Abstract
We have previously shown that TNF–tumor necrosis factor receptor-2/p75 (TNFR2/p75) signaling plays a critical role in ischemia-induced neovascularization in skeletal muscle and heart tissues. To determine the role of TNF-TNFR2/p75 signaling in ischemia-induced inflammation and muscle regeneration, we subjected wild-type (WT) and TNFR2/p75 knockout (p75KO) mice to hind limb ischemia (HLI) surgery. Ischemia induced significant and long-lasting inflammation associated with considerable decrease in satellite-cell activation in p75KO muscle tissue up to 10 d after HLI surgery. To determine the possible additive negative roles of tissue aging and the absence of TNFR2/p75, either in the tissue or in the bone marrow (BM), we generated 2 chimeric BM transplantation (BMT) models where both young green fluorescent protein (GFP)-positive p75KO and WT BM-derived cells were transplanted into adult p75KO mice. HLI surgery was performed 1 mo after BMT, after confirming complete engraftment of the recipient BM with GFP donor cells. In adult p75KO with the WT-BMT, proliferative (Ki67+) cells were detected only by d 28 and were exclusively GFP+, suggesting significantly delayed contribution of young WT-BM cell to adult p75KO ischemic tissue recovery. No GFP+ young p75KO BM cells survived in adult p75KO tissue, signifying the additive negative roles of tissue aging combined with decreased/absent TNFR2/p75 signaling in postischemic recovery.—Sasi, S. P., Rahimi, L., Yan, X., Silver, M., Qin, G., Losordo, D. W., Kishore, R., Goukassian, D. A. Genetic deletion of TNFR2 augments inflammatory response and blunts satellite-cell-mediated recovery response in a hind limb ischemia model.
Keywords: apoptosis, muscle regeneration, proliferation, TNF-TNFR2/p75 signaling
TNF-α, a proinflammatory cytokine, is highly expressed in ischemic tissue (1–5) and plays a critical role in ischemia-induced recovery and regeneration processes in skeletal muscle and heart tissues (6–12). TNF-α mediates activation of divergent intracellular signaling pathways through 2 of its receptors, TNFR1 (p55) and TNFR2 (p75) (13–16). Because p55 signaling mediates cytotoxic effects and p75 facilitates protective effects of TNF-α (17, 18), TNF signaling through its 2 receptors may have opposing actions in the recovery after an ischemic event.
Age-related impairment of postischemic recovery, including decreased expression of angiogenic growth factors (19–22) and inhibition of endothelial cell proliferation and function (19, 23–25), has been documented previously (19, 26–29). Because aging has also been shown to be associated with increased expression of p55 and decreased expression of p75 in human lymphocytes (30), prior studies from our laboratory examined ischemia-induced neovascularization and aging in p75 knockout (p75KO) mice (6). Through this model, we demonstrated that signaling through the p75 receptor plays a critical role in ischemia-induced neovascularization with advanced age via modulation of several angiogenic growth factors (6). The role of ischemia-induced inflammation and skeletal muscle regeneration remains to be characterized.
Monocyte/macrophage accumulation, which produces a variety of cytokines, including TNF-α, is critical for neovascularization in the ischemic tissue and ultimately tissue regeneration (1, 31–33). TNF-α is a potent mediator of inflammatory responses (1, 34, 35) and induces the expression of many angiogenesis-related and immunologically relevant genes through its 2 receptors (36–40). Because aging is associated with a steady decline in immune functions (41, 42) along with increased expression of p55 and decreased expression of p75 (30), the present study examined the specific role of tumor necrosis factor receptor-2/p75 (TNFR2/p75) signaling in ischemia-induced inflammation and skeletal muscle recovery.
We hypothesized that ischemia-induced inflammatory responses are impaired in p75KO mice after hind limb ischemia (HLI) surgery and that p75 deficiency affects satellite-cell activation at the time of ischemic recovery. To test these hypotheses, we studied neutrophil and macrophage infiltration in satellite-cell activation after HLI surgery in young and adult age-matched wild-type (WT) and p75KO mice. We examined a possibility of additive negative roles of tissue aging and the absence of TNFR2/p75, either in tissue or bone marrow (BM), by transplanting green fluorescent protein (GFP)-positive BM-derived cells from young WT and p75KO mice into recipient adult p75KO mice.
MATERIALS AND METHODS
Experimental animal model
Young (4 to 6 wk old) and adult (10 to 12 mo old) male mice used for both HLI and BM transplantation (BMT) studies were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The animals used for this study included young WT (C57BL/6J) and p75KO (B6. 129S2-Tnfrsf1btm1Mwm/J) mice, young WT (C57BL/6J)/GFP+ and p75KO/GFP+ mice, and adult p75KO mice. To create young homozygous p75KO/GFP+ mice, we crossbred young WT/GFP+ with p75KO/GFP− until GFP+ homozygous breeders were obtained. Any GFP−, WT, or heterozygous littermates were excluded. All TNFR2/p75 homozygous knockout and GFP+ mice were genotyped according to Jackson Laboratories’ protocols and guidelines. All animals were handled and housed in accordance with protocols approved by the GeneSys Research Institute Inc. Institutional Animal Care and Use Committee (Boston, MA, USA).
BMT studies
We established 2 chimeric BMT models in which BM-derived mononuclear cells (BM-MNC) from young (4 to 6 wk old) WT/GFP+ and p75KO/GFP+ donors were injected into adult (10 to 12 mo old) p75KO animals. BM cells from young GFP+ donor mice obtained by flushing tibiae and femurs and were subjected to density centrifugation using Histopaque-1083 (Sigma-Aldrich, St. Louis, MO, USA) (43, 44). Isolated BM-MNC at a concentration of 3 × 106 cells per mouse were administered by tail-vein injection into 9 Gy lethally γ-irradiated adult p75KO recipient mice (Supplemental Fig. S1A) (45). To evaluate the engraftment of recipient BM with donor GFP+ cells, BM-MNC as well as peripheral blood mononuclear cells (PBMNC) from the recipient adult p75KO mice were processed for fluorescent-activated cell sorting analysis (Supplemental Fig. S1B–J) 1 and 2 mo after BMT. HLI surgery was performed on the recipient mice 30 d after BMT, at which time the BM of the recipient mice was regenerated by donor GFP+ BM cells (6).
Hind limb surgery and tissue collection
Unilateral HLI models in young WT and p75KO and adult p75KO BMT mice were established by ligation and excision of femoral artery as previously described (6, 46). This model allows observation of the recovery processes after the development of HLI (46). Animals found to exhibit severe infection or pain, i.e., limited mobility, reduced consumption of food and water, and weight loss of 15% or more, were killed immediately. All mice at respective time points were killed using a pentobarbital-based euthanasia solution (200 mg/kg delivered intraperitoneally). Presurgery hind limb (HL) samples at d 0 as well as operated HL muscle samples from both young WT and p75KO genotypes were collected at 1, 3, 7, and 10 d after HLI surgery. HL muscle samples from adult p75KO BMT model studies were collected 28 d after HLI surgery. Muscle samples were bisected completely and divided into 2 parts. One part was fixed overnight in buffered formalin for paraffin embedding. Another part of the ischemic muscle sample was fixed in 4% paraformaldehyde overnight, followed by subsequent 15% and 30% sucrose incubation overnight at 4°C and embedded in optimal cutting temperature compound (Tissue-Tek, Torrance, CA, USA). All animals had free access to food and water before and after surgery.
Histology, immunohistochemistry, and immunofluorescence staining
Paraffin-embedded sections of HL muscle at d 0 (before surgery) and at d 1, 3, 7, and 10 after HLI surgery were processed for hematoxylin and eosin (H&E) staining according to the manufacturer’s protocol (Electron Microscopy Sciences, Hatfield, PA, USA). Macrophage infiltration in the ischemic tissue was assessed by F4/80 staining. In brief, after enzyme pretreatment using proteinase K solution (20 μg/ml), tissue sections were incubated with rat anti-mouse F4/80 monoclonal antibody (AbD Serotec, Raleigh, NC, USA) and then biotinylated secondary antibody, ABC Vectastain (Vector Laboratories, Burlingame, CA, USA), followed by 3,3′-diaminobenzidine (Vector Laboratories) for visualization of F4/80 staining (47, 48) and counterstained with hematoxylin to visualize nuclei. Paraformaldehyde-prefixed and sucrose-treated (15% then 30%, 1 d each) frozen muscle tissue sections obtained at 6 to 8 μm thickness were fixed in cold acetone (4°C) for 10 min (6, 49) and then processed for immunofluorescent staining. Neutrophil infiltration in ischemic tissue after HLI surgery at d 0 (before surgery), and at postsurgery d 1, 3, 7, and 10 was evaluated by staining with myeloperoxidase-1 (MPO-1; Biocare Medical, Concord, CA, USA) antibody and Alexa-Fluor 488 goat anti-rabbit secondary antibody (Life Technologies).
To evaluate satellite-cell activation in ischemic tissue after HLI surgery, frozen sections from d 0 (before surgery) and d 1, 3, 7, and 10 after HLI were stained with polyclonal rabbit Myf5 (C-20) antibody (Santa Cruz Biotechnology, Dallas, TX, USA) in conjunction with rabbit laminin antibody (basement membrane marker) to identify satellite cells on the basis of their anatomic position before and after ischemia. Muscle tissue sections were sequentially stained, first with anti-Myf5 primary antibody followed by Alexa-Fluor 555 goat anti-rabbit secondary antibody (Life Technologies), then with anti-laminin primary antibody followed by Alexa Fluor 488 goat anti-rabbit secondary antibody (Life Technologies). Muscle sections were also separately stained using monoclonal rat anti-mouse neural cell adhesion molecule (NCAM; BD Bioscience, San Jose, CA, USA) and Alexa-Fluor 555 goat anti-rat secondary antibody (Life Technologies).
For BMT studies in adult p75KO mice, paraffin-embedded sections of HL muscle after HLI surgery at d 28 along with respective presurgery controls were processed and evaluated for both proliferation and apoptosis of BM-derived cells in ischemic tissue after BMT. Tissue sections were first stained for rabbit polyclonal Ki67 antibody (Vector Labs) along with Alexa-Fluor 555 goat anti-rabbit secondary antibody (Life Technologies) and ApopTag Red In-Situ Apoptosis TUNEL Kit (Millipore, Billerica, MA, USA) to evaluate proliferation and apoptosis respectively. TO-PRO-3 nuclear staining (Life Technologies) was used in combination with all immunofluorescent staining to visualize cell nuclei.
Imaging and analysis
Post-HLI surgery muscle samples stained with H&E and F4/80 were imaged by light microscopy at ×40 and ×20 magnification, respectively. All immunofluorescent stained muscle slides were imaged using a Laser Scanning Confocal Microscope (Zeiss 510; Carl Zeiss, Oberkochen, Germany). The numbers of TUNEL+ cells and Myf5+ cells were evaluated in at least 4 to 5 animals per group by ImageJ software (v1.40, NIH) in 7 to 8 separate visual fields of 17,689 μm2 per mouse per time point for each genotype (×63 images). The number of MPO-1+ cells was also evaluated in at least 4 to 5 animals per group by ImageJ software in 7 to 8 separate visual fields of 44,100 μm2 per mouse per time point for each genotype (×40 images). Results were plotted as a graph between the mean number of cells and the treatment time point after HLI surgery.
Quantification of central nuclei/muscle fiber
Post-HLI surgery muscle tissue from young WT and p75KO mouse HL were carefully bisected, cross-sectioned through the middle, and fixed in formalin. H&E-stained tissue sections (6 µm) were visualized using a light microscope (Leica Microsystems GmbH, Wetzlar, Germany). Images of the full circumference of the cross sections of the above-mentioned muscles were taken at ×20 magnification across the board, and the average number of muscle fibers per visual field was 85 individual fibers (Nikon Instruments Inc., Melville, NY, USA). Muscle tissue samples from 4 to 5 mice per genotype for WT and p75KO were coded and analyzed by 2 independent laboratory members in a blind study. Results were represented as mean number of central nuclei/muscle fiber.
Statistical analysis
All results were expressed as means ± sem, and plots were obtained from at least 4 to 5 mice per group for each time point. Statistical analysis was performed on the data by 1-way ANOVA (StatView Software, SAS Institute Inc., Middleton, MA, USA). Differences were considered significant at P < 0.05.
RESULTS
Postischemic recovery is diminished in young p75KO mice
Compared to presurgery normal muscle tissue (Fig. 1A and Supplemental Fig. S2), H&E staining of young WT ischemic tissue showed decreased muscle fiber density and increased nonspecific cellular infiltrates on d 3 (Fig. 1C, dotted line). There was a mild inflammatory response, myofibroblastic proliferation, and early immature fibrosis by d 7 (Fig. 1D, dotted line). There was a substantial increase in regenerative activity of muscle tissue by d 10 after HLI, which was evident by a large number of muscle fibers with 1 or more centrally located nuclei (Fig. 1E, black arrows) accompanied by cellular expansion (Fig. 1E, dotted line). This could be attributed to early and mild inflammatory response followed by active muscle regeneration in WT tissue.
Figure 1.
Diminished postischemic recovery in young p75KO mice. Representative ×40 bright field microscopy images for H&E staining in HL muscle tissue from WT and p75KO mice respectively at (A, F) presurgery control, (B, G) d 1 after HLI surgery, (C, H) d 3 after HLI surgery, (D, I) d 7 after HLI surgery and (E, J) d 10 after HLI surgery. Dotted lines indicate in (C) decreased muscle fiber density and increased cellular infiltration, in (D) myofibroblastic proliferation and immature fibrosis, and in (E) intermysial cellular expansion. Small arrows indicate in (E) 1 or more centrally located nuclei in muscle fibers and in (I) residual centrally located nuclei. Arrows in (H) indicate cytoplasmic vacuolization.
Compared to presurgery normal p75KO tissue (Fig. 1F), ischemic young p75KO tissue revealed a significant loss of normal skeletal muscle fiber morphology and features of degeneration such as cytoplasmic vacuolization by d 3 (Fig. 1H, black arrows), and there was a significant loss of muscle fiber that was replaced by cellular infiltration by d 7 and 10 (Fig. 1I, J). Within the cellular infiltrate, there were residual muscle fibers with centralized nuclei on d 7 (Fig. 1I, black arrows) that were no longer observed on d 10 (Fig. 1J). These histologic findings further signify our earlier observations in an identical HLI model, wherein we observed a significant increase in apoptosis in the ischemic HL tissue on d 10 in p75KO mice (6), an indication of impaired recovery in p75KO mice.
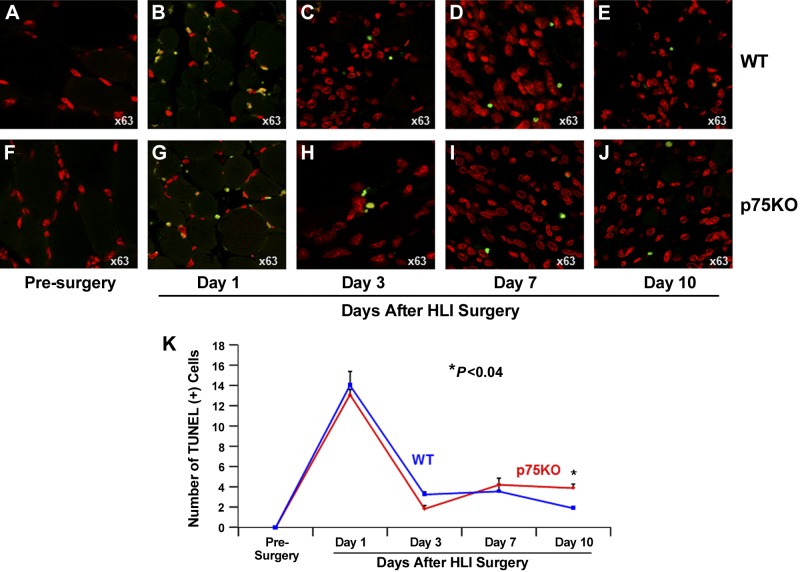
Ischemia-induced apoptosis is comparably increased in both WT and p75KO tissue by d 7 but remains higher in p75KO tissue on d 10 after HLI surgery
To evaluate the apoptotic processes in the ischemic limb of WT and p75KO mice, we immunostained sections of optimal cutting temperature compound–embedded muscle tissue for TUNEL, a marker of apoptosis, and TO-PRO-3 staining to visualize nuclei. No apoptosis was detected in the muscle tissue of WT or p75KO mice before HLI surgery (Fig. 2A, F, K). There were comparable increases in TUNEL+ apoptotic cells in WT and p75KO mice on d 1 through 7 after HLI surgery, with peak increase in apoptosis on d 1 for both genotypes (Fig. 2B–D and Fig. 2G–I, K). Between d 3 and 10, apoptosis was decreased in both WT and p75KO muscle tissue; however, on d 10, the number of apoptotic cells was twice as high in p75KO vs. WT tissue (Fig. 2E, J, K), suggesting that ischemia-induced apoptosis may be augmented in p75KO mice.
Figure 2.
Ischemia-induced apoptosis is increased in the muscle tissue of both WT and p75KO mice after HLI surgery. A–J) Representative ×63 confocal images of double-immunostained ischemic muscle tissue to visualize and quantify apoptotic cells. TUNEL+ cells (green) identify apoptotic cells, and TO-PRO-3+ cells (red) visualize nuclei in WT and p75KO tissue before surgery and on d 1, 3, 7, and 10 after HLI surgery. K) Representative images show significant and comparable increases in the number of TUNEL+ cells in both WT and p75KO tissue on d 1 after HLI surgery. By d 3 and 7, the number of TUNEL+ cell was decreased comparably in mice of both genotypes. However, by d 10, p75KO tissue revealed a small but statistically significant increase in the number of TUNEL+ cells. All results are presented as means ± sem for WT (solid blue line) and p75KO (solid red line) groups. Statistical significance was assigned when P < 0.05.
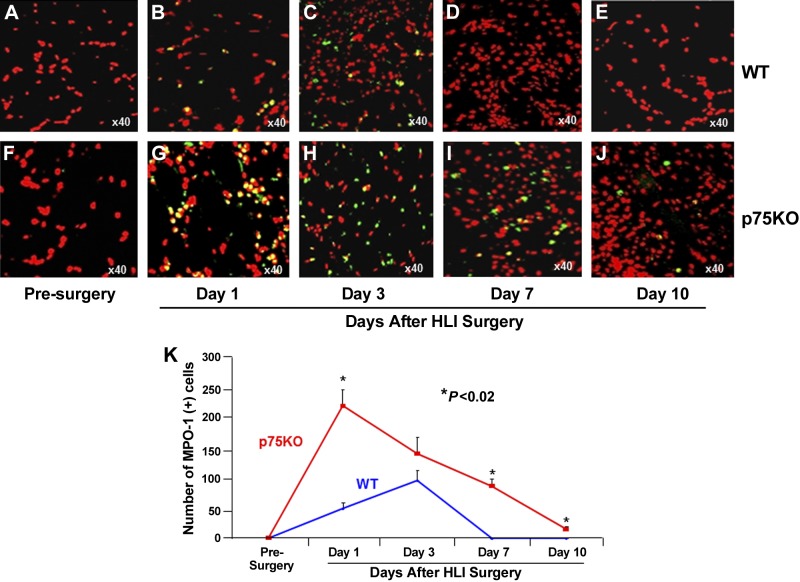
Increased and prolonged neutrophil and macrophage infiltration is evident in young p75KO mice after HLI surgery
To evaluate postischemic inflammatory responses in WT and p75KO mice, HL ischemic muscle sections were stained for MPO-1, a neutrophil marker (Fig. 3 and Supplemental Fig. S3). TO-PRO-3 staining was used to visualize nuclei. WT and p75KO presurgery control muscle tissue did not reveal any MPO-1+ cells (Fig. 3A, F). WT ischemic tissue had a gradual increase in MPO-1+ cells from 1 to 3 d with a peak at d 3 (Fig. 3B, C, K). Compared to WT mice, p75KO ischemic tissue on d 1 demonstrated a significant increase (4.4-fold, ∼340%) in MPO-1+ cells (Fig. 3B, G, K). The MPO-1+ cells in p75KO muscle tissue decreased to ∼47% on d 3 compared with d 1, yet it was still ∼50% higher compared to WT at the same point (Fig. 3C, H, K). By d 7 and 10, neutrophil infiltration returned to presurgery control levels in WT ischemic tissue (Fig. 3D, E, K), while p75KO ischemic tissue continued to exhibit a significantly higher number of MPO-1+ cells during d 7 to 10 (∼100% and ∼25% higher at d 7 and 10, respectively, WT vs. p75KO tissue, Fig. 3I–K).
Figure 3.
Ischemia-induced neutrophil infiltration is increased and longer-lasting in p75KO mice. Representative ×40 confocal microscopy images for immunostaining with neutrophil marker: MPO-1– (green) and TO-PRO-3–stained nuclei (red) in HL muscle tissue from WT and p75KO mice respectively at (A, F) presurgery control, (B, G) d 1 after HLI surgery, (C, H) d 3 after HLI surgery, (D, I) d 7 after HLI surgery, and (E, J) d 10 after HLI surgery. K) Graphic representation of the number of MPO-1+ cells in the HL muscle tissue from WT and p75KO mice before surgery and up to 10 d after HLI surgery. All results are presented as means ± sem; n = 3–5 animals per time point per group for WT (solid blue line) and p75KO (solid red line) groups. Statistical significance was assigned when P < 0.05.
Quantification of F4/80+ cells, a macrophage marker, showed no macrophage infiltration in either p75KO or WT ischemic tissue by d 1 after HLI surgery, which was comparable to respective presurgery control tissues (Fig. 4A, B, F, G, K). By d 3 after HLI surgery, both WT and p75KO ischemic tissues revealed a significant increase in macrophage infiltration with a substantial 5-fold (∼400%) increase in p75KO tissue, when compared to WT (Fig. 4C, H, K). From 7 to 10 d after surgery, both WT and p75KO ischemic tissues had a decreasing trend in F4/80+ cells, with p75KO tissue still showing a significantly higher macrophage infiltration, ∼4- and 5-fold higher at 7 and 10 d, in WT vs. p75KO tissue, respectively (Fig. 4D, E, I, J, K). Taken together, these data suggest that in the absence of TNFR2/p75 signaling, post-HLI surgery inflammatory responses are increased and longer lasting.
Figure 4.
Ischemia-induced macrophage infiltration is increased and long-lasting in young p75KO mice. Representative ×20 bright field microscopy images for immunostaining with macrophage marker: F4/80- (brown) and hematoxylin-stained nuclei (blue) in HL muscle tissue from WT and p75KO mice, respectively at (A, F) presurgery control, (B, G) d 1 after HLI surgery, (C, H) d 3 after HLI surgery, (D, I) d 7 after HLI surgery, and (E, J) d 10 after HLI surgery. K) Graphic representation of the number of F4/80+ cells in the HL muscle tissue isolated from WT and p75KO mice before surgery and up to 10 d after HLI surgery. All results are presented as means ± sem; n = 3–5 animals per time point per group for WT (solid blue line) and p75KO (solid red line) groups. Statistical significance was assigned when P < 0.05.
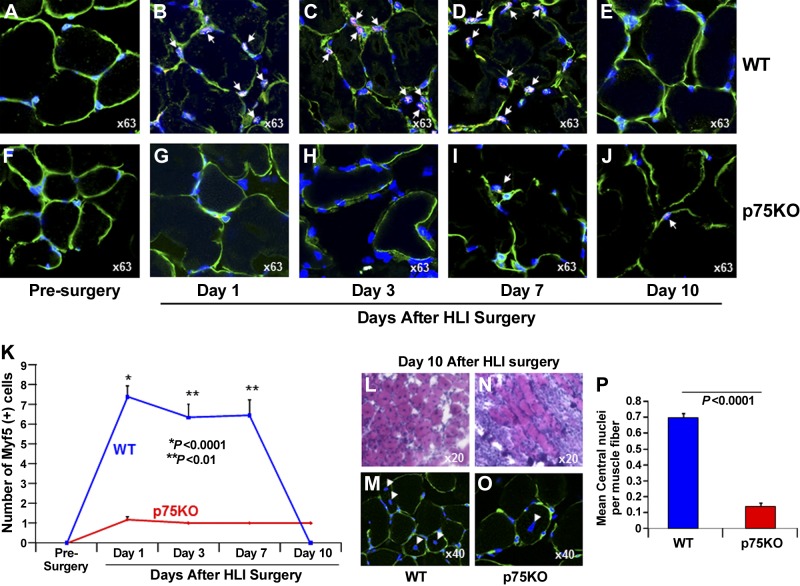
Impaired activation of satellite cells occurs in young p75KO mice after HLI surgery
To evaluate whether the absence of TNFR2/p75 signaling affects satellite-cell activation during postischemic recovery, we double-stained p75KO and WT muscle sections postsurgery with Myf5 (50, 51) and NCAM, markers of satellite-cell (52, 53) and TO-PRO-3, staining to visualize nuclei. Before HLI surgery, the number of NCAM+ cells was similar in WT and p75KO muscle tissue (Supplemental Fig. S4A, F, K), and Myf5+ cells were not detected in muscle tissue from either genotype (Fig. 5A, F, K). Compared to presurgery controls, on d 1, 3, and 7 after HLI surgery, there was a 5- to 6-fold increase in the number of satellite cells in WT HLI muscle tissue, demonstrated by positive staining for both Myf5 and NCAM markers (Fig. 5B–D, K and Supplemental Fig. S4B–D, K). In p75KO ischemic tissue, however, from d 1 through 10, there were occasional satellite cells detectable by Myf5 staining (Fig. 5G–K), whereas no NCAM+ cells were detected in the adjacent section of the same tissue (Supplemental Fig. S4G–K). Satellite cells positive for NCAM marker were detectable only at d 10 in p75KO ischemic tissue (Supplemental Fig. S4J, K).
Figure 5.
Ischemia-induced satellite-cell activation is impaired in young p75KO mice. Representative ×63 confocal microscopy images for triple immunostaining with satellite-cell marker: Myf5 (red), laminin (green) to visualize the basement membrane, and TO-PRO-3 (blue) to identify nuclei in HL muscle tissue from WT and p75KO mice, respectively, at (A, F) presurgery control, (B, G) d 1 after HLI surgery, (C, H) d 3 after HLI surgery, (D, I) d 7 after HLI surgery, and (E, J) d 10 after HLI surgery. White arrows indicate Myf5/TO-PRO-3+ satellite cells in the muscle tissue, and the green laminin staining identifies the basement membrane. K) Graphic representation of number of Myf5+ cells in the HL muscle tissue isolated from WT and p75KO mice before surgery and up to 10 d after HLI surgery. Representative ×20 bright field microscopy images of H&E staining in HL muscle tissue from (L) WT and (N) p75KO mice 10 d after HLI surgery. Representative ×40 confocal images of laminin (green) and TO-PRO-3 (blue) double staining in HL muscle tissue from (M) WT and (O) p75KO to confirm migratory properties and centralized of myonuclei in WT and p75KO muscle fibers on d 10 after HLI surgery. N) Graphic representation of mean number of central nuclei/muscle fiber in the HL muscle tissue isolated from WT and p75KO mice. All results are presented as means ± sem; n = 3–5 animals per time point per group for WT (blue bar) and p75KO (red bar) groups. Statistical significance was assigned when P < 0.05.
The histologic analysis of regenerating muscle tissue after injury may reveal newly formed smaller-size myofibers with centralized myonuclei (54). We quantified the number of muscle fibers with centralized nuclei in WT and p75KO on d 10 after HLI surgery. Post-HLI muscle samples on d 10 from WT mice showed a significant increase (7-fold) in the mean number of centralized nuclei per muscle fiber compared to p75KO at the same time point (Fig. 5L, M vs. Fig. 5N, O and Fig. 5P). Taken together, these findings indicate that ischemia-induced activation of satellite cells and muscle regeneration are impaired in p75KO ischemic muscle tissue, further signifying the role of TNFR2/p75 signaling in postischemic recovery.
Ischemia-induced proliferation is decreased and apoptosis is increased in adult p75KO mice transplanted with young p75KO BM cells
To determine the contribution of young BM-derived WT and p75KO cells to adult ischemic muscle tissue recovery, we created 2 chimeric BMT models (Supplemental Fig. S1A), where BM-MNC from young WT/GFP+ or p75KO/GFP+ mice were transplanted into lethally γ-irradiated adult p75KO mice (Supplemental Fig. S1A). At 4 and 8 wk after BMT, fluorescence-activated cell sorting analysis showed that recipient adult p75KO mice and control GFP mice had a similar percentage of GFP+ cells (Supplemental Fig. S1C–G). Eight weeks after BMT, the number of circulating PBMNC was similar in control GFP mice and BMT recipient mice (Supplemental Fig. S1I, J). Negative control BM-derived cells and PBMNC showed no GFP+ cells (Supplemental Fig. S1B, E, H).
We evaluated the possibility of additive negative roles of tissue aging and the absence of TNFR2/p75, either in the tissue or in the BM cells, using these 2 chimeric BMT models (Fig. 6). In the ischemic tissue of adult p75KO recipients that were transplanted with young WT GFP+ BM cells, there was a significant difference in the number of Ki67+ cells, in GFP+ BM-derived (Fig. 6A–C, arrowheads) vs. GFP− resident cells (Fig. 6B, C, circles) 28 d after HLI. In the muscle tissue of adult p75KO mice transplanted with young p75KO GFP+ BM, the number of Ki67+ cells was exclusively GFP+ (Fig. 6D–F, arrowheads). No Ki67+ resident cells were detected (Fig. 6F). These results suggest an additive negative role of tissue aging and the absence of TNFR2/p75 in BM-derived cells and muscle tissue, which may contribute to impaired proliferation in adult p75KO ischemic skeletal muscle tissue.
Figure 6.
Proliferation of BM-derived and resident muscle cells is decreased in adult p75KO tissue transplanted with young p75KO but not young WT BM cells. Representative ×20 confocal microscopy images of HL muscle tissue from 2 chimeric BMT models: young WT/GFP+ BMT to adult p75KO and young p75KO/GFP+ BMT to adult p75KO mice 28 d after HLI surgery. Representative images of young WT (A) and young p75KO (D) BMT GFP+ cells (green) identified in adult p75KO tissue; Ki67+ (red) cells in adult p75 mice transplanted with young WT (B) and p75KO (E) BM cells. C, F) Triple-overlaid images of GFP+/Ki67+/TO-PRO-3+ immunostained cells. White arrowheads show BM-derived cells transplanted from GFP+ young WT and p75KO donor mice that are also proliferative (Ki67+) in adult p75KO HLI tissue. These are also shown as double-positive yellow-colored cells in the overlaid images. White circles represent GFP− and Ki67+, proliferative, resident adult p75KO cells (magenta) in HLI tissue.
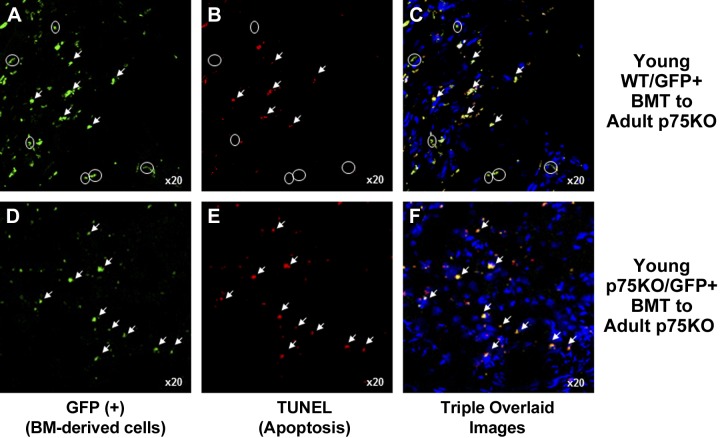
Analysis of apoptosis showed that in the adult p75KO ischemic tissue transplanted with young WT GFP+ donor BM cells (Fig. 7A, arrowheads), there was an equal number of GFP+ BM-derived cells that were both apoptotic (TUNEL+, Fig. 7A–C, arrowheads) and nonapoptotic (TUNEL−; Fig. 7A–C, circles). However, in adult p75KO mice transplanted with young p75KO GFP+ BM cells, none of the GFP+ young p75KO donor BM cells (Fig. 7D, arrowheads) survived in adult p75KO tissue (Fig. 7E, F, arrowheads). Taken together, the Ki67 and TUNEL data showed decreased proliferative activity and increased apoptosis in adult p75KO mice transplanted with young p75KO BM cells. These findings strongly suggest an additive negative role of tissue aging combined with absent TNFR2/p75 signaling in postischemic recovery.
Figure 7.
Young WT but not young p75 BM cells survive in adult p75KO tissue. Representative ×20 confocal microscopy images of HL muscle tissue from 2 chimeric BMT models, young WT/GFP+ BMT to adult p75KO and young p75KO/GFP+ BMT to adult p75KO mice 28 d after HLI surgery. Representative images of young WT (A) and p75KO (D) BMT GFP+ cells (green) identified in adult p75KO tissue; TUNEL+ (red) cells in adult p75 mice transplanted with young WT (B) and young p75KO (E) BM cells. C, F) Triple-overlaid images of GFP+/TUNEL+/TO-PRO-3+ immunostained cells. White arrowheads represent BM-derived cells transplanted from GFP+ young WT and p75KO donor mice that are also apoptotic in adult p75KO HLI tissue, shown as double-positive yellow-colored cells in the overlaid images. A–C) White circles represent GFP+ and TUNEL−, nonapoptotic, BM-derived young p75KO cells (yellow) surviving in adult p75KO HLI tissue.
DISCUSSION
TNF-α mediates expression of various immune and angiogenic genes (36, 37) through ubiquitously expressed tumor necrosis factor receptor-1/p55 (TNFR1/p55) and hematopoietically restricted TNFR2/p75 receptors (55–57) that are known to have opposing effects (17, 18, 39, 40). Alterations in cytokine signaling pathways associated with aging can result in enhanced apoptosis and tissue damage (30, 58). Although the underlying mechanisms of TNFR2/p75 signaling are not clearly understood, previous studies have suggested that this receptor facilitates neuro-, cardio-, and osteoprotective effects (59) and promotes survival after myocardial infarction in mice (7). The protective effect of TNF-α through TNFR2/p75 receptor has been well documented in endothelial and hematopoietic lineage cells (60, 61). Prior studies from our laboratory have shown that TNFR2/p75 signaling mediates several angiogenic growth factors in mice, promoting ischemia-induced angiogenesis (6, 7) and at the same time blocking TNFR2/p75 activity in Lewis lung carcinoma cells, which led to increases in TNF-α mediated apoptosis and tumor growth suppression in mouse models (49). On the other hand, TNFR1/p55-mediated effects have been implicated predominantly in TNF-induced cytotoxicity (59, 62). Upon stimulation with TNF-α, adaptor proteins are recruited to the cytosolic death domain of TNFR1/p55 (59, 63), which is known to mediate cytotoxic effects (64). The additional cytosolic adaptor proteins that are recruited mediate the activation of inflammatory signaling and the expression of TNF-α target genes as a part of the proinflammatory response (59, 63). Our results demonstrated that compared to WT mice, p75KO resulted in increased and long-lasting infiltration of neutrophils and macrophages in p75KO ischemic muscle tissue, with substantial inflammatory infiltrate and significant loss of muscle tissue between d 7 and 10 after HLI surgery. Young WT muscle tissue showed mild and transient inflammatory response during the first 3 d after HLI surgery and significant regenerative activity by d 10. In addition to previously reported findings by our laboratory on impaired neovascularization and increased apoptosis in p75KO young and old mice using HLI and acute myocardial infarct models (7), these results further elaborate a significantly increased and long-lasting ischemia-induced inflammatory response in p75KO vs. WT tissue.
Because TNFR2/p75 facilitates the protective effects of TNF-α and TNFR1/p55 has been shown to modulate the cytotoxic effects, including apoptotic signaling (7, 65), increased muscle damage and impaired inflammatory responses in the young p75KO mice are conceivably due to up-regulated signaling through the p55 receptor, which could result in a microenvironment with increased cytotoxic effects (64). TNFR1/p55 is shown to initiate proinflammatory signaling pathways by activating the proinflammatory NF-κB and MAP kinases in response to TNF-α (59, 62, 66, 67). Earlier in vivo studies by Taubitz et al. have demonstrated the predominant role of p55 in promoting TNF-α–induced leukocyte accumulation by induction of adhesion molecules, chemokines, and proinflammatory mediators, which was absent in p55-knockout mice (68). These observations, together with the fact that TNF-α signaling through TNFR2/p75 promotes proliferation (69) and migration (70) while TNFR1/p55 induces cell death and inflammation (71), further corroborate our findings in young p75KO mice.
Previous studies have demonstrated contradictory roles of proinflammatory cytokines in either inducing or inhibiting the skeletal muscle regeneration and differentiation process (72, 73). Studies using TNF-knockout mice revealed that TNF-α deficiency significantly reduced muscle mass and contributed to impaired muscle regeneration (74). Reports have also shown that TNF-α is a requirement for myogenesis; however, the concentration of this cytokine appears to play an important role in myogenesis process, and high vs. low levels of TNF-α result in contradictory actions regarding differentiation (75). In addition to TNF-α being predominantly produced by activated macrophages, muscle fibers have also been shown to constitutively produce this cytokine (75, 76). Myoblasts synthesize TNF-α during differentiation. In cases of muscle injury, TNF-α expression is up-regulated by both increased myoblast differentiation and macrophage infiltration (77). Although muscle regeneration may be generally successful in a high TNF-α environment (75), studies have shown that exogenously added TNF-α can have an inhibitory effect on myoblast differentiation (78–80). Chen et al. (75) concluded that while temporary increases in TNF-α during differentiation may stimulate myogenesis, prolonged pathologic increases in TNF-α can have an inhibitory effect on muscle regeneration. Earlier findings by our laboratory have demonstrated that blocking or absence of TNFR2/p75 receptor with or without significant increases in systemic levels of TNF-α results in increased apoptosis with subsequent inhibition of ischemia-induced angiogenesis (6, 49). Taken together, our previous and current results suggest that the absence of TNFR2/p75 signaling may impair regenerative capacity of skeletal muscle, specifically activation of satellite cells during postischemic recovery.
Satellite cells are undifferentiated progenitor precursors that carry out myogenesis (81–83) and are responsible for muscle regeneration, maintenance, growth, and regeneration of skeletal muscle cells (84). Satellite cells provide damaged muscle fibers with the extra nuclei needed for initiation of rapid recovery process to prevent cell death, loss of function, and subsequent muscle loss (83). The recovery and ultimately regeneration sequence of damaged muscle tissue involves the initial influx of leukocytes followed by phagocytosis by inflammatory cells (i.e., macrophages) to remove dead tissue to support muscle fiber regeneration (83). Under normal physiologic conditions in adult muscle, satellite cells are mitotically quiescent (83, 85) and are attached to muscle fibers between the basal lamina and sarcolemma (81, 83, 85, 86). Upon muscle injury, satellite cells rapidly up-regulate the expression of myogenic regulatory factor (MRFs) such as Myf5, MyoD, Mrf4, and Myogenin (87). Once activated by injury or exercise, these cells proliferate extensively (81, 88), move to the site of injury, and differentiate into myocytes to replace damaged myofibers by fusing with each other or existing muscle fibers to form regenerating fibers (81, 89). In mouse strains with individual genetic ablation of Myf5, MyoD, Mrf4, and Myogenin (90–92), it was shown that Myf5 and MyoD can independently initiate the myogenic signaling and thus act as myogenic/regeneration determinant genes (93). In our current studies, immunofluorescence staining of the ischemic muscle tissue with Myf5 showed near-complete silencing of Myf5 expression in p75KO mice, strongly suggesting an important role of the TNF-TNFR2/p75 signaling axis in the activation of myogenic/regeneration program in the damaged muscle tissue.
Earlier studies in animal models of ischemia (43, 94, 95) have documented that transplantation of BM cells results in recruitment and retention of these cells to the areas of ischemia. This significantly improves ischemia-induced neovascularization and development of collateral vessels, which subsequently improves blood flow in ischemic regions (43, 94–96). To evaluate the possibility of additive negative roles of tissue aging and the absence of TNFR2/p75 in either the tissue or BM, we used 2 chimeric BMT models: adult p75KO mice transplanted with young WT/GFP+ BM cells, and adult p75KO mice transplanted with young p75KO/GFP+ BM cells. In the adult p75KO-WT/GFP+ model, there was an equal number of proliferative cells from both the donor and recipient—that is, Ki67+/GFP+ and Ki67+/GFP− cells (Fig. 6A–C). In addition, there were increased number of TUNEL−/GFP+ (nonapoptotic cells) young WT/GFP+ BM-derived donor cells that were capable of surviving in the adult p75KO muscle tissue 28 d after HLI surgery (Fig. 7D–F). On the contrary, in the adult p75KO-p75KO/GFP+ model, there were substantially fewer proliferating cells, and these were exclusively GFP+ BM-derived cells (Fig. 6D, E). The inability of resident cells to proliferate even after receiving BMT from young p75KO mice may represent an additive negative role of aging combined with absence of TNFR2/p75, which can ultimately result in muscle loss. In addition, a large number of young p75KO/GFP+ donor BM-derived cells did not survive in adult p75KO ischemic muscle tissue (Fig. 7D–F), suggesting the importance of signaling through TNFR2/p75 in cell survival and recovery of muscle tissue after ischemia. These findings corroborate our earlier work, in which we observed that BM cells from young WT mice helped rescue limbs of 16 to 18-mo-old p75KO mice from ischemia-induced autoamputation (6), and which also validated that functional TNFR2/p75 is required in old WT tissue for efficient postischemic recovery (6).
Our findings are as follows: ischemia-induced recovery in skeletal muscle tissue is impaired in young p75KO mice; ischemia-induced inflammatory responses are significantly increased and long-lasting in p75KO mice; in the absence of TNFR2/p75 signaling, activation of satellite cells is affected in p75KO mice; and during postischemic recovery, tissue aging combined with decreased/absent TNFR2/p75 signaling may play additive negative roles on survival, proliferation, and ultimately regeneration of the damaged muscle tissue.
Supplementary Material
Acknowledgments
The authors thank A. Perepletchikov, clinical pathologist and our collaborator from Steward St. Elizabeth’s Medical Center. Dr. Perepletchikov submitted full histopathologic reports of all H&E-stained samples, analyzed the data, and contributed to the writing of the sections pertaining to these data. The authors would also like to thank A. Kiladjian and J. Schawb, graduate interns in our laboratory, for all their help with frozen tissue sectioning, immunofluorescence staining, confocal microscopy. and data quantification. Supported by U.S. National Institutes of Health (NIH) Grant R21-AG02677, U.S. American Heart Association Grants 0630113N and 14GRNT18860032, and NASA Grant NNJ10ZSA001N to D.A.G.; and by grants from NIH National Heart, Lung, and Blood Institute HL106098 to X.Y. and HL091983 to R.K.
Glossary
- BM
bone marrow
- BM-MNC
BM-derived mononuclear cells
- BMT
BM transplantation
- GFP
green fluorescent protein
- H&E
hematoxylin and eosin
- HL
hind limb
- HLI
HL ischemia
- MPO-1
myeloperoxidase-1
- MRF
myogenic regulatory factor
- NCAM
neural cell adhesion molecule
- p75KO
p75 knockout
- PBMNC
peripheral blood mononuclear cells
- TNFR1/p55
tumor necrosis factor receptor-1/p55
- TNFR2/p75
tumor necrosis factor receptor-2/p75
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Arras M., Ito W. D., Scholz D., Winkler B., Schaper J., Schaper W. (1998) Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J. Clin. Invest. 101, 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu T., Clark R. K., McDonnell P. C., Young P. R., White R. F., Barone F. C., Feuerstein G. Z. (1994) Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 25, 1481–1488 [DOI] [PubMed] [Google Scholar]
- 3.Gu Q., Yang X. P., Bonde P., DiPaula A., Fox-Talbot K., Becker L. C. (2006) Inhibition of TNF-alpha reduces myocardial injury and proinflammatory pathways following ischemia–reperfusion in the dog. J. Cardiovasc. Pharmacol. 48, 320–328 [DOI] [PubMed] [Google Scholar]
- 4.Durán W. N. (2008) The double-edge sword of TNF-alpha in ischemia–reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 295, H2221–H2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sack M. (2002) Tumor necrosis factor-alpha in cardiovascular biology and the potential role for anti-tumor necrosis factor-alpha therapy in heart disease. Pharmacol. Ther. 94, 123–135 [DOI] [PubMed] [Google Scholar]
- 6.Goukassian D. A., Qin G., Dolan C., Murayama T., Silver M., Curry C., Eaton E., Luedemann C., Ma H., Asahara T., Zak V., Mehta S., Burg A., Thorne T., Kishore R., Losordo D. W. (2007) Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation 115, 752–762 [DOI] [PubMed] [Google Scholar]
- 7.Kishore R., Tkebuchava T., Sasi S. P., Silver M., Gilbert H. Y., Yoon Y. S., Park H. Y., Thorne T., Losordo D. W., Goukassian D. A. (2011) Tumor necrosis factor-α signaling via TNFR1/p55 is deleterious whereas TNFR2/p75 signaling is protective in adult infarct myocardium. Adv. Exp. Med. Biol. 691, 433–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monden Y., Kubota T., Inoue T., Tsutsumi T., Kawano S., Ide T., Tsutsui H., Sunagawa K. (2007) Tumor necrosis factor-alpha is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 293, H743–H753 [DOI] [PubMed] [Google Scholar]
- 9.Maddahi A., Kruse L. S., Chen Q. W., Edvinsson L. (2011) The role of tumor necrosis factor-α and TNF-α receptors in cerebral arteries following cerebral ischemia in rat. J. Neuroinflammation 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jasielska M., Semkova I., Shi X., Schmidt K., Karagiannis D., Kokkinou D., Mackiewicz J., Kociok N., Joussen A. M. (2010) Differential role of tumor necrosis factor (TNF)-alpha receptors in the Development of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 51, 3874–3883 [DOI] [PubMed] [Google Scholar]
- 11.Tan J., Weil B. R., Abarbanell A. M., Wang Y., Herrmann J. L., Dake M. L., Meldrum D. R. (2010) Ablation of TNF-alpha receptors influences mesenchymal stem cell–mediated cardiac protection against ischemia. Shock 34, 236–242 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Zhao J., Lau W. B., Jiao L. Y., Liu B., Yuan Y., Wang X., Gao E., Koch W. J., Ma X. L., Wang Y. (2013) Tumor necrosis factor-α and lymphotoxin-α mediate myocardial ischemic injury via TNF receptor 1, but are cardioprotective when activating TNF receptor 2. PLoS ONE 8, e60227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leibovich S. J., Polverini P. J., Shepard H. M., Wiseman D. M., Shively V., Nuseir N. (1987) Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 329, 630–632 [DOI] [PubMed] [Google Scholar]
- 14.Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. (1987) Tumor necrosis factor type α, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc. Natl. Acad. Sci. USA 84, 5277–5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato N., Fukuda K., Nariuchi H., Sagara N. (1987) Tumor necrosis factor inhibiting angiogenesis in vitro. J. Natl. Cancer Inst. 79, 1383–1391 [PubMed] [Google Scholar]
- 16.BenEzra D., Hemo I., Maftzir G. (1990) In vivo angiogenic activity of interleukins. Arch. Ophthalmol. 108, 573–576 [DOI] [PubMed] [Google Scholar]
- 17.Smith C. A., Farrah T., Goodwin R. G. (1994) The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 76, 959–962 [DOI] [PubMed] [Google Scholar]
- 18.Tartaglia L. A., Weber R. F., Figari I. S., Reynolds C., Palladino M. A. Jr., Goeddel D. V. (1991) The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc. Natl. Acad. Sci. USA 88, 9292–9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivard A., Fabre J. E., Silver M., Chen D., Murohara T., Kearney M., Magner M., Asahara T., Isner J. M. (1999) Age-dependent impairment of angiogenesis. Circulation 99, 111–120 [DOI] [PubMed] [Google Scholar]
- 20.Augustin-Voss H. G., Voss A. K., Pauli B. U. (1993) Senescence of aortic endothelial cells in culture: effects of basic fibroblast growth factor expression on cell phenotype, migration, and proliferation. J. Cell. Physiol. 157, 279–288 [DOI] [PubMed] [Google Scholar]
- 21.Beck L. S., DeGuzman L., Lee W. P., Xu Y., Siegel M. W., Amento E. P. (1993) One systemic administration of transforming growth factor-beta 1 reverses age- or glucocorticoid-impaired wound healing. J. Clin. Invest. 92, 2841–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarzani R., Arnaldi G., Chobanian A. V. (1991) Hypertension-induced changes of platelet-derived growth factor receptor expression in rat aorta and heart. Hypertension 17, 888–895 [DOI] [PubMed] [Google Scholar]
- 23.Mogford J. E., Tawil N., Chen A., Gies D., Xia Y., Mustoe T. A. (2002) Effect of age and hypoxia on TGFbeta1 receptor expression and signal transduction in human dermal fibroblasts: impact on cell migration. J. Cell. Physiol. 190, 259–265 [DOI] [PubMed] [Google Scholar]
- 24.Nissen N. N., Polverini P. J., Koch A. E., Volin M. V., Gamelli R. L., DiPietro L. A. (1998) Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 152, 1445–1452 [PMC free article] [PubMed] [Google Scholar]
- 25.Reed M. J., Corsa A. C., Kudravi S. A., McCormick R. S., Arthur W. T. (2000) A deficit in collagenase activity contributes to impaired migration of aged microvascular endothelial cells. J. Cell. Biochem. 77, 116–126 [PubMed] [Google Scholar]
- 26.Edelberg J. M., Reed M. J. (2003) Aging and angiogenesis. Front. Biosci. 8, s1199–s1209 [DOI] [PubMed] [Google Scholar]
- 27.Marinho A., Soares R., Ferro J., Lacerda M., Schmitt F. C. (1997) Angiogenesis in breast cancer is related to age but not to other prognostic parameters. Pathol. Res. Pract. 193, 267–273 [DOI] [PubMed] [Google Scholar]
- 28.Sadoun E., Reed M. J. (2003) Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J. Histochem. Cytochem. 51, 1119–1130 [DOI] [PubMed] [Google Scholar]
- 29.Yamaura H., Matsuzawa T. (1980) Decrease in capillary growth during aging. Exp. Gerontol. 15, 145–150 [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal S., Gollapudi S., Gupta S. (1999) Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J. Immunol. 162, 2154–2161 [PubMed] [Google Scholar]
- 31.Gordon H. M., Kucera G., Salvo R., Boss J. M. (1992) Tumor necrosis factor induces genes involved in inflammation, cellular and tissue repair, and metabolism in murine fibroblasts. J. Immunol. 148, 4021–4027 [PubMed] [Google Scholar]
- 32.Liu Y., Wang L., Kikuiri T., Akiyama K., Chen C., Xu X., Yang R., Chen W., Wang S., Shi S. (2011) Mesenchymal stem cell–based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med. 17, 1594–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faustman D. L., Davis M. (2013) TNF receptor 2 and disease: autoimmunity and regenerative medicine. Front. Immunol. 4, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Squadrito F., Altavilla D., Zingarelli B., Ioculano M., Calapai G., Campo G. M., Miceli A., Caputi A. P. (1993) Tumor necrosis factor involvement in myocardial ischaemia–reperfusion injury. Eur. J. Pharmacol. 237, 223–230 [DOI] [PubMed] [Google Scholar]
- 35.Kondo S., Sauder D. N. (1997) Tumor necrosis factor (TNF) receptor type 1 (p55) is a main mediator for TNF-alpha-induced skin inflammation. Eur. J. Immunol. 27, 1713–1718 [DOI] [PubMed] [Google Scholar]
- 36.Krönke M., Schütze S., Scheurich P., Pfizenmaier K. (1992) TNF signal transduction and TNF-responsive genes. Immunol. Ser. 56, 189–216 [PubMed] [Google Scholar]
- 37.Yoshida S., Ono M., Shono T., Izumi H., Ishibashi T., Suzuki H., Kuwano M. (1997) Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol. Cell. Biol. 17, 4015–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botchkina G. I., Meistrell M. E. III, Botchkina I. L., Tracey K. J. (1997) Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol. Med. 3, 765–781 [PMC free article] [PubMed] [Google Scholar]
- 39.Hoefer I. E., van Royen N., Rectenwald J. E., Bray E. J., Abouhamze Z., Moldawer L. L., Voskuil M., Piek J. J., Buschmann I. R., Ozaki C. K. (2002) Direct evidence for tumor necrosis factor-alpha signaling in arteriogenesis. Circulation 105, 1639–1641 [DOI] [PubMed] [Google Scholar]
- 40.Leeuwenberg J. F., van Tits L. J., Jeunhomme T. M., Buurman W. A. (1995) Evidence for exclusive role in signalling of tumour necrosis factor p55 receptor and a potentiating function of p75 receptor on human endothelial cells. Cytokine 7, 457–462 [DOI] [PubMed] [Google Scholar]
- 41.Ernst D. N., Weigle W. O., Noonan D. J., McQuitty D. N., Hobbs M. V. (1993) The age-associated increase in IFN-gamma synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J. Immunol. 151, 575–587 [PubMed] [Google Scholar]
- 42.Saini A., Sei Y. (1993) Age-related impairment of early and late events of signal transduction in mouse immune cells. Life Sci. 52, 1759–1765 [DOI] [PubMed] [Google Scholar]
- 43.Kalka C., Masuda H., Takahashi T., Kalka-Moll W. M., Silver M., Kearney M., Li T., Isner J. M., Asahara T. (2000) Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 97, 3422–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asahara T., Takahashi T., Masuda H., Kalka C., Chen D., Iwaguro H., Inai Y., Silver M., Isner J. M. (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow–derived endothelial progenitor cells. EMBO J. 18, 3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frasca D., Guidi F., Arbitrio M., Pioli C., Poccia F., Cicconi R., Doria G. (2000) Hematopoietic reconstitution after lethal irradiation and bone marrow transplantation: effects of different hematopoietic cytokines on the recovery of thymus, spleen and blood cells. Bone Marrow Transplant. 25, 427–433 [DOI] [PubMed] [Google Scholar]
- 46.Couffinhal T., Silver M., Zheng L. P., Kearney M., Witzenbichler B., Isner J. M. (1998) Mouse model of angiogenesis. Am. J. Pathol. 152, 1667–1679 [PMC free article] [PubMed] [Google Scholar]
- 47.Akbarshahi H., Menzel M., Posaric Bauden M., Rosendahl A., Andersson R. (2012) Enrichment of murine CD68+ CCR2+ and CD68+ CD206+ lung macrophages in acute pancreatitis–associated acute lung injury. PLoS ONE 7, e42654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham O., Campion S., Perry V. H., Murray C., Sidenius N., Docagne F., Cunningham C. (2009) Microglia and the urokinase plasminogen activator receptor/uPA system in innate brain inflammation. Glia 57, 1802–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasi S. P., Yan X., Enderling H., Park D., Gilbert H. Y., Curry C., Coleman C., Hlatky L., Qin G., Kishore R., Goukassian D. A. (2012) Breaking the “harmony” of TNF-α signaling for cancer treatment. Oncogene 31, 4117–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zammit P. S., Partridge T. A., Yablonka-Reuveni Z. (2006) The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 54, 1177–1191 [DOI] [PubMed] [Google Scholar]
- 51.Biressi S., Bjornson C. R., Carlig P. M., Nishijo K., Keller C., Rando T. A. (2013) Myf5 expression during fetal myogenesis defines the developmental progenitors of adult satellite cells. Dev. Biol. 379, 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kadi F., Charifi N., Denis C., Lexell J. (2004) Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29, 120–127 [DOI] [PubMed] [Google Scholar]
- 53.Renault V., Thornell L. E., Eriksson P. O., Butler-Browne G., Mouly V. (2002) Regenerative potential of human skeletal muscle during aging. Aging Cell 2, 71–78 [DOI] [PubMed] [Google Scholar]
- 54.Cabral A. J. V., Machado V., Farinha R., Cabrita A. (2008) Skeletal muscle regeneration: a brief review. Exp. Pathol. Health Sci. 2, 9–17 [Google Scholar]
- 55.Harrison M. L., Obermueller E., Maisey N. R., Hoare S., Edmonds K., Li N. F., Chao D., Hall K., Lee C., Timotheadou E., Charles K., Ahern R., King D. M., Eisen T., Corringham R., DeWitte M., Balkwill F., Gore M. (2007) Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose. J. Clin. Oncol. 25, 4542–4549 [DOI] [PubMed] [Google Scholar]
- 56.Kulbe H., Thompson R., Wilson J. L., Robinson S., Hagemann T., Fatah R., Gould D., Ayhan A., Balkwill F. (2007) The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 67, 585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charles K. A., Kulbe H., Soper R., Escorcio-Correia M., Lawrence T., Schultheis A., Chakravarty P., Thompson R. G., Kollias G., Smyth J. F., Balkwill F. R., Hagemann T. (2009) The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J. Clin. Invest. 119, 3011–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann J., Haendeler J., Aicher A., Rössig L., Vasa M., Zeiher A. M., Dimmeler S. (2001) Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ. Res. 89, 709–715 [DOI] [PubMed] [Google Scholar]
- 59.Ruspi G., Schmidt E. M., McCann F., Feldmann M., Williams R. O., Stoop A. A., Dean J. L. (2014) TNFR2 increases the sensitivity of ligand-induced activation of the p38 MAPK and NF-κB pathways and signals TRAF2 protein degradation in macrophages. Cell. Signal. 26, 683–690 [DOI] [PubMed] [Google Scholar]
- 60.Jacobsen S. E., Jacobsen F. W., Fahlman C., Rusten L. S. (1994) TNF-alpha, the great imitator: role of p55 and p75 TNF receptors in hematopoiesis. Stem Cells 12Suppl 1, 111–126 [PubMed] [Google Scholar]
- 61.Hohenberger P., Latz E., Kettelhack C., Rezaei A. H., Schumann R., Schlag P. M. (2003) Pentoxifyllin attenuates the systemic inflammatory response induced during isolated limb perfusion with recombinant human tumor necrosis factor-alpha and melphalan. Ann. Surg. Oncol. 10, 562–568 [DOI] [PubMed] [Google Scholar]
- 62.Oshima H., Ishikawa T., Yoshida G. J., Naoi K., Maeda Y., Naka K., Ju X., Yamada Y., Minamoto T., Mukaida N., Saya H., Oshima M. (2014) TNF-alpha/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene 33, 3820–3829 [DOI] [PubMed] [Google Scholar]
- 63.Chandrasekharan U. M., Dechert L., Davidson U. I., Waitkus M., Mavrakis L., Lyons K., Beach J. R., Li X., Egelhoff T. T., Fox P. L., DiCorleto P. E. (2013) Release of nonmuscle myosin II from the cytosolic domain of tumor necrosis factor receptor 2 is required for target gene expression. Sci. Signal. 6, ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong G. H., Goeddel D. V. (1994) Fas antigen and p55 TNF receptor signal apoptosis through distinct pathways. J. Immunol. 152, 1751–1755 [PubMed] [Google Scholar]
- 65.Speeckaert M. M., Speeckaert R., Laute M., Vanholder R., Delanghe J. R. (2012) Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am. J. Nephrol. 36, 261–270 [DOI] [PubMed] [Google Scholar]
- 66.Wajant H. (2009) The role of TNF in cancer. Results Probl. Cell Differ. 49, 1–15 [DOI] [PubMed] [Google Scholar]
- 67.Wajant H., Pfizenmaier K., Scheurich P. (2003) Tumor necrosis factor signaling. Cell Death Differ. 10, 45–65 [DOI] [PubMed] [Google Scholar]
- 68.Taubitz A., Schwarz M., Eltrich N., Lindenmeyer M. T., Vielhauer V. (2013) Distinct contributions of TNF receptor 1 and 2 to TNF-induced glomerular inflammation in mice. PLoS ONE 8, e68167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizoguchi E., Mizoguchi A., Takedatsu H., Cario E., de Jong Y. P., Ooi C. J., Xavier R. J., Terhorst C., Podolsky D. K., Bhan A. K. (2002) Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology 122, 134–144 [DOI] [PubMed] [Google Scholar]
- 70.Kaiser G. C., Polk D. B. (1997) Tumor necrosis factor alpha regulates proliferation in a mouse intestinal cell line. Gastroenterology 112, 1231–1240 [DOI] [PubMed] [Google Scholar]
- 71.Kaiser G. C., Yan F., Polk D. B. (1999) Conversion of TNF alpha from antiproliferative to proliferative ligand in mouse intestinal epithelial cells by regulating mitogen-activated protein kinase. Exp. Cell Res. 249, 349–358 [DOI] [PubMed] [Google Scholar]
- 72.Trendelenburg A. U., Meyer A., Jacobi C., Feige J. N., Glass D. J. (2012) TAK-1/p38/nNFκB signaling inhibits myoblast differentiation by increasing levels of Activin A. Skelet. Muscle 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen S. E., Gerken E., Zhang Y., Zhan M., Mohan R. K., Li A. S., Reid M. B., Li Y. P. (2005) Role of TNF-alpha signaling in regeneration of cardiotoxin-injured muscle. Am. J. Physiol. Cell Physiol. 289, C1179–C1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spencer M. J., Marino M. W., Winckler W. M. (2000) Altered pathological progression of diaphragm and quadriceps muscle in TNF-deficient, dystrophin-deficient mice. Neuromuscul. Disord. 10, 612–619 [DOI] [PubMed] [Google Scholar]
- 75.Chen S. E., Jin B., Li Y. P. (2007) TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am. J. Physiol. Cell Physiol. 292, C1660–C1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuru S., Inukai A., Kato T., Liang Y., Kimura S., Sobue G. (2003) Expression of tumor necrosis factor-alpha in regenerating muscle fibers in inflammatory and non-inflammatory myopathies. Acta Neuropathol. 105, 217–224 [DOI] [PubMed] [Google Scholar]
- 77.Li Y. P., Schwartz R. J. (2001) TNF-alpha regulates early differentiation of C2C12 myoblasts in an autocrine fashion. FASEB J. 15, 1413–1415 [DOI] [PubMed] [Google Scholar]
- 78.Guttridge, D. C., Mayo, M. W., Madrid, L. V., Wang, C. Y., and Baldwin, A. S., Jr. (2000) NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science289, 2363–2366 [DOI] [PubMed] [Google Scholar]
- 79.Langen R. C., Schols A. M., Kelders M. C., Wouters E. F., Janssen-Heininger Y. M. (2001) Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 15, 1169–1180 [DOI] [PubMed] [Google Scholar]
- 80.Langen R. C., Schols A. M., Kelders M. C., Van Der Velden J. L., Wouters E. F., Janssen-Heininger Y. M. (2002) Tumor necrosis factor-alpha inhibits myogenesis through redox-dependent and -independent pathways. Am. J. Physiol. Cell Physiol. 283, C714–C721 [DOI] [PubMed] [Google Scholar]
- 81.Capkovic K. L., Stevenson S., Johnson M. C., Thelen J. J., Cornelison D. D. (2008) Neural cell adhesion molecule (NCAM) marks adult myogenic cells committed to differentiation. Exp. Cell Res. 314, 1553–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi X., Garry D. J. (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev. 20, 1692–1708 [DOI] [PubMed] [Google Scholar]
- 83.Hill M., Wernig A., Goldspink G. (2003) Muscle satellite (stem) cell activation during local tissue injury and repair. J. Anat. 203, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuang S., Rudnicki M. A. (2008) The emerging biology of satellite cells and their therapeutic potential. Trends Mol. Med. 14, 82–91 [DOI] [PubMed] [Google Scholar]
- 85.Grounds, M. D., White, J. D., Rosenthal, N., and Bogoyevitch, M. A. (2002) The role of stem cells in skeletal and cardiac muscle repair. J. Histochem. Cytochem.50, 589–610 [DOI] [PubMed]
- 86.Bischoff R. (1989) Analysis of muscle regeneration using single myofibers in culture. Med. Sci. Sports Exerc. 21(5, Suppl)S164–S172 [PubMed] [Google Scholar]
- 87.Pownall M. E., Gustafsson M. K., Emerson C. P. Jr (2002) Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu. Rev. Cell Dev. Biol. 18, 747–783 [DOI] [PubMed] [Google Scholar]
- 88.Crameri R. M., Langberg H., Magnusson P., Jensen C. H., Schrøder H. D., Olesen J. L., Suetta C., Teisner B., Kjaer M. (2004) Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J. Physiol. 558, 333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schultz E., McCormick K. M. (1994) Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 123, 213–257 [DOI] [PubMed] [Google Scholar]
- 90.Braun T., Rudnicki M. A., Arnold H. H., Jaenisch R. (1992) Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71, 369–382 [DOI] [PubMed] [Google Scholar]
- 91.Kassar-Duchossoy L., Gayraud-Morel B., Gomès D., Rocancourt D., Buckingham M., Shinin V., Tajbakhsh S. (2004) Mrf4 determines skeletal muscle identity in Myf5:Myod double-mutant mice. Nature 431, 466–471 [DOI] [PubMed] [Google Scholar]
- 92.Valdez M. R., Richardson J. A., Klein W. H., Olson E. N. (2000) Failure of Myf5 to support myogenic differentiation without myogenin, MyoD, and MRF4. Dev. Biol. 219, 287–298 [DOI] [PubMed] [Google Scholar]
- 93.Haldar M., Karan G., Tvrdik P., Capecchi M. R. (2008) Two cell lineages, myf5 and myf5-independent, participate in mouse skeletal myogenesis. Dev. Cell 14, 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi T., Kalka C., Masuda H., Chen D., Silver M., Kearney M., Magner M., Isner J. M., Asahara T. (1999) Ischemia- and cytokine-induced mobilization of bone marrow–derived endothelial progenitor cells for neovascularization. Nat. Med. 5, 434–438 [DOI] [PubMed] [Google Scholar]
- 95.Kamihata H., Matsubara H., Nishiue T., Fujiyama S., Tsutsumi Y., Ozono R., Masaki H., Mori Y., Iba O., Tateishi E., Kosaki A., Shintani S., Murohara T., Imaizumi T., Iwasaka T. (2001) Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 104, 1046–1052 [DOI] [PubMed] [Google Scholar]
- 96.Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S. M., Li B., Pickel J., McKay R., Nadal-Ginard B., Bodine D. M., Leri A., Anversa P. (2001) Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.