Abstract
Akt signaling regulates diverse physiologies in a wide range of organisms. We examine the impact of increased Akt signaling in the fat body of 2 mosquito species, the Asian malaria mosquito Anopheles stephensi and the yellow fever mosquito Aedes aegypti. Overexpression of a myristoylated and active form of A. stephensi and Ae. aegypti Akt in the fat body of transgenic mosquitoes led to activation of the downstream signaling molecules forkhead box O (FOXO) and p70 S6 kinase in a tissue and blood meal–specific manner. In both species, increased Akt signaling in the fat body after blood feeding significantly increased adult survivorship relative to nontransgenic sibling controls. In A. stephensi, survivorship was increased by 15% to 45%, while in Ae. aegypti, it increased 14% to 47%. Transgenic mosquitoes fed only sugar, and thus not expressing active Akt, had no significant difference in survivorship relative to nontransgenic siblings. Expression of active Akt also increased expression of fat body vitellogenin, but the number of viable eggs did not differ significantly between transgenic and nontransgenic controls. This work demonstrates a novel mechanism of enhanced survivorship through increased Akt signaling in the fat bodies of multiple mosquito genera and provides new tools to unlock the molecular underpinnings of aging in eukaryotic organisms.—Arik, A. J., Hun, L. V., Quicke, K., Piatt, M., Ziegler, R., Scaraffia, P. Y., Badgandi H., Riehle, M. A. Increased Akt signaling in the mosquito fat body increases adult survivorship.
Keywords: Aedes aegypti, aging, Anopheles stephensi, insulin signaling, vitellogenin
Akt is a critical signaling molecule operating in multiple signaling pathways, including the insulin/insulin growth factor 1 signaling (IIS) and target of rapamycin (TOR) cascades that regulate diverse physiologies in a wide range of eukaryotic organisms (1). One of the most intriguing and least understood physiologic processes regulated by Akt is its impact on survivorship and aging. Disruption of the IIS cascade extends life span in a variety of model organisms, whereas overstimulation of the IIS cascade typically reduces life span (2–4). It has been demonstrated that this life span extension is tissue dependent, although the specific tissues involved vary within and across genera (2, 5–7). In Drosophila melanogaster, the fat body, nervous system, and gut are key IIS centers regulating life span. In the nervous system, 3 insulin-like peptides (ILPs) expressed in medial neurosecretory cells of the brain—dILP2, 3, and 5—have been shown to influence life span, with ablation of the medialneurosecretory cells expressing these ILPs leading to life span extension (8, 9). In the fat body of Drosophila, overexpression of dFOXO (forkhead box O), a transcription factor phosphorylated and inactivated by Akt, led to significant increases in life span (10, 11). It has also been argued that the Drosophila midgut is a key IIS tissue regulating life span (12). In Caenorhabditis elegans, reduction of IIS and ablation of the germ-line extended life span by increasing DAF-16/FOXO activity (13). Phosphorylation of DAF-16/FOXO by the IIS cascade inhibits DAF-16/FOXO activity by preventing its movement into the nucleus, whereas reduced IIS allows unphosphorylated DAF-16/FOXO to translocate into the nucleus and transcribe target genes, resulting in life span extension (1). Mutations in DAF-16/FOXO reverse the life span extension phenotype caused by reduced IIS (14). Furthermore, disruption of IIS and overexpression of the IIS antagonists FOXO and phosphatase and tensin homolog (PTEN) in specific tissues such as fat body and intestine led to significant extensions in life span (10, 11, 15). The importance of IIS on aging extends into vertebrates as well. In mice, deletion of the insulin receptor in adipose tissue led to a significant increase in life span (16). More recently, deletion of mouse insulin receptor substrate 1 significantly extended the life span of both male and female mice (17, 18).
In mosquitoes, the midgut is an IIS control center that, among other things, regulates aging (19). Exogenous insulin from the vertebrate blood meal activates the midgut IIS cascade and shortens mosquito life span. Similarly, overexpression of active Akt in the mosquito midgut significantly reduced adult life span (20), while overexpression of the IIS inhibitor PTEN significantly extended life span (21). However, the effects of manipulating IIS in other mosquito tissues have not yet been investigated.
We explored the impact of increased Akt signaling in the mosquito fat body. The fat body in mosquitoes is analogous to human liver and adipose tissue, and it plays a critical role in immunity, reproduction, and metabolism (22). Work with Drosophila (10, 11) has indicated that the fat body is also likely to be a key regulator of mosquito aging. IIS signaling, including Akt, in the mosquito fat body also controls reproduction and lipid metabolism (22, 23). Insulin-like peptides are essential for both vitellogenesis in the fat body and ecdysteroid production in ovarian follicle cells (24). However, the impact of fat body Akt signaling on adult survivorship has not yet been explored. We engineered an active variant of Akt into 2 mosquito species, the Asian malaria mosquito Anopheles stephensi and the yellow fever mosquito Aedes aegypti. We regulated its expression in a fat body– and blood meal–dependent manner and examined the effects of increased Akt activity on downstream signaling molecules, survival, and reproduction.
MATERIALS AND METHODS
Mosquito rearing
A. stephensi (Liston) and Ae. aegypti (UGAL) mosquitoes were reared as described previously (20). Heterozygous transgenic (TG) mosquito lines were outcrossed with wild-type colony mosquitoes each generation to enhance genetic diversity, resulting in a 50:50 mix of heterozygous TG and nontransgenic (NTG) sibling mosquitoes reared together under identical conditions. TG and NTG mosquitoes were separated at the pupal stage using an Olympus SZX10 fluorescent stereomicroscope (Olympus, Tokyo, Japan) and appropriate filters for detecting enhanced green fluorescent protein (EGFP). For line maintenance and experiments, female mosquitoes were provided human blood (American Red Cross; IBC protocol 2010-014) via artificial membrane feeders.
Generation of TG mosquitoes with increased IIS in the fat body
A. stephensi and Ae. aegypti TG lines were generated that expressed a modified Akt protein regulated by the fat body and blood meal–specific vitellogenin (VG) promoter. The modified, active AsteAkt was generated by adding a myristoylation sequence and HA epitope to the amino and carboxyl termini of AsteAkt, respectively, as described in Corby-Harris et al. (20). A similar construct was generated for AaegAkt using an identical myristoylation sequence and HA epitope. The myr-AsteAkt-HA and myr-AaegAkt-HA genes were linked to VG promoters from A. stephensi [from Dr. Tony James, University of California (UC)-Irvine, CA, USA] and Ae. aegypti (from Dr. Alex Raikhel, UC-Riverside, CA, USA), respectively, on the 5′ end and the SV40 UTR at the 3′ end (25, 26). The complete construct was ligated into the pBac[3xP3-EGFPafm] vector (26) using AscI. The final constructs (Fig. 1A) were sent to the University of Maryland Biotechnology Institute, Insect Transformation Facility (UMBI-ITF), and transformed into the respective mosquito genomes. A total of 8 EGFP-positive A. stephensi lines and a single Ae. aegypti line were generated. All 8 A. stephensi lines had similar blood meal–induced transgene expression patterns (Supplemental Fig. S1), and we selected a representative line (M9) for subsequent studies. Transgene insertion sites in the mosquito genome were identified by inverse PCR following the protocol of Buchholz et al. (Exelxis, Inc., South, San Francisco, CA, USA; http://flystocks.bio.indiana.edu/pdfs/Exel_links/5__fly_iPCR_piggyBac_pub.pdf). Life span and reproduction experiments were only initiated after outcrossing the TG lines to their respective wild-type laboratory strain a minimum of 5 generations.
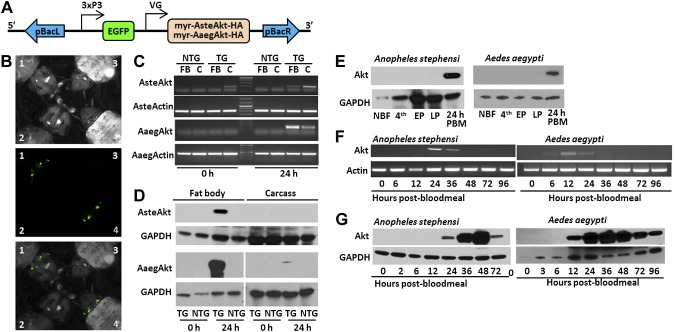
Figure 1.
Generation and expression profiles of TG A. stephensi and Ae. aegypti mosquitoes with increased IIS in fat body. A) Schematic of construct engineered into TG mosquito lines. Myristoylated Akts were linked to an HA epitope to facilitate protein identification and engineered downstream of the A. gambiae or Ae. aegypti VG (VG) promoter. The EGFP fluorescent marker was linked to the synthetic 3XP3 promoter to drive expression in the eyes and nervous system. These 2 genes were flanked by the left and right arms of the piggyback (pBac) transposon. B) Example of EGFP expression in the eyes of larval TG Ae. aegypti (1) and A. stephensi (4) compared to NTG Ae. aegypti (2) and A. stephensi (3). The top panel shows the larvae under white light, the middle with a green fluorescent protein filter, and the third a merging of the top 2. C) Transcript expression in the fat body (FB) and carcass (C) of TG and NTG mosquitoes before blood feeding (0 hours) and 24 hours after blood feeding (24 hours). Actin was used as a positive control. D) Protein expression in the fat body and carcass of TG and NTG mosquitoes before blood feeding (0 hours) and 24 hours after blood feeding (24 hours). E) Expression profile of myr-AsteAkt-HA protein during mosquito development. Non-blood-fed adult females (NBF), fourth-instar larva (4th), early pupae (EP), late pupae (LP), and 24 hours after blood meal for A. stephensi (AsteAkt) and Ae. aegypti (AaegAkt). F) Postblood meal time course of transcript expression in the fat body of adult TG A. stephensi females utilizing myr-AsteAkt-specific primers (top) showed that transcript expression occurred from 12 to 36 hours after blood meal. AsteActin-specific primers were used as a positive control. Postblood meal time course of protein expression in the fat bodies of TG A. stephensi females (bottom) shows that myr-AsteAkt-HA protein is first present 24 hours after blood meal, reaches maximal expression between 36 and 48 hours after blood meal, and begins to decline by 72 hours after blood meal. GAPDH protein expression was used as a loading control. G) Postblood meal time course of transcript expression in the fat body of TG Ae. aegypti mosquitoes utilizing myr-AaegAkt-specific primers (top) showed that expression occurred at 12 and 24 hours after blood meal. AaegActin-specific primers were used as positive control. Postblood meal time course of protein expression in the fat bodies of TG A. stephensi females (bottom) shows that myr-AsteAkt-HA protein is first present 12 hours after blood meal, reaches maximal expression between 24 and 48 hours after blood meal, and begins to decline by 96 hours after blood meal. GAPDH was used as a loading control for protein analysis. All transcript and protein expression studies were replicated a minimum of 3 times.
Transcript and protein expression analysis
Tissue samples were dissected from TG and NTG mosquitoes at a variety of developmental and physiologic stages, transferred to RNAlater (Life Technologies, Grand Island, NY, USA) for RNA isolation or a ×10 solution of Complete Protease/PhosStop protease Inhibitor (Roche Diagnostics, Indianapolis, IN, USA) for protein isolation, and stored at −80°C until processed. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA) and DNase treated (Fermentas, Thermo Scientific, West Palm Beach, FL, USA) to remove contaminating genomic DNA. cDNA was prepared from DNase-treated RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Woburn, MA, USA).
The cDNA samples were PCR amplified using myr-AsteAkt-HA- or myr-AaegAkt-HA-specific primers to detect the transgenes in fat bodies and carcasses (myr-AsteAkt-HA for: 5′-TTACCGGTGAAAGTGTGGAGCTGA-3′; myr-AsteAkt-HA rev:5′-CCATACGATGTGCCAGATTACGCT-3′; myr-AaegAkt-HA for:5′-TCGACCTCGAGTTTACCACTCCCTAT-3′; myr-AaegAkt-HA rev:5′-TCGTCGCGCAGAATGAAATAACGC-3′). Positive controls to confirm amplification were carried out with A. stephensi- and Ae. aegypti-specific actin primers (AsteActin for: 5′-AGCGTGGTATCCTGACGCTGAAAT-3′; AsteActin rev:5′-AACCTTCGTAGATCGGCACGGTAT-3′; AaegActin for:5′-AAGGCCAACCGTGAGAAGATGACT-3′; AaegActin rev:5′-GAAACGCTCGTTGCCAATGGTGAT-3′). Negative controls to detect genomic DNA contamination were performed on DNase-treated RNA samples using actin primers.
Immunoblot analysis was performed to assess protein expression patterns of myr-Akt and the phosphorylation status of downstream IIS molecules as described previously (20). Primary antibodies were used at the following concentrations: HA 1:5000 (Pierce); antiphospho-FOXO 1:50,000 (Cell Signaling Technologies, Danvers, MA, USA); anti-VG (1:12,500 dilution; Brown Laboratory, University of Georgia, Athens, GA, USA), and anti–p70 S6 kinase (p70S6k; 1:2000, Cell Signaling Technologies). A primary antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:10,000 dilution) was used as a loading control.
Life span studies
TG mosquitoes heterozygous for the myr-AsteAkt-HA or myr-AaegAkt-HA constructs were mated with NTG mosquitoes to generate 50% heterozygous TG and 50% NTG sibling mosquitoes reared together under identical conditions. Four treatments were established for each study: blood-fed TG, blood-fed NTG, sugar-fed TG, and sugar-fed NTG. Sugar-fed treatments served as controls because mosquitoes not receiving a blood meal did not express the transgene. For the blood meal treatments, A. stephensi mosquitoes were provided with human blood 3 times a week (Monday, Wednesday, and Friday) via membrane feeders and Ae. aegypti twice weekly (Monday and Thursday) due to more persistent expression of the transgene. Oviposition substrates were present in the blood-fed treatments at all times after the first blood feeding and replaced every 48 hours. Mosquitoes in all treatments were allowed to feed on 10% dextrose ad libitum. Dead females were counted and removed daily. Survivorship and mortality curves were generated for each experiment following Promislow et al. (27). In brief, daily survivorship (Nx) was calculated by determining the total number of dead mosquitoes each day (dx) divided by the initial cohort (N0) for each experiment. To obtain the mortality curve, we determined the probability of surviving 1 day (Px) and calculated mortality rate (µx) as µx = −ln (Px), followed by the ln(µx) transformation. Survival curves were conducted by the Kaplan-Meier method, and significant differences were detected using the Wilcoxon test as described previously (19). These experiments were replicated a minimum of 3 times.
Reproduction studies
Adult female mosquitoes from a heterozygous TG/wild-type cross were separated into 2 cages: NTG and TG females. Both treatments were provided with whole human blood via membrane feeders. Engorged females were collected, and those that did not feed or partially fed were discarded. At 48 hours after feeding, previously engorged females were placed into individual cages with an oviposition cup and allowed to oviposit for 48 hours (48 to 96 hours after blood meal). Oviposition cups were subsequently removed and eggs counted by hand under a light microscope. This procedure was repeated for 2 to 3 gonotrophic cycles and replicated 3 times.
RESULTS
Generation and validation of myr-Akt TG mosquito lines
TG mosquitoes expressing the Akt transgene in fat body tissue were generated for both Ae. aegypti and A. stephensi mosquitoes (Fig. 1B) at the UMBI-ITF. For the VG-myr-AaegAkt-HA construct, a total of 510 Ae. aegypti embryos were injected, resulting in 193 surviving adults (38%) and a single TG line. For the VG-myr-AsteAkt-HA construct, a total of 1,091 A. stephensi embryos were injected, resulting in 100 surviving adults (9%) and a total of 8 TG A. stephensi lines. All lines were established at the University of Arizona and maintained as heterozygous lines by outcrossing the mosquitoes every generation to the appropriate wild-type colony to increase genetic diversity.
To verify transgene expression, we assessed transcript and protein expression levels throughout mosquito development, in various mosquito tissues, and throughout a gonotrophic cycle for both the single Ae. aegypti line and the A. stephensi M9 line (all 8 A. stephensi lines shared similar expression patterns [Supplemental Fig. S1], and M9 was selected as a representative line). Importantly, transgenes were expressed in a blood meal–specific manner consistent with known expression patterns of the VG promoters (25, 26). The myr-AaegAkt-HA and myr-AsteAkt-HA transcripts were predominantly expressed in the fat body of blood-fed, adult mosquitoes, although some transcript was detected in the carcass, likely as a result of small amounts of fat body in the thorax and head (Fig. 1C). Interestingly, a small amount of transgene transcript was observed in the carcasses, but not the fat body, of non-blood-fed A. stephensi M9 mosquitoes. In contrast, myr-AaegAkt-HA and myr-AsteAkt-HA proteins were only expressed in the fat body (Fig. 1D). They were not detected in fourth instar larvae, early pupae, late pupae, or non-blood-fed adult female mosquitoes (Fig. 1E). After a blood meal, transgene transcript expression in A. stephensi mosquitoes was first observed at 12 h after blood meal (Fig. 1F), whereas protein expression first occurred at ∼24 hours after blood meal, peaked at 48 h, and was present at low levels at 72 hours after blood meal (Fig. 1G). For Ae. aegypti mosquitoes, both transgene transcript and protein were first observed by 12 h after blood meal (Fig. 1F). In contrast to the A. stephensi transgene, the Ae. aegypti myr-AaegAkt-HA protein persisted considerably longer, up to at least 96 hours after blood meal (Fig. 1G). In summary, both transgenes were expressed in a blood meal– and fat body–specific manner, with transcript and protein expression beginning 12 to 24 hours after the blood meal, peaking by 36 to 48 hours, and persisting through the reproductive cycle.
Identification of transgene insertion sites using inverse PCR
Inverse PCR and sequencing of the myr-AsteAkt-HA genomic integration site in the A. stephensi M9 line demonstrated that the transgene inserted into a TTAA sequence, the preferred site of piggyBac transposition (Supplemental Fig. S2A). A single insertion site was identified in the M9 line. BLAST analyses of the 5′ and 3′ sequences flanking the insertion site identified identical sequences in the A. stephensi genome. Most importantly, the insertion site was not associated with any predicted gene in the A. stephensi genome annotation and was not identified in A. stephensi transcript databases. This indicates that the transgene was inserted into an intergenic region, minimizing any potential fitness costs due to transgene insertion.
Likewise, inverse PCR of the myr-AaegAkt genomic integration site verified its insertion into a TTAA site (Supplemental Fig. S2B). Although an accurate sequence could not be identified for the 3′ end of the insertion, BLAST analysis of the 5′ sequence revealed an exact match to the Ae. aegypti genome. As with A. stephensi, the insertion site did not disrupt any predicted genes, nor was the sequence found in Ae. aegypti transcript databases.
Activation of downstream signaling molecules by the myr-Akt transgene
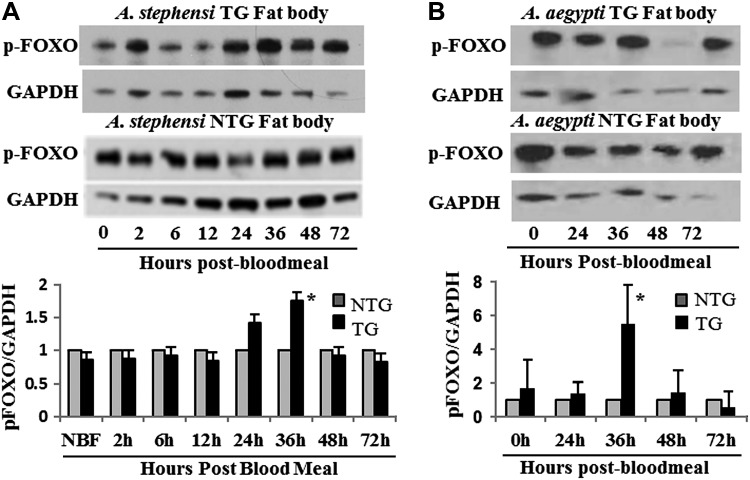
Active Akts expressed in Ae. aegypti and A. stephensi were predicted to activate the downstream signaling molecules FOXO and p70S6k. The transcription factor FOXO is directly downstream of and phosphorylated by Akt. When phosphorylated by Akt, FOXO cannot enter the nucleus to transcribe its target genes and is thus inactivated. We observed a significant increase in AsteFOXO and AaegFOXO phosphorylation in the respective TG lines 36 hours after blood meal relative to nontransgenic (NTG) controls (Fig. 2). This timing corresponds with high levels of myr-Akt expression in the fat body and demonstrates that expression of myr-Akt in the fat body overstimulates the IIS cascade in this tissue, as expected. A consistent, but nonsignificant, increase in FOXO phosphorylation was also observed 24 hours after blood meal in A. stephensi TG mosquitoes. Akt also activates TOR signaling by inhibiting the Tsc complex, leading to the phosphorylation and activation of p70S6 kinase (p70S6k). Although not as strongly stimulated, a significant increase in phosphorylated p70S6k was observed in Ae. aegypti TG mosquitoes 24 hours after blood meal compared to NTG controls (Supplemental Fig. S3B). TG A. stephensi had a modest, but not significant, increase in p70S6k phosphorylation at 24 hours after blood meal (Supplemental Fig. S3A).
Figure 2.
Phosphorylation of the downstream IIS signaling molecule FOXO by myr-Akt. A) Fat bodies from 3 to 5 days old TG and NTG A. stephensi females were dissected to assess the phosphorylation of the downstream IIS molecule FOXO (pFOXO). Relative levels of pFOXO were measured by immunoblotting using pFOXO and GAPDH (control) antibodies (top). Shown is a representative immunoblot showing pFOXO levels throughout a reproductive cycle. In the bottom graph, the relative levels of pFOXO (determined by the ratio of pFOXO to GAPDH controls) are shown. To highlight differences between TG and NTG samples, pFOXO levels in the NTG controls were normalized to 1. B) Levels of pFOXO in TG and NTG Ae. aegypti are shown in a representative immunoblot and densitometry analysis graph as described in (A). Each experiment was replicated a minimum of 3 times. Asterisks above bar in each graph indicates a significant difference (P < 0.05) between TG and NTG relative to GAPDH control.
Effect of myr-AsteAkt-HA expression on mosquito survival
It was hypothesized that overexpression of an active Akt and subsequent stimulation of downstream signaling molecules in the fat body would result in a significant decrease in the life span of blood-fed TG mosquitoes versus NTG mosquitoes. Surprisingly, we found that in both Ae. aegypti and A. stephensi, myr-Akt expression significantly extended adult survivorship of TG mosquitoes relative to NTG siblings. In 4 replicated A. stephensi and 5 of 6 replicated Ae. aegypti survival experiments, we observed that TG females fed on blood multiple times each week, and thus with nearly constant induction of the IIS cascade by myr-Akt, survived significantly longer than their NTG siblings reared under identical conditions (Fig. 3A, C; Tables 1 and 2; survivorship and mortality curves for all A. stephensi and Ae. aegypti blood-fed replicates can be found in Supplemental Figs. S4A and S5A, respectively). Blood-fed TG A. stephensi mosquitoes survived for an average of 23.0 (range of means, 20.9 to 25.12) days compared to 18.1 d (14.39 to 19.87) days for NTG control siblings, resulting in a significant increase in survivorship of 15% to 45% (P < 0.0001 to P = 0.0184). Similarly, TG Ae. aegypti mosquitoes survived for an average of 32.7 (17.3 to 39.7) days compared with 25.9 (15.7 to 34.7) days for NTG control siblings, resulting in a significant increase in survivorship of 14% to 47% (P < 0.0001 to P = 0.0380). In 1 of the 6 Ae. aegypti blood-fed replicates, TG mosquitoes had an increase in survivorship of only 10%, which was not significantly different from the NTG controls (replicate 4; P = 0.945). However, life spans for both the TG and NTG treatments were noticeably shorter in this replicate, suggesting that an external factor may have artificially reduced survivorship.
Figure 3.
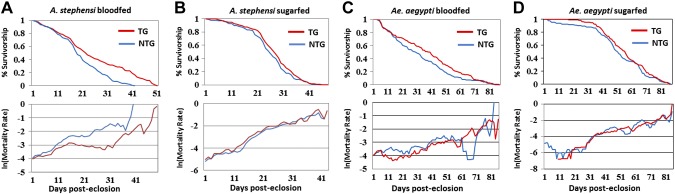
Impact of myr-Akt expression on mosquito survival. A) Representative survivorship and mortality curves of A. stephensi NTG and TG mosquitoes reared under identical conditions and provided with a blood meal 3 times a week in addition to 10% dextrose solution ad libitum (top). B) Representative survivorship and mortality curves of A. stephensi NTG and TG mosquitoes reared under the same conditions provided with only 10% dextrose solution (top). C) Representative survivorship and mortality curves of Ae. aegypti NTG and TG mosquitoes reared under identical conditions and provided with a blood meal twice weekly in addition to 10% dextrose solution ad libitum (top). D) Representative survivorship and mortality curves of Ae. aegypti NTG and TG mosquitoes reared under the same conditions provided with 10% dextrose solution (top). See Tables 1 and 2.
TABLE 1.
Statistical significance of biological replicates for blood- and sugar-fed A. stephensi
| Repetition no. | NTG |
TG |
Wilcoxon P | ||||
|---|---|---|---|---|---|---|---|
| n | Median | Mean ± se | n | Median | Mean ± se | ||
| Blood fed | |||||||
| 1 | 92 | 18.5 | 19.53 ± 0.98 | 122 | 22 | 22.41 ± 0.87 | 0.0184* |
| 2 | 108 | 18 | 18.87 ± 1.15 | 103 | 23 | 23.79 ± 1.41 | 0.0145* |
| 3 | 155 | 19 | 19.57 ± 1.15 | 159 | 22 | 25.12 ± 1.12 | <0.002* |
| 4 | 143 | 14 | 14.39 ± 0.66 | 141 | 21 | 20.9 ± 1.01 | <0.001* |
| Sugar fed | |||||||
| 1 | 114 | 28.5 | 27.7 ± 0.8 | 95 | 27 | 26.5 ± 0.9 | 0.2441 |
| 2 | 148 | 32 | 31.5 ± 0.8 | 156 | 31 | 30.1 ± 0.7 | 0.1882 |
| 3 | 148 | 22.5 | 21.1 ± 1.2 | 147 | 24 | 22.5 ± 0.8 | 0.1716 |
Significant increase in life span of TG mosquitoes.
TABLE 2.
Statistical significance of biologic replicates for blood- and sugar-fed Ae. aegypti
| Repetition no. | NTG |
TG |
Wilcoxon P | ||||
|---|---|---|---|---|---|---|---|
| n | Median | Mean ± se | n | Median | Mean ± se | ||
| Blood fed | |||||||
| 1 | 192 | 27.5 | 34.7 ± 2.0 | 200 | 37.5 | 39.7 ± 1.7 | 0.0100* |
| 2 | 178 | 25 | 28.9 ± 1.6 | 196 | 32 | 36.8 ± 1.9 | 0.0105* |
| 3 | 219 | 11 | 18.5 ± 1.2 | 192 | 24.5 | 27.2 ± 1.4 | <0.0001* |
| 4 | 198 | 8 | 15.7 ± 1.1 | 206 | 7 | 17.3 ± 1.2 | 0.9450 |
| 5 | 141 | 30 | 31.8 ± 1.8 | 149 | 38 | 37.4 ± 1.9 | 0.0380* |
| 6 | 105 | 21 | 25.9 ± 1.7 | 103 | 40 | 37.7 ± 2.0 | <0.0001* |
| Sugar fed | |||||||
| 1 | 198 | 30 | 35.1 ± 2.2 | 211 | 19 | 36.0 ± 2.3 | 0.0976 |
| 2 | 215 | 42 | 37.3 ± 1.5 | 202 | 17 | 26.1 ± 1.4 | <0.0001** |
| 3 | 131 | 30 | 28.1 ± 1.8 | 131 | 30 | 28.4 ± 1.8 | 0.7338 |
| 4 | 110 | 50.5 | 50.8 ± 1.9 | 108 | 55 | 54.5 ± 1.5 | 0.2830 |
| 5 | 111 | 44 | 39.8 ± 1.6 | 107 | 53 | 45.5 ± 1.9 | 0.0009* |
Significant increase in life span of TG mosquitoes. **Significant decrease in life span of TG mosquitoes.
Myr-Akt expression is regulated by the VG promoter and is only present in the fat body after a blood meal. Thus, mosquitoes fed exclusively sugar never express the myr-Akt transgene, and no effect on survivorship was expected. Appropriately, in 3 A. stephensi replicates, no significant difference in survivorship was observed between TG and NTG females (Fig. 3B; P = 0.1716 to 0.2441). Sugar-fed TG A. stephensi lived an average of 26.4 (22.5 to 30.1) days, while NTG siblings lived an average of 26.8 (21.1 to 31.5) days (Fig. 3B). Likewise, sugar-fed TG Ae. aegypti did not differ significantly from NTG siblings in 3 of the 5 replicates (Fig. 3D; P = 0.0976 to 0.7338; survivorship and mortality curves for all A. stephensi and Ae. aegypti sugar-fed replicates can be found in Supplemental Figs. S4B and S5B, respectively). In a fourth replicate, TG mosquitoes survived significantly longer (14%) than NTG controls (replicate 5; P = 0.0009), and in the last replicate, TG mosquitoes lived significantly shorter (30%) than NTG controls (replicate 2; P < 0.0001). Combined, these results indicate that the expression of the myr-Akt transgene is directly responsible for the enhanced survivorship observed in TG blood-fed mosquitoes. Furthermore, sugar-fed treatments indicate that insertion of the transgene and expression of the EGFP marker had no deleterious effects on mosquito survival.
The unexpected increase in survivorship is contrary to several other studies that show that disruption, not overstimulation, of IIS in the fat body extends life span (1, 18, 28). To assess whether sustained activation of IIS in blood-fed mosquitoes actually inhibited signaling downstream of Akt through a negative feedback mechanism as the mosquitoes aged, we examined myr-Akt expression, FOXO phosphorylation, and p70S6k phosphorylation in older (18 d) mosquitoes. In A. stephensi, myr-AsteAkt-HA was still expressed in a tissue- and blood meal-dependent manner, although at somewhat lower levels (Supplemental Fig. S6A). In Ae. aegypti, high levels of the myr-AaegAkt-HA protein could still be detected (Supplemental Fig. S6D; note the expression in non-blood-fed sample due to previous blood meals). Similarly, FOXO phosphorylation was significantly higher at 36 hours after blood meal in both mosquito species (Supplemental Figs. S6B, E). We did observe a significant increase in p70S6k phosphorylation at 36 hours after blood meal that was not observed in young A. stephensi but was observed in young Ae. aegypti (Supplemental Figs. S6C, F). Overall, these expression and phosphorylation patterns are consistent with younger mosquitoes, indicating that negative feedback of Akt signaling in mosquitoes with sustained myr-Akt expression is not responsible for the increased survival that we observed.
Effect of myr-Akt expression on mosquito reproduction
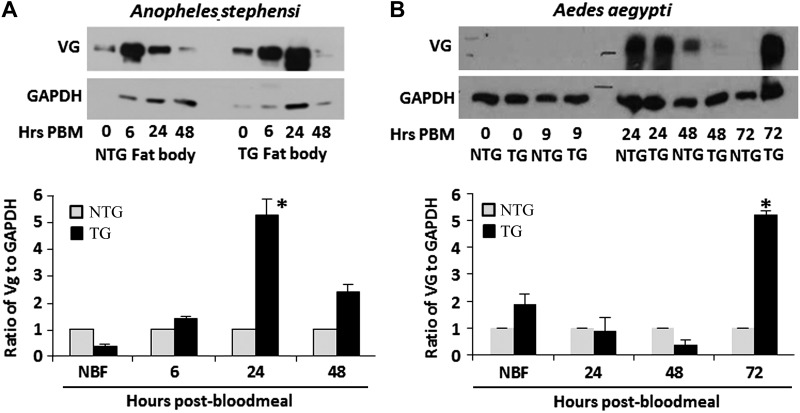
Insulin signaling regulates reproduction in a broad range of organisms. In mosquitoes, studies have shown the IIS cascade, including Akt, regulates both steroidogenesis in the ovaries and vitellogenesis in the fat body (24, 29). To determine if enhanced Akt signaling in the fat body affects mosquito reproduction, we examined both the amount of VG expressed and the number of viable eggs produced in TG and NTG mosquitoes. VG protein expression was compared in the fat bodies of TG and NTG females at several time points after blood feeding (A. stephensi, 0 [nonfed], 6, 24, and 48 hours; Ae. aegypti, 0, 24, 48, and 72 hours). In heterozygous A. stephensi TG mosquitoes, we observed a significant increase in VG protein expression at 24 hours after blood meal relative to NTG mosquitoes (Fig. 4A). Heterozygous Ae. aegypti TG mosquitoes had significantly higher VG expression at 72 hours after blood meal, which is after egg development is completed (Fig. 4B). This may be because myr-AaegAkt-HA persists up to 96 hours in the Ae. aegypti fat body. Interestingly, we did not observe a significant increase in A. stephensi egg production during either the first reproductive cycle (when myr-AsteAkt-HA is first being expressed partway through the gonotrophic cycle) or during a second reproductive cycle initiated immediately after the first (when myr-AsteAkt-HA was present at the onset of the gonotrophic cycle) (Table 3). Similarly, we did not observe differences in egg production between TG and NTG Ae. aegypti during the first, second, or third reproductive cycles (Table 4).
Figure 4.
Impact of myr-Akt expression on VG synthesis. A) Representative immunoblot of VG protein levels (VG) in the abdominal body walls/fat body of A. stephensi during a reproductive cycle (top). Graph indicates relative levels of A. stephensi VG (determined by the ratio of VG to GAPDH controls). To highlight differences between TG and NTG samples, VG levels in the NTG controls were normalized to 1. B) Representative immunoblot of VG protein levels (VG) in the abdominal body walls of Ae. aegypti during a reproductive cycle (top). Immunoblots were replicated a minimum of 3 times.
TABLE 3.
Average number of eggs laid by A. stephensi NTG vs. TG females during the first and second reproductive cycles
| Repetition no. | NTG |
TG |
Wilcoxon P | ||
|---|---|---|---|---|---|
| n | Mean ± se | n | Mean ± se | ||
| First reproductive cycle | |||||
| 1 | 25 | 72.28 ± 45.46 | 25 | 87.32 ± 32.48 | 0.0933 |
| 2 | 20 | 59.25 ± 38.54 | 20 | 63.00 ± 32.66 | 0.6898 |
| 3 | 25 | 82.59 ± 47.58 | 22 | 85.68 ± 43.79 | 0.8337 |
| Second reproductive cycle | |||||
| 1 | 12 | 63.38 ± 46.85 | 13 | 62.75 ± 43.88 | 0.9725 |
| 2 | 15 | 46.20 ± 41.01 | 14 | 50.92 ± 44.06 | 0.7673 |
| 3 | 12 | 59.66 ± 39.59 | 11 | 68.39 ± 4.58 | 0.6085 |
TABLE 4.
Average number of eggs laid by Ae. aegypti NTG vs. TG females during the first, second, and third reproductive cycles
| Repetition no. | NTG |
TG |
Wilcoxon P | ||
|---|---|---|---|---|---|
| n | Mean ± se | n | Mean ± se | ||
| First reproductive cycle | |||||
| 1 | 8 | 67.50 ± 44.26 | 9 | 42.89 ± 33.22 | 0.1774 |
| 2 | 16 | 59.50 ± 42.79 | 20 | 69.15 ± 47.02 | 0.3624 |
| 3 | 19 | 62.16 ± 51.66 | 22 | 82.41 ± 38.60 | 0.3776 |
| Second reproductive cycle | |||||
| 1 | 19 | 51.00 ± 30.09 | 18 | 52.00 ± 23.37 | 0.7960 |
| 2 | 28 | 97.89 ± 20.33 | 30 | 94.00 ± 18.19 | 0.2691 |
| 3 | 16 | 64.56 ± 46.38 | 28 | 84.39 ± 30.84 | 0.2826 |
| Third reproductive cycle | |||||
| 1 | 10 | 33.20 ± 16.34 | 8 | 26.87 ± 14.42 | 0.2645 |
| 2 | 16 | 91.19 ± 28.01 | 16 | 88.81 ± 31.58 | 0.8801 |
| 3 | 10 | 32.80 ± 38.14 | 27 | 67.85 ± 35.70 | 0.0257* |
Significant differences in egg production between TG and NTG mosquitoes.
DISCUSSION
In mosquitoes and other species, insulin signaling regulates numerous physiologic processes, including longevity, reproduction, metabolism, and immunity. Studies with the fruit fly D. melanogaster, the nematode Caenorhabditis elegans, and the mosquito A. stephensi have shown that by up-regulating insulin signaling, it is possible to reduce life span (10, 20, 30). On the other hand, down-regulating the IIS cascade in Drosophila, C. elegans, mice, and A. stephensi leads to an extension of life span (1, 10, 13, 18, 21). It has also been suggested that an inverse relationship exists between life span and reproduction (31, 32), with a reduction in life span leading to increased reproduction and life span extension reducing reproduction. However, exceptions to this exist, such as in honeybees, where queens produce large numbers of offspring yet are long lived relative to their worker siblings. Evidence also suggests that the link between reproduction and life span can be uncoupled in a variety of organisms (33).
In our experiments, we sought to enhance Akt signaling, a key signaling molecule in the IIS and other pathways, in the fat bodies of mosquitoes by expressing a myristoylated Akt regulated by the VG promoter. We expected to increase FOXO phosphorylation immediately downstream of Akt, thereby preventing its entry into the nucleus, in turn decreasing longevity and possibly increasing reproduction. Our TG mosquitoes did in fact have increased FOXO phosphorylation, although only at 24 to 36 hours after the blood meal, suggesting that a negative feedback loop or phosphatase may be suppressing IIS even though active Akt was present at later time points. To our surprise, we found that TG mosquitoes actually lived longer than their NTG siblings, leading us to speculate that perhaps Akt signaling was not up-regulated throughout the mosquito’s life span, as expected. However, examination of older mosquitoes showed increased levels of FOXO phosphorylation comparable to young mosquitoes, suggesting that increased Akt signaling does not decrease as the mosquito ages. Another possible explanation is that increased Akt signaling in the fat body affects the expression or activity of mosquito ILPs in other tissues. A recent study found that expression of the insulin-like peptide dILP6 within the fat body of Drosophila increased insulin receptor–mediated phosphosignaling, which led to reduced circulation of a second insulin-like peptide, DILP2, and an increase in life span (34), suggesting a possible mechanism for our observed life span extension. IIS has also been shown to affect life span by increasing or mitigating oxidative damage, providing yet another possible mechanism for our observed life span extension (35). In C. elegans, mutation of the insulin receptor ortholog, daf-2, extended life span and increased resistance to oxidative damage, while worms with daf-16 (FOXO) mutations are short lived and more susceptible to oxidative damage relative to wild-type individuals (14, 36). Similar studies using C. elegans showed that provisioning of antioxidants increased life span by reducing oxidative damage in stressed, but not unstressed, nematodes (37). In D. melanogaster, flies provisioned with exogenous antioxidants had extended life spans compared with superoxide dismutase-deficient flies that were less tolerant to oxidative stress (38). In mosquitos, the analysis of H2O2 synthesis of A. stephensi cells in vitro showed that enhanced oxidative stress reduced mosquito life span (19). IIS is also involved in the regulation of autophagy, with increased IIS suppressing autophagy and reducing life span [reviewed in (39)]. Indeed, in the A. stephensi midgut, increased IIS led to mitochondrial damage and inhibition of autophagy, which together combined to increase oxidative damage and decrease life span (21). However, it is possible that the impact of Akt signaling on fat body autophagy or oxidative stress differs from other tissues such as the midgut. Finally, intermittent fat body expression of myr-Akt during the metabolically intensive process of vitellogenesis may mitigate the oxidative stress this tissue experiences. The impact of fat body Akt signaling on endogenous ILP expression, oxidative stress, and autophagy are all important avenues of future research to determine why expression of an active Akt in the fat body extends life span in the mosquito.
In addition to its impact on life span, IIS also regulates reproduction in the fat body and other tissues. Egg production in the mosquito is a carefully orchestrated series of hormonal and cell signaling events that controls 2 major physiologic processes: steroidogenesis in the ovaries and vitellogenesis in the fat body. One of the key signaling cascades regulating both steroidogenesis and vitellogenesis is the IIS cascade. In our reproductive studies, we observed no significant difference in egg production between the TG and NTG sibling mosquitoes during the first few gonotrophic cycles. However, we observed a significant increase of VG protein in the TG A. stephensi at 24 hours after blood meal and in TG Ae. aegypti at 72 hours after blood meal relative to their NTG siblings. It has been demonstrated that IIS stimulates 20-hydroxyecdysone (20HE) production by the ovarian follicle cells of Ae. aegypti (29), and 20HE is necessary to initiate vitellogenesis in the fat body (40). Furthermore, it has been shown that in addition to amino acids, both insulin and 20HE are required for full activation of vitellogenesis in fat body (24). Previously, we demonstrated that the knockdown of the IIS inhibitor PTEN using RNAi, and thus globally increasing IIS in a wide range of tissues, increased egg production in mosquitoes (41). In contrast, for this study, increased Akt signaling was specifically targeted to the fat body. Although this was sufficient for an increase in yolk protein synthesis, it did not translate to an overall increase in egg production. This suggests that IIS from multiple tissues is likely required to increase egg clutch size.
Both life span and reproduction are key factors determining overall organismal fitness and provide opportunities for improving the fitness of malaria resistant TG mosquitoes for mosquito population replacement strategies. The successful replacement of wild vector populations with ones refractory to Plasmodium parasites will depend strongly on the fitness costs of the transgene. Substantial fitness costs could impede the effectiveness of genetic drive mechanisms and thus the spread of transgenes into target field population. The insertion and expression of antimalaria effector molecules and marker genes often have a negative impact on mosquito fitness (20, 42–46), although not always (46–48). Furthermore, effective genetic drive systems have still not been developed that can overcome fitness costs and provide the released TG mosquitoes with a genetic advantage. In our study, we successfully engineered TG mosquitoes with a significant increase in life span and an increase in the production of yolk proteins, although not egg production itself. By manipulating Akt activity or genes regulated downstream of Akt, it may be possible to offset fitness costs associated with transgene insertion, or perhaps even enhance overall mosquito fitness. Continued research into the molecular and hormonal regulators of these processes is essential to developing novel control strategies for malaria control.
In summary, this work identified a novel mechanism of controlling survival that is conserved between mosquito genera and provides a unique opportunity to explore the impact of Akt signaling on life span not only in mosquitoes but in a wide range of organisms. The ability to activate Akt signaling, and in turn enhance survival, in the mosquito fat body through blood feeding will allow us to explore the molecular underpinnings of life span extension. Furthermore, we have developed a possible mechanism for manipulating the fitness of mosquitoes, which should facilitate the development of population replacement strategies to control vector-borne disease transmission.
Supplementary Material
Acknowledgments
This work was funded by the U.S. National Institutes of Health National Institute of Allergies and Infectious Diseases Grants AI073745 to M.A.R. and AI088092 to P.Y.S.; and a Grand Challenges Exploration grant from the Bill and Melinda Gates Foundation. The authors thank Dr. Frank Ramberg for his assistance in maintaining the TG mosquito colonies and Dr. Jenya Antonova for her advice and guidance.
Glossary
- 20HE
20-hydroxyecdysone
- EGFP
enhanced green fluorescent protein
- FOXO
forkhead box O
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IIS
insulin/insulin growth factor 1 signaling
- ILP
insulin-like peptide
- Myr
myristoylation
- NTG
nontransgenic
- p70S6k
p70 S6 kinase
- PTEN
phosphatase and tensin homolog
- TG
transgenic
- TOR
target of rapamycin
- UMBI-ITF
University of Maryland Biotechnology Institute, Insect Transformation Facility
- VG
vitellogenin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Antonova Y., Arik A., Moore W., Riehle M., Brown M. (2012) Insulin-like peptides: structure, signaling, and function. In Insect Endocrinology (Gilbert L., ed.), pp. 63–92, Elsevier, New York [Google Scholar]
- 2.Toivonen J. M., Partridge L. (2009) Endocrine regulation of aging and reproduction in Drosophila. Mol. Cell. Endocrinol. 299, 39–50 [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay A., Tissenbaum H. A. (2007) Reproduction and longevity: secrets revealed by C. elegans. Trends Cell Biol. 17, 65–71 [DOI] [PubMed] [Google Scholar]
- 4.Hyun J., Hashimoto C. (2011) Physiological effects of manipulating the level of insulin-degrading enzyme in insulin-producing cells of Drosophila. Fly (Austin) 5, 53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piper M. D., Partridge L. (2007) Dietary restriction in Drosophila: delayed aging or experimental artefact? PLoS Genet. 3, e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paaby A. B., Schmidt P. S. (2009) Dissecting the genetics of longevity in Drosophila melanogaster. Fly (Austin) 3, 29–38 [DOI] [PubMed] [Google Scholar]
- 7.Fontana L., Partridge L., Longo V. D. (2010) Extending healthy life span—from yeast to humans. Science 328, 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grönke S., Clarke D. F., Broughton S., Andrews T. D., Partridge L. (2010) Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 6, e1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broughton S. J., Piper M. D., Ikeya T., Bass T. M., Jacobson J., Driege Y., Martinez P., Hafen E., Withers D. J., Leevers S. J., Partridge L. (2005) Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. USA 102, 3105–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwangbo D. S., Gershman B., Tu M. P., Palmer M., Tatar M. (2004) Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429, 562–566 [DOI] [PubMed] [Google Scholar]
- 11.Giannakou M. E., Goss M., Jünger M. A., Hafen E., Leevers S. J., Partridge L. (2004) Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 305, 361. [DOI] [PubMed] [Google Scholar]
- 12.Rera M., Bahadorani S., Cho J., Koehler C. L., Ulgherait M., Hur J. H., Ansari W. S., Lo T. Jr., Jones D. L., Walker D. W. (2011) Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 14, 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 [DOI] [PubMed] [Google Scholar]
- 14.Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L., Tissenbaum H. A., Ruvkun G. (1997) The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999 [DOI] [PubMed] [Google Scholar]
- 15.Wessells R. J., Fitzgerald E., Cypser J. R., Tatar M., Bodmer R. (2004) Insulin regulation of heart function in aging fruit flies. Nat. Genet. 36, 1275–1281 [DOI] [PubMed] [Google Scholar]
- 16.Blüher M., Kahn B. B., Kahn C. R. (2003) Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299, 572–574 [DOI] [PubMed] [Google Scholar]
- 17.Selman C., Lingard S., Choudhury A. I., Batterham R. L., Claret M., Clements M., Ramadani F., Okkenhaug K., Schuster E., Blanc E., Piper M. D., Al-Qassab H., Speakman J. R., Carmignac D., Robinson I. C., Thornton J. M., Gems D., Partridge L., Withers D. J. (2008) Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 22, 807–818 [DOI] [PubMed] [Google Scholar]
- 18.Selman C., Partridge L., Withers D. J. (2011) Replication of extended lifespan phenotype in mice with deletion of insulin receptor substrate 1. PLoS ONE 6, e16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang M. A., Mott T. M., Tapley E. C., Lewis E. E., Luckhart S. (2008) Insulin regulates aging and oxidative stress in Anopheles stephensi. J. Exp. Biol. 211, 741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corby-Harris V., Drexler A., Watkins de Jong L., Antonova Y., Pakpour N., Ziegler R., Ramberg F., Lewis E. E., Brown J. M., Luckhart S., Riehle M. A. (2010) Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 6, e1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauck E. S., Antonova-Koch Y., Drexler A., Pietri J., Pakpour N., Liu D., Blacutt J., Riehle M. A., Luckhart S. (2013) Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi. Microbes Infect. 15, 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrese E. L., Soulages J. L. (2010) Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clements A. N., Clements A. N. (1992) The Biology of Mosquitoes. Chapman & Hall, New York [Google Scholar]
- 24.Roy S. G., Hansen I. A., Raikhel A. S. (2007) Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 37, 1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nirmala X., Marinotti O., Sandoval J. M., Phin S., Gakhar S., Jasinskiene N., James A. A. (2006) Functional characterization of the promoter of the vitellogenin gene, AsVg1, of the malaria vector, Anopheles stephensi. Insect Biochem. Mol. Biol. 36, 694–700 [DOI] [PubMed] [Google Scholar]
- 26.Kokoza V., Ahmed A., Wimmer E. A., Raikhel A. S. (2001) Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac[3xP3-EGFP afm]. [3xP3-EGFP afm]Insect Biochem. Mol. Biol. 31, 1137–1143 [DOI] [PubMed] [Google Scholar]
- 27.Promislow D. E. L., Tatar M., Khazaeli A. A., Curtsinger J. W. (1996) Age-specific patterns of genetic variance in Drosophila melanogaster. I. Mortality. Genetics. 143, 839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piper M. D., Selman C., McElwee J. J., Partridge L. (2008) Separating cause from effect: how does insulin/IGF signalling control lifespan in worms, flies and mice? J. Intern. Med. 263, 179–191 [DOI] [PubMed] [Google Scholar]
- 29.Riehle M. A., Brown M. R. (1999) Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 29, 855–860 [DOI] [PubMed] [Google Scholar]
- 30.Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G. (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 [DOI] [PubMed] [Google Scholar]
- 31.Sgrò C. M., Partridge L. (1999) A delayed wave of death from reproduction in Drosophila. Science 286, 2521–2524 [DOI] [PubMed] [Google Scholar]
- 32.Jenkins N. L., McColl G., Lithgow G. J. (2004) Fitness cost of extended lifespan in Caenorhabditis elegans. Proc. Biol. Sci. 271, 2523–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flatt T. (2011) Survival costs of reproduction in Drosophila. Exp. Gerontol. 46, 369–375 [DOI] [PubMed] [Google Scholar]
- 34.Bai H., Kang P., Tatar M. (2012) Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 11, 978–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gems D., Partridge L. (2013) Genetics of longevity in model organisms: debates and paradigm shifts. Annu. Rev. Physiol. 75, 621–644 [DOI] [PubMed] [Google Scholar]
- 36.Garsin D. A., Villanueva J. M., Begun J., Kim D. H., Sifri C. D., Calderwood S. B., Ruvkun G., Ausubel F. M. (2003) Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300, 1921. [DOI] [PubMed] [Google Scholar]
- 37.Keaney M., Matthijssens F., Sharpe M., Vanfleteren J., Gems D. (2004) Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 37, 239–250 [DOI] [PubMed] [Google Scholar]
- 38.Magwere T., West M., Riyahi K., Murphy M. P., Smith R. A., Partridge L. (2006) The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech. Ageing Dev. 127, 356–370 [DOI] [PubMed] [Google Scholar]
- 39.Petrovski G., Das D. K. (2010) Does autophagy take a front seat in lifespan extension? J. Cell. Mol. Med. 14, 2543–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raikhel A. S., Kokoza V. A., Zhu J., Martin D., Wang S. F., Li C., Sun G., Ahmed A., Dittmer N., Attardo G. (2002) Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochem. Mol. Biol. 32, 1275–1286 [DOI] [PubMed] [Google Scholar]
- 41.Arik A. J., Rasgon J. L., Quicke K. M., Riehle M. A. (2009) Manipulating insulin signaling to enhance mosquito reproduction. BMC Physiol. 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catteruccia F., Godfray H. C., Crisanti A. (2003) Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes. Science 299, 1225–1227 [DOI] [PubMed] [Google Scholar]
- 43.Irvin N., Hoddle M. S., O’Brochta D. A., Carey B., Atkinson P. W. (2004) Assessing fitness costs for transgenic Aedes aegypti expressing the GFP marker and transposase genes. Proc. Natl. Acad. Sci. USA 101, 891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreira L. A., Wang J., Collins F. H., Jacobs-Lorena M. (2004) Fitness of anopheline mosquitoes expressing transgenes that inhibit Plasmodium development. Genetics 166, 1337–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C., Marrelli M. T., Yan G., Jacobs-Lorena M. (2008) Fitness of transgenic Anopheles stephensi mosquitoes expressing the SM1 peptide under the control of a vitellogenin promoter. J. Hered. 99, 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amenya D. A., Bonizzoni M., Isaacs A. T., Jasinskiene N., Chen H., Marinotti O., Yan G., James A. A. (2010) Comparative fitness assessment of Anopheles stephensi transgenic lines receptive to site-specific integration. Insect Mol. Biol. 19, 263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong Y., Das S., Cirimotich C., Souza-Neto J. A., McLean K. J., Dimopoulos G. (2011) Engineered anopheles immunity to Plasmodium infection. PLoS Pathog. 7, e1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isaacs A. T., Jasinskiene N., Tretiakov M., Thiery I. Zettor, Agnes, Bourgouin C., James A. A. (2012) Transgenic Anopheles stephensi co-expressing single-chain antibodies resist Plasmodium falciparum development. Proc. Natl. Acad. Sci. USA109, E1922–E1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.