Abstract
The reason co-morbid methamphetamine use and HIV infection lead to more rapid progression to AIDS is unclear. We used a model of methamphetamine self-administration to measure the effect of methamphetamine on the systemic immune system to better understand the comorbidity of methamphetamine and HIV. Catheters were implanted into the jugular veins of male, Sprague Dawley rats so they could self-administer methamphetamine (n = 18) or be given saline (control; n = 16) for 14 days. One day after the last self-administration session, blood and spleens were collected. We measured serum levels of pro-inflammatory cytokines, intracellular IFN-γand TNF-α, and frequencies of CD4+, CD8+, CD200+ and CD11b/c+ lymphocytes in the spleen. Rats that self-administer methamphetamine had a lower frequency of CD4+ T cells, but more of these cells produced IFN-γ. Methamphetamine did not alter the frequency of TNF-α-producing CD4+ T cells. Methamphetamine using rats had a higher frequency of CD8+ T cells, but fewer of them produced TNF-α. CD11b/c and CD200 expression were unchanged. Serum cytokine levels of IFN-γ, TNF-α and IL-6 in methamphetamine rats were unchanged. Methamphetamine lifetime dose inversely correlated with serum TNF-α levels. Or data suggest that methamphetamine abuse may exacerbate HIV disease progression by activating CD4 T cells, making them more susceptible to HIV infection, and contributing to their premature demise. Methamphetamine may also increase susceptibility to HIV infection, explaining why African American men who have sex with men (MSM) and frequently use methamphetamine are at the highest risk of HIV infection.
Keywords: Methamphetamine, Self-administration, Rat, CD4 T cells, Inflammatory cytokines
1. Introduction
Abuse of methamphetamine correlates with increased transmission and altered pathogenesis of HIV infection (Boddiger, 2005; Drumright et al., 2006; Gorbach et al., 2006) and other sexually transmitted diseases (Gonzales et al., 2006; Semple et al., 2004). These findings are responsible for our interest the effects of methamphetamine on the systemic immune response, which may have an impact on individuals who are at high-risk of HIV infection or are already infected. A recent report from the multicenter AIDS cohort study (the MACS) correlates cocaine and methamphetamine abuse with an inversion of the CD4+/CD8+ T cell ratio and increased HIV replication in infected individuals (Shoptaw et al., 2012). In addition, in vitro studies using human cells show that macrophages (Liang et al., 2008; Wang et al., 2012) and monocytederived dendritic cells (DC) from peripheral blood (Nair et al., 2006; Nair and Saiyed, 2011; Nair et al., 2009) are more susceptible to HIV infection after acute treatment with methamphetamine (1–250 µM). These studies also show that the expression of co-receptors necessary for HIV entry to cells, such as CXCR4 and CCR5, increases after acute methamphetamine treatment. These in vitro reports demonstrate the direct effects of methamphetamine on cells in culture. Other studies show that rats that are given investigator selected doses of methamphetamine have lower numbers of CD4 and CD8 T cells, natural killer cells (NK) and DC cells (Harms et al., 2012). Our studies bring a new dimension to this question because of the rats in our studies self-administer methamphetamine which enables us to evaluate the effect of methamphetamine in the context of addiction.
Animal models have been used to evaluate the immunosuppressive properties of methamphetamine at the cellular and molecular levels; the inconsistent findings of these studies are potentially due to the wide range of methamphetamine doses evaluated, from 1 to 40 mg/kg, whether the investigators used an acute or chronic model, and finally, whether the investigator used an active or passive protocol for drug administration. Most studies involve acute, passive (experimenter administered) treatments with high doses of methamphetamine and these protocols show increased production of serum IFN-γ and TNF-α impaired proliferation and function in splenocytes, and enhanced cytokine levels in the striatum and other brain regions of mice (Flora et al., 2002; Goncalves et al., 2008; Saito et al., 2008; Valencia et al., 2012; Yu et al., 2002). However, these immunosuppressive effects of methamphetamine have not been investigated using contingent, self-administration protocols. This represents a significant knowledge gap as self-administered and passively administered psychostimulants can result in significantly different outcomes in laboratory rodents (Galici et al., 2000; Jacobs et al., 2003; Reichel et al., 2012). Thus, to better emulate the scenario of human drug taking, we used rats that self-administered methamphetamine for 14 days, and used this model to determine the consequence on T and non-T cell populations in the spleen and levels of circulating pro-inflammatory cytokines, TNF-α, IL-6, and IFN-γ. We used the self-administration model and immunological tools to determine the effects of chronic methamphetamine abuse on the systemic immune cells that are the primary cells infected by HIV. Depletion of this same cell subpopulation is also responsible for progression to AIDS in HIV infected individuals. Results from these studies help understand the immunological mechanisms responsible for increase risk of infection in methamphetamine abusers and also provide light on the reason HIV disease progression is more rapid in the co-morbid condition.
2. Materials and methods
2.1 Animals model for methamphetamine self-administration
Sprague-Dawley rats (Harlan, Indianapolis, IN; n = 34) were implanted with a jugular vein catheter using the protocol previously described by Graves and Napier (Graves and Napier, 2011, 2012); they were allowed to recover for 5 days. They were then randomly assigned to either a methamphetamine self-administration group (n = 18) or to a control group (n = 16). The operant protocol was used to train Rats in the methamphetamine group were trained to self-administer drug. In brief, rats were placed in operant chambers enclosed in ventilated, sound attenuating cabinets (Med-Associates, St. Albans, VT) and trained for 14 consecutive days to self-administer methamphetamine (as the hydrochloride salt, Sigma, St. Louis, MO) for 3 h/day by the use of active and inactive lever presses. On days 1 thru 7, rats self-administered methamphetamine at a fixed ratio of 1 (FR1), during which, one active lever press resulted in delivery of 0.1 mg/kg of methamphetamine in a 0.1 ml infusion. The FR1 protocol assured rapid task acquisition, but to increase the effort (work load) required to receive the drug, on days 8 thru 14, we switched the task to an FR5 so that five active lever presses were required for one methamphetamine infusion. A 20 second timeout period followed each infusion during which lever presses had no programmed consequence. This task profile allowed the rats to self-titrate their preferred intake of methamphetamine (as is done by human methamphetamine abusers) and avoids overdosing. Pressing the inactive lever had no consequence at any time. Comparing responses on active vs. inactive levers is used to illustrate that lever pressing was not random, but that the rats actually have learned to associate drug infusion with the active lever. Saline control rats were administered saline infusions (0.1 ml/kg) according to the behavioral pattern of methamphetamine self-administering rats. One day after the last operant session, all rats were euthanized using rapid, conscious decapitation and blood and organs were collected for this study and for other studies. Personnel were blinded to the treatment history of animals until all data collection was completed.
When methamphetamine is injected, it is widely distributed throughout the body and organs. Peak concentrations in plasma and spleen are similar (Riviere et al., 2000; Volkow et al., 2010). Molar concentrations of methamphetamine in the blood of 105 human subjects who were using methamphetamine ranged from 0.13–11.12uM with a mean of 1.98uM and median of 1.25uM (Melega et al., 2007). Taking the route of injection (Gentry et al., 2004) and the dose of methamphetamine self-administered into consideration, we estimate the peak blood concentration in our rats to be approximately 1uM, which is within the range reported for human who use this drug.
Rats were maintained in specific pathogen-free conditions at Rush University Medical Center and the Institutional Animal Care and Use Committee (IACUC) approved protocols for animal use.
2.2 Collection of tissues
Spleens were harvested from rats one day after the last operant session. Single cell suspensions of splenocytes were obtained from each spleen by gently pushing them through a metal screen until the cells were completely dispersed. They were then resuspended in 50 ml of RPMI-1640 (Mediatech Inc., Manassas, VA) with 10% FBS (complete media; Atlanta Biologicals, Lawrenceville, GA). Splenocytes were incubated for 5 min in TAC lysis buffer (2.4 mg/L Tris, 8.3 g/L ammonium chloride, pH 7.2) to remove the red blood cells. After incubation, cells were washed again and resuspended in complete media. An automatic cell counter (Beckman Coulter, Inc., Indianapolis, IN) was used to count the number of mononuclear cells per spleen. Cells were used immediately in experiments.
Trunk blood was collected in 50 ml centrifuge tubes following decapitation, refrigerated to allow clotting and then serum was collected. Each serum sample was centrifuged twice to remove remaining cells. Aliquots of serum were stored at −80 °C, prior to use in the assay.
2.3 PMA/Ionomycin stimulation
In order to assess surface marker expression and cytokine production, 1 × 106 fresh splenocytes were added to duplicate wells of a 24-well plate with complete media containing 1 µL/ml of monensin in the presence or absence of 25 ng/ml of PMA and 500 ng/ml of Ionomycin (Sigma). The plate was incubated for 5 h in a 37 °C/5%CO2 humidified atmosphere. Following incubation, cells were harvested from each well and transferred into flow cytometry tubes for staining.
2.4 Staining for phenotype and intracellular cytokine expression
To exclude dead cells from analysis, all splenocytes were washed twice in PBS and incubated with 1 µL of the fixable viability dye eFlour-450 (eBioscience, San Diego, CA) for 30 min at room temperature (RT) in the dark prior to incubation with antibodies. For phenotyping T cells, cells were washed twice in FACS buffer (PBS + 0.5% BSA and 0.1% NaN3) and incubated with anti-CD3 FITC, anti-CD8 PerCP, and anti-CD4 PE Cy7 (all from Biolegend, San Diego, CA) for 30 min at 4 °C in the dark. A duplicate set of cells was used for phenotyping non-T cells; in this case, cells were incubated with anti-CD3 FITC, anti-CD200 PE (Biolegend) and anti-CD11b/c PerCP-Flour 710 (eBioscience, San Diego, CA). Cells were fixed by incubating them in 2% paraformaldehyde for 30 min at RT in the dark; they were then permeabilized with perm/wash buffer (Biolegend) for 30 min at RT in the dark. Cells were washed, resuspended in perm/wash buffer and incubated with anti-TNF-α PE and anti-IFN-γ Alexa Flour 647 (eBioscience) for 30 min at RT in the dark. All intracellular staining was compared against a staining panel with corresponding isotype matched controls: PE Armenian hamster IgG isotype control used for TNF-α and Alexa Fluor 647 mouse IgG, κ for IFN-γ (Biolegend). Cells were washed once more in FACS buffer and evaluated using an LSRII flow cytometer with FACS diva software (BD Biosciences, San Jose, California). LSRII flow cytometer settings were corrected using anti-rat Ig CompBeads set (BD Biosciences) according to manufacture’s instructions; samples were further analyzed using Flojo 8.8.7 (Treestar, La Jolla, CA).
2.5 Serum cytokine analysis
Pre-coated ELISA kits (Biolegend) were used to assess the relative amounts of TNF-α, IL-6 and IFN-γ in the serum from rats that self-administered methamphetamine (n = 18) and saline controls (n = 16). Samples were thawed on assay days and run twice in duplicate following manufacturer instructions. Concentrations of each cytokine were plotted against a standard curve fitting of the corresponding cytokine using Excel (Microsoft).
2.6 Statistical analysis
Splenocyte phenotype and intracellular cytokine production were analyzed using Flowjo software version 8.8.7 (Treestar). Data were graphed using Prism software (Graphpad, La Jolla, CA) and reported as mean ± S.E.M. unless otherwise noted. Statistical differences between methamphetamine self-administration and saline groups were determined using a two-tailed Student’s t- test. A Pearson’s correlation was used to determine relationship between methamphetamine intake and serum cytokine concentrations. Differences were considered significant when the P values were < 0.05.
3. Results
3.1 Methamphetamine self-administration
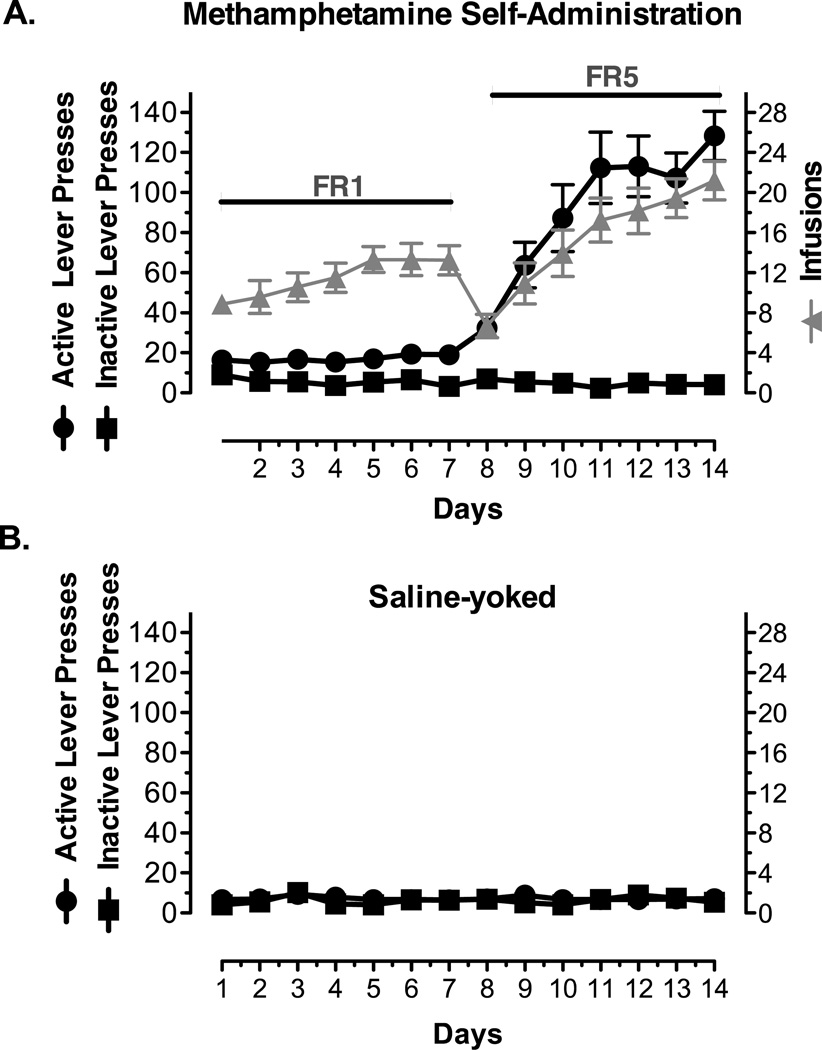
Fig. 1 illustrates the methamphetamine self-administration pattern of 18 rats during 14 consecutive days. Active lever pressing and the corresponding quantity of methamphetamine self-administered by the rats increased on days 1–7. Day 8 responses decreased due to switching to the higher schedule demand, i.e., FR5, but animals rapidly reacquired the operant procedure over days 8–14. Inactive lever pressing was negligible throughout behavioral assessments, which indicates a clear differentiation between the reinforced (active) and non-reinforced (inactive) levers. Lever pressing behavior for saline subjects emulated that seen on the inactive lever in rats trained to self-administer methamphetamine (data not shown).
Figure 1. Methamphetamine Self-Administration.
Male Sprague-Dawley rats (n = 18) were trained to self-administer methamphetamine (methamphetamine) inside operant chambers, with an inactive (no consequence) and an active lever (methamphetamine infusion). At a fixed ratio of 1 (FR1), rats received one methamphetamine infusion at 0.1mg/kg/0.1mL for each active lever press on days 1 through 7 and at a FR5 (days 8 through 14) they received 1 methamphetamine infusion for every 5 active lever presses. A) The number of active lever presses (circles, black), inactive lever presses (squares, black), and active lever presses associated with methamphetamine intake (triangles, gray) for the 14 consecutive days of methamphetamine self-administration. Rats did not learn to press the inactive lever. B) The number of active (circles, black) and inactive (squares, black) lever presses in saline-yoked control rats (n =16). Data is presented as Mean ± S.D.
3.2 Methamphetamine self-administration lowered the percentage of splenic CD4+ T cells
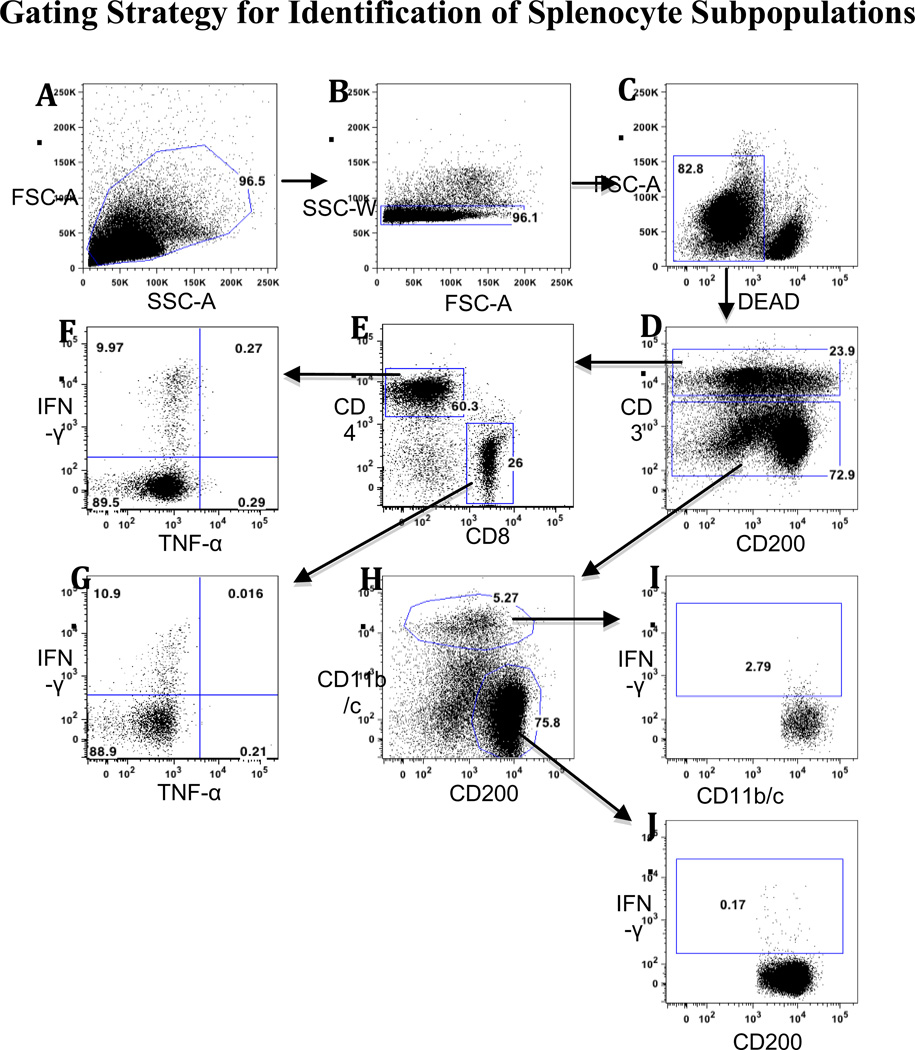
We evaluated the effects of chronic methamphetamine self-administration on the phenotype distribution of resting and activated (PMA/Ionomycin; PMA/I) splenic subpopulations. Splenocytes from methamphetamine self-administering rats and saline controls were analyzed for changes the distribution of subpopulations identified by the following rat cell surface markers: total T cells (CD3), helper T cells (CD4), cytotoxic T cells (CD8), monocytes and dendritic cells (DC; CD11b/c) and B cells (CD200) antibodies. Fig. 2 illustrates, in detail, the gating strategy used for flow cytometric analysis. Briefly, to focus the assessment on T cells, helper T cells were defined as CD3+/CD4+ cells, and cytotoxic T cells as CD3+/CD8+ cells. When evaluating non-T cell subpopulations, a negative gate on CD3+ cells was used. CD3− CD11b/c+ cells were recognized as monocytes and DCs, and live CD3−CD200+ populations were recognized as B cells.
Figure 2. Gating Strategy for Identification of Splenocyte Subpopulations.
Two different antibody panels were used for analysis of T and non-T cells in Figs. 3 and 4. Representative histograms show the analysis of unstimulated splenocytes from a rat that self-administered meth in presence of PMA/Ionomycin. Positive gates for intracellular cytokine production were established according to the unstimulated response from the same animal. A) Lymphocytes were identified using FSC-A/SSC-A. B) Singlets were defined using SSC-W/FSC-A. C) Dead cells were discriminated using a fixable viability dye. D) T cells were defined as CD3+, and non-T cells as CD3−. E) CD4 and CD8 subpopulations within the CD3+ cells were identified. IFN-γ and TNF-α production were determined for F) CD4+ cells and G) CD8+ cells. H) CD11b/c and CD200 antibodies were used to identify subpopulations within the CD3− cell subpopulation. IFN-γ production was determined for I) CD11b/c+ cells and J) CD200+ cells.
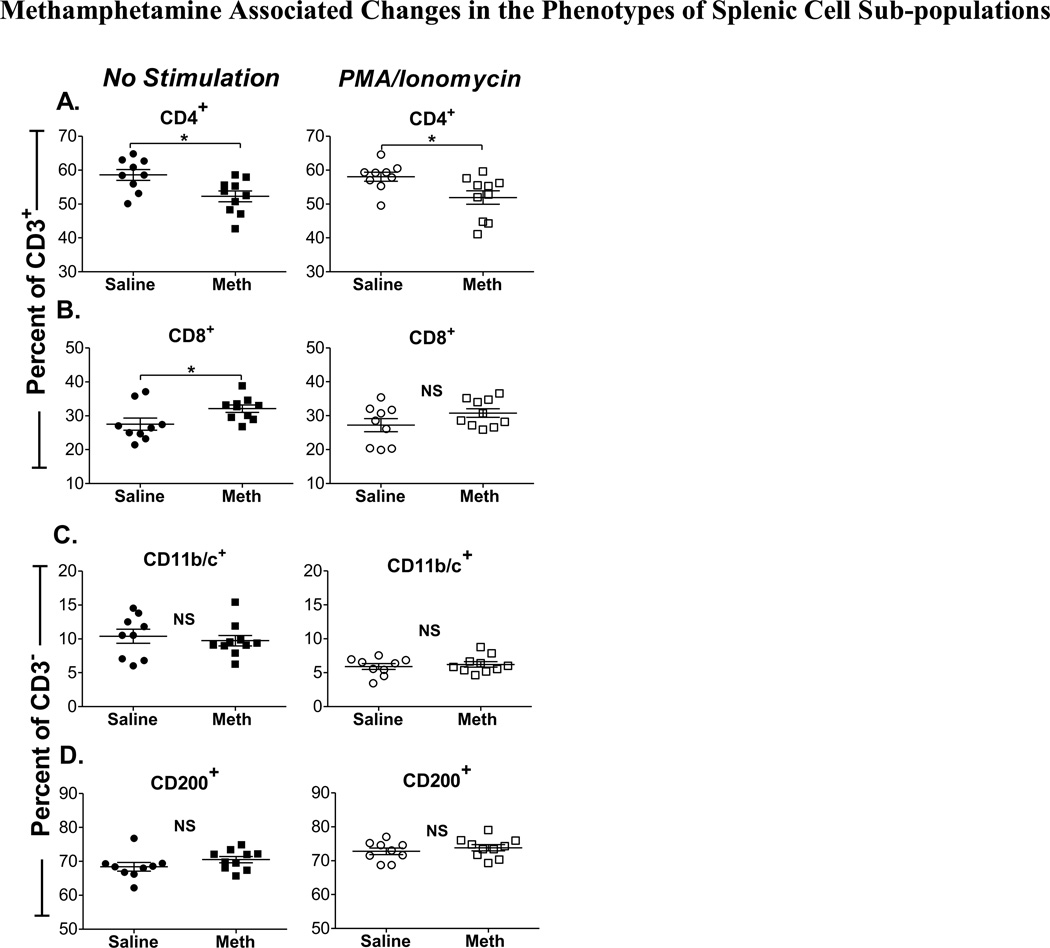
Within the CD3+ subpopulation, we observed a lower percentage of CD4+ cells in rats that self-administered methamphetamine (52.3 ± 1.6) when compared to saline controls (58.6 ±1.6, p = 0.01) (Fig. 3A, left). Similarly, a decrease in CD4 expression was observed even in PMA/Ionomycin stimulated splenocytes (p = 0.02) (Fig. 3A, right). PMA/I stimulation did not exacerbate the decrease in CD4+ T splenocytes, suggesting that this effect is associated with methamphetamine self-administration independently of PMA/I stimulation. The expected corresponding increase in expression of CD8+ T cells is shown in Fig. 3B (left), the increase in unstimulated CD3+ CD8+ T cells was significant when compared to saline controls (p = 0.04); this effect was similar but not statistically significant in PMA/I stimulated cells (Fig. 3B, right).
Figure 3. Methamphetamine Associated Changes in the Phenotypes of Splenic Cell Sub-populations.
Methamphetamine self-administering rats (n = 11) and saline-yoked controls (n = 9) were killed and splenocytes were isolated one day after their last methamphetamine (meth) infusion. Splenocytes were gated on forward and side scatter for singlet analysis; dead cells were excluded with a fixable viability dye. Data represent the percentage of CD3+ or CD3− cell subpopulations. The percentages of (A) CD3+CD4+, (B) CD3+CD8+, (C) CD3−CD11b/c+, and (D) CD3−/CD200+ cells are shown for meth and saline groups in unstimulated (left panel, closed symbols) and PMA/Ionomycin stimulated splenocytes (right panel, open symbols). *, P < 0.05, Student’s t-test. (NS) indicates that differences were not significant.
Contrary to what we observed in T cell subpopulations, our results show that methamphetamine self-administration had no impact on the percentage of CD3− cells (Figs. 3C and D), regardless of PMA/I stimulation (P = 0.62 and 0.19, CD11b/c+ and CD200+, respectively). Interestingly, a decrease in the percentage of CD11b/c+ cells was observed in both methamphetamine and control rats after stimulation with PMA/I (Fig. 3C, right); this observation seems to be an effect of PMA/I stimulation on these cells that cannot be attributed to methamphetamine self-administration. In this study, we also determined the total number of cells per spleen for all splenocyte cell subpopulations; however, there were no significant differences between methamphetamine self-administered rats and saline controls, regardless of PMA/I treatment (data not shown).
3.3 Methamphetamine self-administration altered intracellular cytokine production
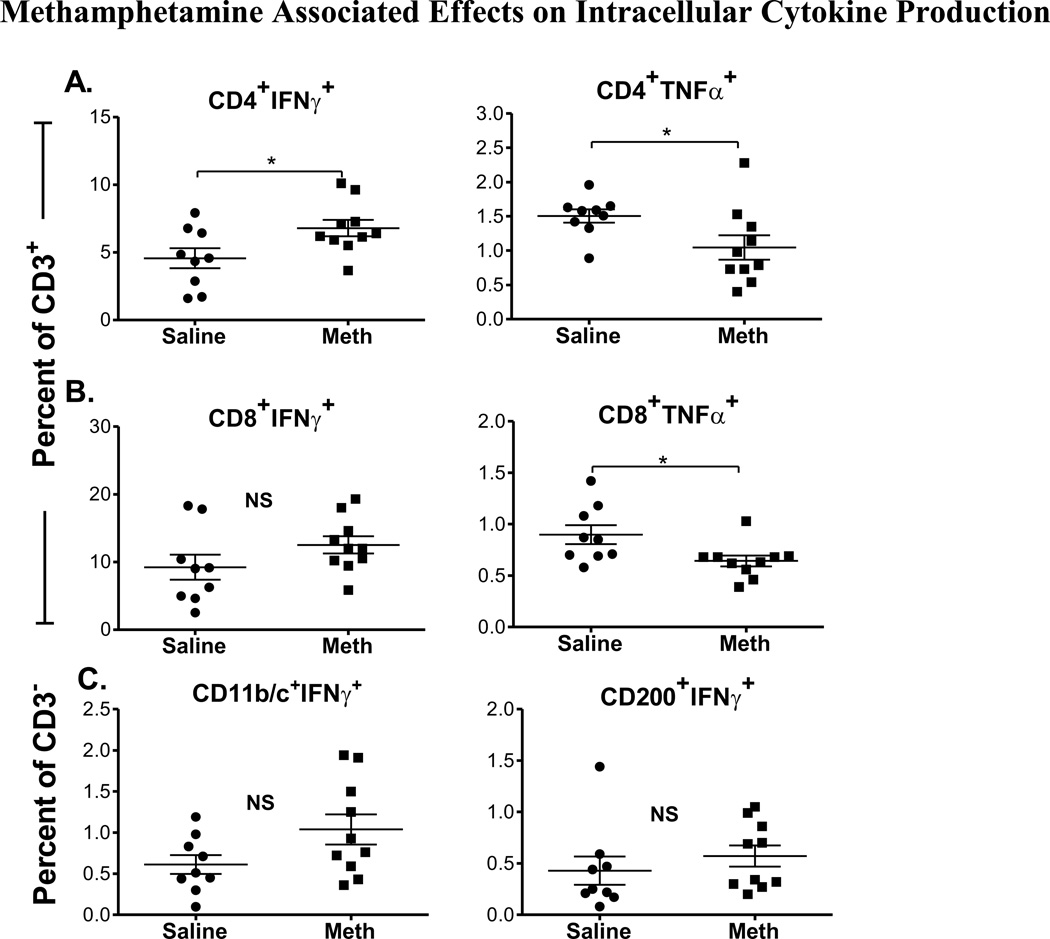
In order to assess the effects of chronic methamphetamine self-administration on immune cell function, splenocytes from methamphetamine self-administering rats and saline controls were incubated in the presence or absence of PMA/I to non-specifically activate the production of cytokines. We compared changes in the intracellular production of pro-inflammatory cytokines IFN-γ and TNF-α in CD4+ and CD8+ T cells within the CD3+ cell population, and in CD11b/c+ cells within the CD3− cell population. In Fig. 4A, we show that a significantly higher proportion of CD4+ T cells from rats that self-administered methamphetamine produced IFN-γ when compared to saline controls (P = 0.03). Conversely, a significantly lower proportion of CD4+ T cells from the rats that self-administered methamphetamine produced TNF-α when compared to saline controls (P = 0.04). A mild increase in IFN-γ production by CD8+ T cells was observed from rats that self-administered methamphetamine; however, this change did not reach statistical significance when compared to saline controls (Fig. 4B, left). On the other hand, TNF-α production was decreased in CD8+ T cells in methamphetamine self-administered rats when compared to saline controls (P = 0.02) (Fig. 4B, right).
Figure 4. Methamphetamine Associated Effects on Intracellular Cytokine Production.
Splenocytes from methamphetamine (meth) self-administering rats and saline-yoked controls were isolated and stimulated with PMA and Ionomycin. We compared intracellular cytokine production by live CD3+ and CD3− subpopulations in rats that self administered methamphetamine and saline controls. The percentage of cells that produce IFN-γ (left) and TNF-α (right) for (A) CD3+CD4+ and (B) CD3+CD8+ T cells are shown for saline (circles) and methamphetamine (squares) groups. (C) The percentage of IFN-γ+ producing CD3− CD11b/c+ (monocytes) and CD3−CD200+ B cells is shown for saline (circles) and methamphetamine (squares) groups. *, P <0.05, Student’s t-test. (NS) indicates that differences were not significant.
Within the CD3− cell subpopulation, we observed an increase in the proportion of CD11b/c+ cells that produced IFN-γ in methamphetamine self-administering rats when compared to saline controls, but this trend did not reach statistical significance (P = 0.07). No statistical differences were observed in intracellular production of IFN-γ by CD200+ cells between methamphetamine self-administered rats and saline controls (Fig. 4C).
Taken together, the splenocyte analysis indicated that even though CD4+ T cells were in lower proportions in methamphetamine self-administering rats (Fig. 3A), they were producing more IFN-γ than CD4+ T cells from saline animals (Fig. 4A). Moreover, even though the results did not reach statistical significance, trends suggest that this may also have been true for CD8+ T cells, monocytes and DCs, but not for B cells. Interestingly, TNF-α production was significantly lower in both CD4+ and CD8+ T cell subpopulations from spleens of methamphetamine-self administering rats, which diverges from observations on TNF-α in methamphetamine studies of brain cells (Galici et al., 2000; Graves and Napier, 2011; Jacobs et al., 2003; Reichel et al., 2012).
3.4 The concentration of pro-inflammatory cytokines in serum was altered by methamphetamine self-administration
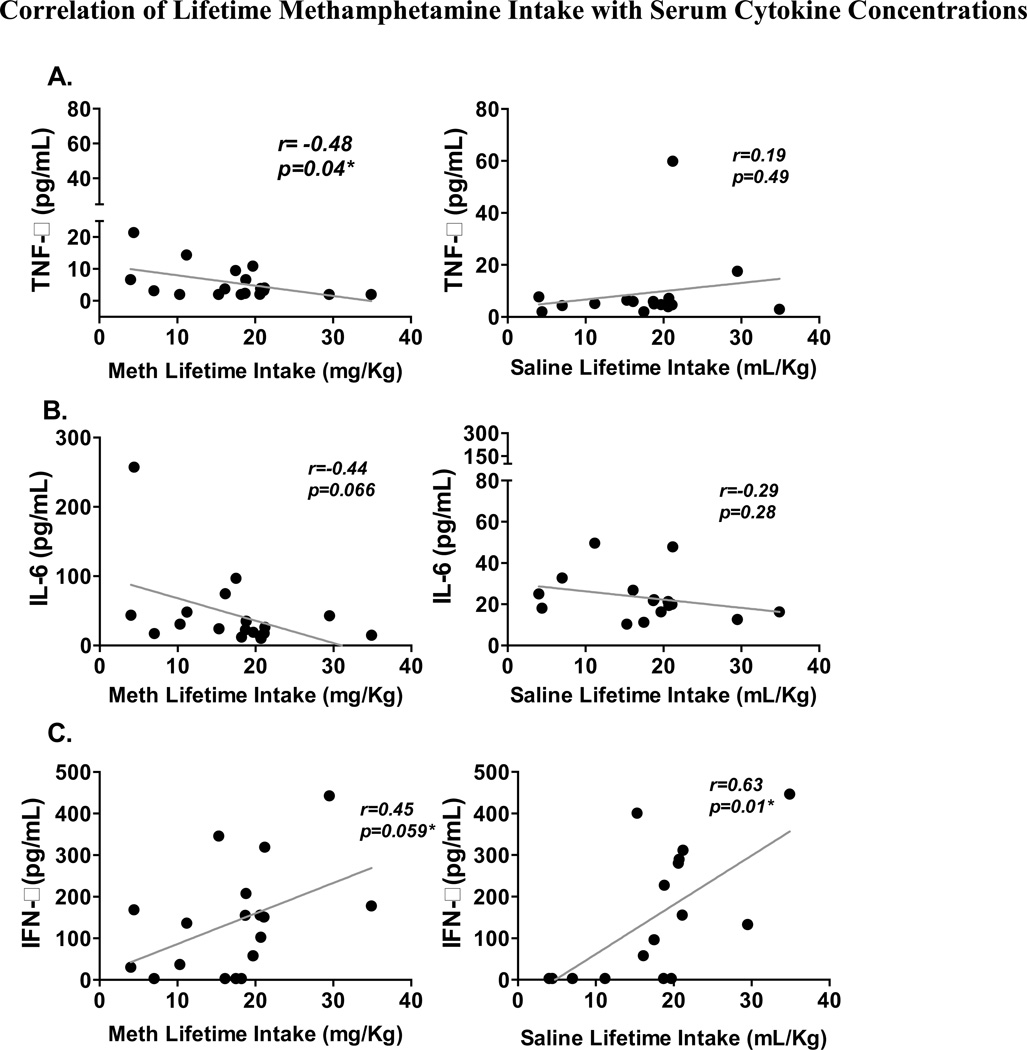
We determined the serum levels of TNF-α, IL-6 and IFN-γ in rats that self-administered methamphetamine and in saline controls, and observed no significant difference between groups. When considering these results, it is important to recognize that even though all self-administering rats had the same opportunity to take methamphetamine, there was a wide range in the lifetime methamphetamine intake among the animals. Accordingly, we addressed two questions: (A) do serum concentrations of pro-inflammatory cytokines TNF-α, IL-6 and IFN-γ correlate with the lifetime dose of methamphetamine taken by self-administering rats, and (B) does the fluid volume of saline used to administer influence the serum concentrations of these cytokines? As shown in Fig. 5A (left), we observed that lifetime intake of methamphetamine in self-administering rats negatively correlated with serum concentrations of TNF-α (R = −0.48, P = 0.04). This observation is particularly interesting when taking into account the impact of comparable volumes of saline intake, which did not correlate significantly with TNF-α production (R = 0.19, P = 0.49). We also observed a negative trend between lifetime methamphetamine dose and serum IL-6 concentration, but this did not reach statistical significance (R = −0.44, P = 0.07) (Fig. 5B). We observed an almost statistically significant correlation between IFN-γ levels and methamphetamine lifetime intake (r = 0.45, p = 0.06); however, this observation is less interesting because IFN-γ levels also positively correlated with lifetime saline intake (R = 0.63, P = 0.01), this control shows that instrumentation of the rats with indwelling catheters and/or fluid/saline intake was possibly sufficient to influence IFN-γ levels (Fig. 5C). Fig. 5A confirms the decrease in TNF-α concentration seen in Fig. 4 in rats that self-administer methamphetamine and demonstrates that not only are T cells producing less TNF-α on an individual basis, but that this is reflected in the concentration of TNF-α in the serum.
Figure 5. Correlation of Lifetime Methamphetamine Intake with Serum Cytokine Concentrations.
Serum from methamphetamine (meth) self-administering (n = 18; left panel) and saline-yoked (n = 16; right panel) rats were assayed by ELISA to determine relative levels of TNF-α, IL-6 and IFN-γ. Saline and methamphetamine lifetime intake values were correlated to the relative levels of (A) TNF-α, (B) IL-6 and (C) IFN-γ. Results from methamphetamine rats are in the left hand column and from saline controls are on the right. *, P <0.05; Pearson’s correlation.
4. Discussion
Methamphetamine is a highly abused illicit drug and its abuse increases the morbidity and mortality of HIV infection; therefore, it is important to understand the impact of methamphetamine on the consequences of HIV infection. Typically, preclinical studies on the effects of methamphetamine, are either in vitro, or if in vivo, evaluate acute, high doses of passively administered methamphetamine. However, acute administration does not mimic the chronic exposure experienced by methamphetamine addicts, and these models do not capture the impact of the biology underlying highly motivated behaviors, like the drive, desire or stress that is associated with self-administration. A recent report by Harms et al. (Harms et al., 2012) shows that chronic administration of methamphetamine leads to lower numbers of activated CD4 and CD8 T cells. In our study, we found that methamphetamine self-administration reduced the frequency of CD4 T cells even in absence of PMA/I stimulation while it increased the frequency of CD8 T cells. One possible explanation for the different outcomes could be attributed to the model of drug administration used. For example, plasma levels of the stress steroid hormone, corticosterone, are elevated in rats that self-administer the stimulant, cocaine, but not in those that passively receive identical cocaine treatments passively (Galici et al., 2000). Since corticosterone also influences the inflammatory response, including parameters measured here, it is possible that could have influenced the response to methamphetamine that we observed in this study. To our knowledge, this is the first study of spleen and plasma indicators of inflammation in rats that self-administer methamphetamine.
One of the most critical determinants of HIV disease progression is the loss of CD4 T cells in infected individuals. When an individual is infected with HIV, CD4+ T cells are rapidly depleted, undergo a rebound and then gradually decrease as disease progresses. Even though this scenario is elucidated for non drug-taking individuals, the immunological characteristics of chronic methamphetamine abusers are not well understood. In this study, the effect of chronic methamphetamine abuse on immune cells that are important determinants of HIV disease in humans is described in methamphetamine self-administering rats, a model that emulates human drug-taking. Using this model, we were able to show that methamphetamine use decreased the percentage of CD4+ T cells (Fig. 3A) in the spleens of rats. This observation suggests that the loss of CD4+ T cells is potentially exacerbated in individuals who abuse methamphetamine, which in turn shortens the time of progression to AIDS.
Previous studies have demonstrated additional immunosuppressive effects of methamphetamine, including decreased proliferation, decreased NK activity by splenic lymphocytes of mice (Saito et al., 2008; Yu et al., 2002) and increased T cell dysfunction in human cells after passive methamphetamine treatment (Potula et al., 2010). However, methamphetamine effects on circulating levels of cytokines have been more difficult to demonstrate (Buchanan et al., 2010; Loftis et al., 2011; Valencia et al., 2012). In our study, perhaps due to the variability in levels of individual methamphetamine intake, we did not see differences in circulating cytokine levels until we looked at lifetime methamphetamine dose in methamphetamine self-administering rats, which revealed a negative correlation of serum TNF-α with lifetime methamphetamine intake, (a non-significant trend was also noted with IL-6).
The impact of methamphetamine self-administration on the systemic immune response was more evident when we evaluated specific splenic cell subpopulations, i.e., CD4+ and CD8+ T cells, monocytes/DCs and B cells. In this regard, we observed several subpopulations with altered expression of inflammation markers, IFN-γ and TNF-α. Significantly increased levels of intracellular IFN-γ were observed in CD4+ T cells, and a similar (albeit non-significant) trend was observed in CD8+ T cells and macrophages/DCs. Because previous studies attribute increased IFN-γ to CD4+ T cell activation and depletion (Long and Stoddart, 2012) as well as increased HIV susceptibility, it is interesting that we observed IFN-γ increase in CD4+ T cells. This finding is meaningful in understanding HIV pathogenesis in methamphetamine abusing humans.
Intracellular TNF-α production was lower in both CD4+ helper T cells and CD8+ cytotoxic T cells and serum TNF-α concentrations were lower over time with continued methamphetamine use. It is hard to predict the effect that this change in TNF-α concentration would have on HIV resistance or disease progression. TNF-α is an inflammatory cytokine and inflammation activates immune cells making them more susceptible to HIV infection; inflammation is thought to exacerbate HIV disease progression. For these reasons, you might expect that a decrease in TNF-α concentration would be beneficial to the high risk or infected individual. On the other hand, TNF-α itself has antiviral activity; so one part of the innate immune defense against HIV would be missing. It is difficult to predict which of these effects of TNF-α would impact infection and progression of HIV more. In the future, we plan to study a wider range of cytokines and markers of activation to evaluate the interplay of these cytokines and design studies to enable us to better understand the impact of the modified cytokine environment created by methamphetamine use.
The clinical implications of the observations described in this study are an important start in deducing the biological link between methamphetamine abuse and HIV pathogenesis. Our results suggest a physiological role for methamphetamine addiction in susceptibility to HIV infection and progression to AIDS. Finally, we hope that by using a model of self-administration, our studies and future investigations will be able to describe effects consistent with what we would expect to see in methamphetamine-addicted humans.
Acknowledgements
This work was supported by the Daniel F. and Ada L. Rice foundation, USPHSGs DA015760 and DA024923 and the Chicago Developmental Center for AIDS Research, an NIH funded program (P30 AI 082151, PD, Dr. Alan Landay, Department of Immunology/Microbiology, Rush University Medical Center, Chicago, IL 60612), which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NCCAM).
We thank Ryan T. Sowell, Amanda Persons, Sharanya Kousik and Leo Kelly for help with experiments, discussion and critical review of this manuscript. We also thank the Center for Compulsive Behavior and Addiction, Rush University Medical Center Flow Cytometry Core and the Chicago Developmental Center for AIDS Research.
Abbreviations
- DC
dendritic cells
- PMA/I stimulation
PMA/Ionomycin stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Boddiger D. Metamphetamine use linked to rising HIV transmission. Lancet. 2005;365:1217–1218. doi: 10.1016/S0140-6736(05)74794-2. [DOI] [PubMed] [Google Scholar]
- Buchanan JB, Sparkman NL, Johnson RW. A neurotoxic regimen of methamphetamine exacerbates the febrile and neuroinflammatory response to a subsequent peripheral immune stimulus. J Neuroinflammation. 2010;7:82. doi: 10.1186/1742-2094-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumright LN, Little SJ, Strathdee SA, Slymen DJ, Araneta MR, Malcarne VL, Daar ES, Gorbach PM. Unprotected anal intercourse and substance use among men who have sex with men with recent HIV infection. Journal of acquired immune deficiency syndromes. 2006;43:344–350. doi: 10.1097/01.qai.0000230530.02212.86. [DOI] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Maragos W, Hennig B, Toborek M. Methamphetamine-induced TNF-alpha gene expression and activation of AP-1 in discrete regions of mouse brain: potential role of reactive oxygen intermediates and lipid peroxidation. Neuromolecular Med. 2002;2:71–85. doi: 10.1385/NMM:2:1:71. [DOI] [PubMed] [Google Scholar]
- Galici R, Pechnick RN, Poland RE, France CP. Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol. 2000;387:59–62. doi: 10.1016/s0014-2999(99)00780-3. [DOI] [PubMed] [Google Scholar]
- Gentry WB, Ghafoor AU, Wessinger WD, Laurenzana EM, Hendrickson HP, Owens SM. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacology, biochemistry, and behavior. 2004;79:751–760. doi: 10.1016/j.pbb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Goncalves J, Martins T, Ferreira R, Milhazes N, Borges F, Ribeiro CF, Malva JO, Macedo TR, Silva AP. Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Ann N Y Acad Sci. 2008;1139:103–111. doi: 10.1196/annals.1432.043. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Marinelli-Casey P, Shoptaw S, Ang A, Rawson RA. Hepatitis C virus infection among methamphetamine-dependent individuals in outpatient treatment. J Subst Abuse Treat. 2006;31:195–202. doi: 10.1016/j.jsat.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Gorbach PM, Drumright LN, Daar ES, Little SJ. Transmission behaviors of recently HIV-infected men who have sex with men. Journal of acquired immune deficiency syndromes. 2006;42:80–85. doi: 10.1097/01.qai.0000196665.78497.f1. [DOI] [PubMed] [Google Scholar]
- Graves SM, Napier TC. Mirtazapine alters cue-associated methamphetamine seeking in rats. Biological psychiatry. 2011;69:275–281. doi: 10.1016/j.biopsych.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves SM, Napier TC. SB 206553, a putative 5-HT2C inverse agonist, attenuates methamphetamine-seeking in rats. BMC neuroscience. 2012;13:65. doi: 10.1186/1471-2202-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms R, Morsey B, Boyer CW, Fox HS, Sarvetnick N. Methamphetamine administration targets multiple immune subsets and induces phenotypic alterations suggestive of immunosuppression. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172:1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotoxicity research. 2011;20:59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long BR, Stoddart CA. Alpha interferon and HIV infection cause activation of human T cells in NSG-BLT mice. J Virol. 2012;86:3327–3336. doi: 10.1128/JVI.06676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61:216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Nair MP, Mahajan S, Sykes D, Bapardekar MV, Reynolds JL. Methamphetamine modulates DC-SIGN expression by mature dendritic cells. J Neuroimmune Pharmacol. 2006;1:296–304. doi: 10.1007/s11481-006-9027-1. [DOI] [PubMed] [Google Scholar]
- Nair MP, Saiyed ZM. Effect of methamphetamine on expression of HIV coreceptors and CC-chemokines by dendritic cells. Life sciences. 2011;88:987–994. doi: 10.1016/j.lfs.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MP, Saiyed ZM, Nair N, Gandhi NH, Rodriguez JW, Boukli N, Provencio-Vasquez E, Malow RM, Miguez-Burbano MJ. Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J Neuroimmune Pharmacol. 2009;4:129–139. doi: 10.1007/s11481-008-9128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potula R, Hawkins BJ, Cenna JM, Fan S, Dykstra H, Ramirez SH, Morsey B, Brodie MR, Persidsky Y. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J Immunol. 2010;185:2867–2876. doi: 10.4049/jimmunol.0903691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere GJ, Gentry WB, Owens SM. Disposition of methamphetamine and its metabolite amphetamine in brain and other tissues in rats after intravenous administration. The Journal of pharmacology and experimental therapeutics. 2000;292:1042–1047. [PubMed] [Google Scholar]
- Saito M, Terada M, Kawata T, Ito H, Shigematsu N, Kromkhun P, Yokosuka M, Saito TR. Effects of single or repeated administrations of methamphetamine on immune response in mice. Exp Anim. 2008;57:35–43. doi: 10.1538/expanim.57.35. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. A comparison of injection and non-injection methamphetamine-using HIV positive men who have sex with men. Drug and alcohol dependence. 2004;76:203–212. doi: 10.1016/j.drugalcdep.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Stall R, Bordon J, Kao U, Cox C, Li X, Ostrow DG, Plankey MW. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. Int J STD AIDS. 2012;23:576–580. doi: 10.1258/ijsa.2012.011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia F, Bubar MJ, Milligan G, Cunningham KA, Bourne N. Influence of methamphetamine on genital herpes simplex virus type 2 infection in a mouse model. Sex Transm Dis. 2012;39:720–725. doi: 10.1097/OLQ.0b013e31825af129. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Shumay E, Telang F, Thanos PK, Alexoff D. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PloS one. 2010;5:e15269. doi: 10.1371/journal.pone.0015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Y, Ye L, Li J, Zhou Y, Sakarcan S, Ho W. Modulation of intracellular restriction factors contributes to methamphetamine- mediated enhancement of acquired immune deficiency syndrome virus infection of macrophages. Curr HIV Res. 2012;10:407–414. doi: 10.2174/157016212802138797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Zhang D, Walston M, Zhang J, Liu Y, Watson RR. Chronic methamphetamine exposure alters immune function in normal and retrovirus-infected mice. Int Immunopharmacol. 2002;2:951–962. doi: 10.1016/s1567-5769(02)00047-4. [DOI] [PubMed] [Google Scholar]