transcripts from the PAIR elements within the IgH locus that are required for proper V(D)J recombination in early lymphopoiesis.
Loss of Setd1a decreases H3K4me3 levels and disrupts long-range chromatin interactions in the rearranging IgH locus
In the IgH locus, H3K4me3 patterns are exclusively associated with the Eμ enhancer, 4 JH gene segments, and nearby DQ52 in the DH locus (Fig. 5C, top). The enrichment of H3K4me3 on rearranging genes may enforce the ordered rearrangement of DHJH prior to VH to DHJH. To determine the role of Setd1a as an H3K4 methyltransferase in establishing H3K4me3 marks at this rearranging region, cross-linked chromatin prepared from Setd1a-cKO and control BM hematopoietic cells was precipitated with H3K4me3 antibody. The H3K4me3-enriched chromatin was purified and quantitated by qPCR. The levels of H3K4me3 marks were significantly decreased at the Eμ enhancer and the JH gene segments in the Setd1a-cKO BM cells compared with that of the control BM cells (Fig. 5C). This suggests that Setd1a is responsible for establishing H3K4me3 patterns at the Eμ enhancer and nearby 4 JH gene segments in the rearranging IgH locus during pro-B to pre-B cell development.
Recent 3-dimensional FISH studies reported that the IgH locus undergoes repositioning and locus contraction in the nucleus of pro-Bs (35). The IgH locus contraction requires formation of 2 distinct multilooped domains. The first multiple-looped domain involves the interactions of the Eμ enhancer with 5′ DFL and 3′ regulatory regions (3′RRs) in the 3′ end of the rearranging locus that initiates the DHJH rearrangement. In the second multiple-looped domain, the Eμ enhancer brings the DH-JH region in close proximity to both the distal and proximal parts of the VH gene regions, which are spread over large megabase distances in the IgH locus (22, 36). In addition, antisense transcripts from PAIR4/6 in the distal region of VH genes are critical to form the Eμ-PAIR interactions that also facilitate locus compaction, and allow distal VH genes to undergo efficient rearrangement (24). Given that Setd1a deficiency reduced H3K4me3 and accessibility at the Eμ enhancer and the JH regions as well as inhibited antisense PAIR4/6 transcription, we tested whether the loss of Setd1a disrupts the folding of 2 distinct multiple-looped domains at the rearranging IgH locus. Setd1a deficiency impaired the looped contractions of the Eμ enhancer with the 3′ end as well as the 5′ end of the rearranging locus in pro-Bs (Fig. 6). First, Setd1a and its mediated H3K4me3 are critical for the Eμ-HS1/2 and the Eμ-5′ DFL interactions, which bring DHJH and 3′RR into close proximity (Fig. 6B, C). Next, Setd1a ablation also inhibited the looped interaction between Eμ and the distal VH genes at 3′ VHJ558 (Fig. 6B, C). The Eμ-PAIR interactions at the distal VH regions were also affected by Setd1a deficiency (Fig. 6B, D). Thus, the looped contractions that bring distal VH genes into proximity with Eμ enhancer are also dependent on Setd1a HMT activity.
Figure 6.
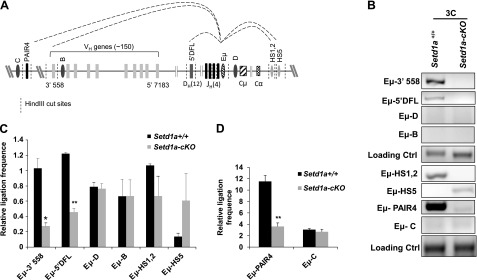
3C analyses of long-range chromatin interactions between Eμ-interacting elements in the rearranging IgH locus. A) Schematic representation of the interacting regions at the IgH locus. Dashed vertical lines indicate HindIII cleavage sites. B) The 3C analyses of long-range interactions of the enhancer Eμ with the distal 3′ VHJ558, PAIR elements, proximal 5′ DFL, 3′RR regions, and the negative control regions across the IgH locus comparing the control and Setd1a-cKO mice. Dark-gray oval regions designated as B, C, and D were used as negative control regions. C and D) A total of 3 independent 3C experiments were quantitated by densitometry. Data are shown as the mean ± sd. *P < 0.05; **P < 0.01.
DISCUSSION
The dynamic regulation of H3K4 HMT activity has been implicated in various biologic processes and plays a fundamental role in the development of solid tumors and leukemia (1, 4, 14, 37, 38). In order to study in vivo function of Setd1a HMT in hematopoietic development, we generated a Setd1a-cKO mouse model. Setd1a and its mediated H3K4me3 control pro-B to pre-B development by regulating B-cell master regulators: Pax5, Rag1, and Rag 2. Setd1a deficiency led to decreases in H3K4me3 levels at the rearranging IgH locus, perturbed long-range chromatin interactions, and impaired V(D)J recombination at the rearranging locus. In addition to the B-cell defect, several hematopoietic lineages are also affected by the BM Setd1a KO, which include erythroid cells, myeloid cells, and T-lymphocytes, and suggest that Setd1a and its mediated H3K4me3 may have an important role in hematopoiesis. However, the molecular mechanism by which Setd1a regulates HSC function and other hematopoietic lineage development remains to be determined. Interestingly, a very similar myeloid phenotype was observed in mice lacking Pax5, which is a master transcription regulator for pro-B to pre-B development (25).
Setd1a deficiency leads to a decrease in the pre-B population by blocking differentiation of pro-Bs in the BM and inhibiting B-cell maturation in the spleen. It is interesting to note that Pax5-mutant mice exhibited the loss of mature B cells, inhibition of pro-B development, and interference of V-to-DJ recombination at the IgH locus (29, 39), a phenotype similar to that of the Setd1a-deficient mice. The cause of impaired B-cell development from pro-B to pre-B transition in the Setd1a-deficient animal is likely due to the loss of the B-cell master regulator Pax5 and the impairment of the IgH locus rearrangement in the BM hematopoietic cells. Moreover, loss of Setd1a also decreases H3K4me3 at the Eμ enhancer and 4 JH gene regions, which leads to a disruption of the Eμ-HS1/2 and the Eμ-5′DFL interactions that bring DHJH and 3′RR into close proximity. Thus, Setd1a activity may be critical for DHJH and VH-to-DHJH rearrangement.
Setd1a is a major HMT responsible for the promoter H3K4me3 patterns at active genes (14, 15, 40). Homozygous Setd1a KO mice die at the epiblast stage shortly after implantation (40), suggesting that Setd1a is critical for organ/tissue development, yet playing a nonoverlapping role with other TrxG-related complexes such as Setd1b and MLLs (7, 40, 41). Setd1a loss significantly reduces H3K4me3 enrichment in the regions comprising Eμ enhancer and 4 JH gene segments, which have the highest levels of activating histone modifications including H3K4me3 for recruiting RAG1 and RAG2 to form a recombination center (22, 42). In addition, H3K4me3 levels are significantly inhibited in the promoters of Pax5 and Rag1/2 genes, which are critical for B-cell identity, development, and IgH recombination. It is noteworthy that a B-lineage acute lymphoblast leukemia expressing the MLL translocation mutant MLL/AF4 blocked pro-B to pre-B differentiation, which underlines the importance of human TrxG-related HMT complexes in B-cell development (43). Recent genome-wide studies revealed that enhancer-associated H3K4me1 and promoter-directed H3K4me3 are implemented by distinct MLLs and Setd1 branches, respectively (1, 13, 14, 16). It remains to be determined whether distinct branches of TrxG-related complexes play a cooperative function in B-cell development.
It has been suggested that transcriptional activity of VH regions, especially transcription from the distal VH part of the IgH locus, makes those regions accessible by forming long-range loops with the Eμ enhancer region during VH to DHJH recombination (24, 33). Pax5 is the regulatory transcription factor that binds to the PAIR elements in the distal VH region and activates transcription of 2 ncRNAs: PAIR4 and PAIR6 (24). In the Setd1a-deficient mice, the reduced Pax5 levels led to a decrease in its recruitment to the distal VH region (Fig. 5A), lower expression levels of PAIR4/PAIR6 ncRNAs (Fig. 5B), and disruption of the Eμ-PAIR long-range interaction (Fig. 6B, D). Thus, B-cell-specific antisense ncRNAs, PAIRs, from these regions provide the accessibility that is needed for long-distance loop between VH and Eμ enhancer in the pro-B stage during VH-to-DHJH rearrangement.
Nuclear processes, such as transcription and recombination, require dynamic chromatin configuration between target sites and their distal regulatory elements. Setd1a-mediated H3K4me3 facilitates long-range promoter-enhancer interaction during transcriptional activation (19), suggesting a role of Setd1a in chromatin organization and locus contraction. We proposed that Setd1a-mediated H3K4me3 is involved in IgH locus contraction and rearrangement in multiple levels. First, Setd1a controls accessibility of the regions comprising Eμ enhancer, 4 JH gene segments, and DQ52 by implementing H3K4me3 in these regions. Second, Setd1a regulates transcription levels of the B-cell master regulator Pax5, which is essential for pro-B to pre-B development and the IgH locus rearrangement. Impairment of PAIR ncRNA expression prevented VH-to-DHJH rearrangement. Finally, both reductions of Rag1/2 expression and H3K4me3 levels at JH genes and Eμ enhancer by Setd1a loss impair the formation of the recombination center. Together, our data reveal a new mechanism of B-cell developmental regulation through modulating V(D)J recombination by Setd1a-mediated histone H3K4 trimethylation.
Supplementary Material
Acknowledgments
The authors are grateful to members of the S.H. and Y.Q. laboratories for their suggestions and comments. The authors thank Ms. Nga Tran for providing technical support during the revision. This work was supported by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grants R01HL091929 and R01HL090589 (to S. H.), and R01HL095674 (to Y.Q.). The authors declare no conflicts of interest.
Glossary
- 3C
chromatin conformation capture
- 3′RR
3′ regulatory region
- BM
bone marrow
- CFC
colony-forming cell
- CFU
colony-forming unit
- ChIP
chromatin immunoprecipitation
- cKO
conditional knockout
- FACS
fluorescence-activated cell sorting
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- H3K4
histone H3 lysine 4
- H&E
hematoxylin and eosin
- HMT
histone methyltransferase
- IgH
Ig heavy chain
- KO
knockout
- MLL
mixed-lineage-leukemia
- ncRNA
noncoding RNA
- PAIR
Pax5-activated intergenic repeat
- pI/pC
polyinosinic/polycytidylic
- pre-B
precursor B cell
- pro-B
progenitor B cell
- qPCR
quantitative PCR
- qRT-PCR
quantitative RT-PCR
- TrxG
trithorax
- TSS
transcription start site
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Shilatifard A. (2012) The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu. Rev. Biochem. 81, 65–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wysocka J., Swigut T., Milne T. A., Dou Y., Zhang X., Burlingame A. L., Roeder R. G., Brivanlou A. H., Allis C. D. (2005) WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell 121, 859–872 [DOI] [PubMed] [Google Scholar]
- 3.Dou Y., Milne T. A., Ruthenburg A. J., Lee S., Lee J. W., Verdine G. L., Allis C. D., Roeder R. G. (2006) Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat. Struct. Mol. Biol. 13, 713–719 [DOI] [PubMed] [Google Scholar]
- 4.Mohan M., Lin C., Guest E., Shilatifard A. (2010) Licensed to elongate: a molecular mechanism for MLL-based leukaemogenesis. Nat. Rev. Cancer 10, 721–728 [DOI] [PubMed] [Google Scholar]
- 5.Muntean A. G., Hess J. L. (2012) The pathogenesis of mixed-lineage leukemia. Annu. Rev. Pathol. 7, 283–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artinger E. L., Mishra B. P., Zaffuto K. M., Li B. E., Chung E. K., Moore A. W., Chen Y., Cheng C., Ernst P. (2013) An MLL-dependent network sustains hematopoiesis. Proc. Natl. Acad. Sci. USA 110, 12000–12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst P., Mabon M., Davidson A. J., Zon L. I., Korsmeyer S. J. (2004) An Mll-dependent Hox program drives hematopoietic progenitor expansion. Curr. Biol. 14, 2063–2069 [DOI] [PubMed] [Google Scholar]
- 8.Gan T., Jude C. D., Zaffuto K., Ernst P. (2010) Developmentally induced Mll1 loss reveals defects in postnatal haematopoiesis. Leukemia 24, 1732–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jude C. D., Climer L., Xu D., Artinger E., Fisher J. K., Ernst P. (2007) Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell Stem Cell 1, 324–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon K. A., Hiew S. Y., Hadjur S., Veiga-Fernandes H., Menzel U., Price A. J., Kioussis D., Williams O., Brady H. J. (2007) Mll has a critical role in fetal and adult hematopoietic stem cell self-renewal. Cell Stem Cell 1, 338–345 [DOI] [PubMed] [Google Scholar]
- 11.Heuser M., Yap D. B., Leung M., de Algara T. R., Tafech A., McKinney S., Dixon J., Thresher R., Colledge B., Carlton M., Humphries R. K., Aparicio S. A. (2009) Loss of MLL5 results in pleiotropic hematopoietic defects, reduced neutrophil immune function, and extreme sensitivity to DNA demethylation. Blood 113, 1432–1443 [DOI] [PubMed] [Google Scholar]
- 12.Madan V., Madan B., Brykczynska U., Zilbermann F., Hogeveen K., Döhner K., Döhner H., Weber O., Blum C., Rodewald H. R., Sassone-Corsi P., Peters A. H., Fehling H. J. (2009) Impaired function of primitive hematopoietic cells in mice lacking the Mixed-Lineage-Leukemia homolog MLL5. Blood 113, 1444–1454 [DOI] [PubMed] [Google Scholar]
- 13.Morgan M. A., Shilatifard A. (2013) Drosophila SETs its sights on cancer: Trr/MLL3/4 COMPASS-like complexes in development and disease. Mol. Cell. Biol. 33, 1698–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salz T., Li G., Kaye F. J., Zhou L., Qiu Y., Huang S. (2014) hSETD1A regulates Wnt target genes and controls tumor growth of colorectal cancer cells. Cancer Res. 74, 775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng C., Li Y., Liang S., Cui K., Salz T., Yang H., Tang Z., Gallagher P. G., Qiu Y., Roeder R., Zhao K., Bungert J., Huang S. (2013) USF1 and hSET1A mediated epigenetic modifications regulate lineage differentiation and HoxB4 transcription. PLoS Genet. 9, e1003524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu D., Gao X., Morgan M. A., Herz H. M., Smith E. R., Shilatifard A. (2013) The MLL3/MLL4 branches of the COMPASS family function as major histone H3K4 monomethylases at enhancers. Mol. Cell. Biol. 33, 4745–4754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardehali M. B., Mei A., Zobeck K. L., Caron M., Lis J. T., Kusch T. (2011) Drosophila Set1 is the major histone H3 lysine 4 trimethyltransferase with role in transcription. EMBO J. 30, 2817–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P., Lin C., Smith E. R., Guo H., Sanderson B. W., Wu M., Gogol M., Alexander T., Seidel C., Wiedemann L. M., Ge K., Krumlauf R., Shilatifard A. (2009) Global analysis of H3K4 methylation defines MLL family member targets and points to a role for MLL1-mediated H3K4 methylation in the regulation of transcriptional initiation by RNA polymerase II. Mol. Cell. Biol. 29, 6074–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel B., Kang Y., Cui K., Litt M., Riberio M. S., Deng C., Salz T., Casada S., Fu X., Qiu Y., Zhao K., Huang S. (2014) Aberrant TAL1 activation is mediated by an interchromosomal interaction in human T-cell acute lymphoblastic leukemia. Leukemia 28, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H. T., Kathrein K. L., Barton A., Gitlin Z., Huang Y. H., Ward T. P., Hofmann O., Dibiase A., Song A., Tyekucheva S., Hide W., Zhou Y., Zon L. I. (2013) A network of epigenetic regulators guides developmental haematopoiesis in vivo. Nat. Cell Biol. 15, 1516–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su I. H., Basavaraj A., Krutchinsky A. N., Hobert O., Ullrich A., Chait B. T., Tarakhovsky A. (2003) Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat. Immunol. 4, 124–131 [DOI] [PubMed] [Google Scholar]
- 22.Degner-Leisso S. C., Feeney A. J. (2010) Epigenetic and 3-dimensional regulation of V(D)J rearrangement of immunoglobulin genes. Semin. Immunol. 22, 346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B. E., Gan T., Meyerson M., Rabbitts T. H., Ernst P. (2013) Distinct pathways regulated by menin and by MLL1 in hematopoietic stem cells and developing B cells. Blood 122, 2039–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma-Gaur J., Torkamani A., Schaffer L., Head S. R., Schork N. J., Feeney A. J. (2012) Noncoding transcription within the Igh distal V(H) region at PAIR elements affects the 3D structure of the Igh locus in pro-B cells. Proc. Natl. Acad. Sci. USA 109, 17004–17009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbánek P., Wang Z. Q., Fetka I., Wagner E. F., Busslinger M. (1994) Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 79, 901–912 [DOI] [PubMed] [Google Scholar]
- 26.Li X., Hu X., Patel B., Zhou Z., Liang S., Ybarra R., Qiu Y., Felsenfeld G., Bungert J., Huang S. (2010) H4R3 methylation facilitates beta-globin transcription by regulating histone acetyltransferase binding and H3 acetylation. Blood 115, 2028–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Testa G., Schaft J., van der Hoeven F., Glaser S., Anastassiadis K., Zhang Y., Hermann T., Stremmel W., Stewart A. F. (2004) A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis 38, 151–158 [DOI] [PubMed] [Google Scholar]
- 28.Hardy R. R., Hayakawa K. (2001) B cell development pathways. Annu. Rev. Immunol. 19, 595–621 [DOI] [PubMed] [Google Scholar]
- 29.Nutt S. L., Urbánek P., Rolink A., Busslinger M. (1997) Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 11, 476–491 [DOI] [PubMed] [Google Scholar]
- 30.Bartholdy B., Matthias P. (2004) Transcriptional control of B cell development and function. Gene 327, 1–23 [DOI] [PubMed] [Google Scholar]
- 31.Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. (1992) RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 [DOI] [PubMed] [Google Scholar]
- 32.Ebert A., McManus S., Tagoh H., Medvedovic J., Salvagiotto G., Novatchkova M., Tamir I., Sommer A., Jaritz M., Busslinger M. (2011) The distal V(H) gene cluster of the Igh locus contains distinct regulatory elements with Pax5 transcription factor-dependent activity in pro-B cells. Immunity 34, 175–187 [DOI] [PubMed] [Google Scholar]
- 33.Bolland D. J., Wood A. L., Johnston C. M., Bunting S. F., Morgan G., Chakalova L., Fraser P. J., Corcoran A. E. (2004) Antisense intergenic transcription in V(D)J recombination. Nat. Immunol. 5, 630–637 [DOI] [PubMed] [Google Scholar]
- 34.Yancopoulos G. D., Alt F. W. (1985) Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell 40, 271–281 [DOI] [PubMed] [Google Scholar]
- 35.Jhunjhunwala S., van Zelm M. C., Peak M. M., Cutchin S., Riblet R., van Dongen J. J., Grosveld F. G., Knoch T. A., Murre C. (2008) The 3D structure of the immunoglobulin heavy-chain locus: implications for long-range genomic interactions. Cell 133, 265–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo C., Gerasimova T., Hao H., Ivanova I., Chakraborty T., Selimyan R., Oltz E. M., Sen R. (2011) Two forms of loops generate the chromatin conformation of the immunoglobulin heavy-chain gene locus. Cell 147, 332–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X., Wang S., Li Y., Deng C., Steiner L. A., Xiao H., Wu C., Bungert J., Gallagher P. G., Felsenfeld G., Qiu Y., Huang S. (2011) Chromatin boundaries require functional collaboration between the hSET1 and NURF complexes. Blood 118, 1386–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salz T., Deng C., Pampo C., Siemann D., Qiu Y., Brown K., Huang S. (2014) Histone methyltransferase hSETD1A is a novel regulator of metastasis in breast cancer. [E-pub ahead of print] Mol. Cancer Res. [DOI] [PubMed] [Google Scholar]
- 39.Horcher M., Souabni A., Busslinger M. (2001) Pax5/BSAP maintains the identity of B cells in late B lymphopoiesis. Immunity 14, 779–790 [DOI] [PubMed] [Google Scholar]
- 40.Bledau A. S., Schmidt K., Neumann K., Hill U., Ciotta G., Gupta A., Torres D. C., Fu J., Kranz A., Stewart A. F., Anastassiadis K. (2014) The H3K4 methyltransferase Setd1a is first required at the epiblast stage, whereas Setd1b becomes essential after gastrulation. Development 141, 1022–1035 [DOI] [PubMed] [Google Scholar]
- 41.Ernst P., Fisher J. K., Avery W., Wade S., Foy D., Korsmeyer S. J. (2004) Definitive hematopoiesis requires the mixed-lineage leukemia gene. Dev. Cell 6, 437–443 [DOI] [PubMed] [Google Scholar]
- 42.Ji Y., Resch W., Corbett E., Yamane A., Casellas R., Schatz D. G. (2010) The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell 141, 419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertrand F. E., Vogtenhuber C., Shah N., LeBien T. W. (2001) Pro-B-cell to pre-B-cell development in B-lineage acute lymphoblastic leukemia expressing the MLL/AF4 fusion protein. Blood 98, 3398–3405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.