Abstract
Purpose
This study investigated the relationships among the plasma levels of catecholamine metabolites, the clinical response to duloxetine treatment, and Val158Met polymorphism of the catechol-O-methyltransferase (COMT) gene.
Subjects and methods
Sixty-four patients and 30 healthy control subjects were recruited. Major depressive episodes were diagnosed using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria. The severity of depression was evaluated using the 17-item Hamilton Rating Scale for Depression (HAMD17). Patients whose HAMD17 scores were 15 or greater were enrolled in the study. Blood sampling and clinical evaluation were performed at week 0 and week 8. The levels of plasma catecholamine metabolites were measured using high-performance liquid chromatography with electrochemical detection. Genotyping was performed using direct sequencing.
Results
Thirty of 45 patients (67%) responded to duloxetine treatment during the 8 weeks of treatment. The baseline plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG), but not homovanillic acid (HVA), were lower in patients with major depressive disorder (MDD) who had the Val/Val genotype than in patients who were Met-carriers. Patients with MDD and the Val/Val genotype, but not Met carriers, had increased plasma levels of MHPG after 8 weeks of duloxetine treatment. The baseline plasma MHPG levels in healthy control subjects with the Val/Val genotype were significantly higher than those in patients with MDD. Among the subjects in the MDD group with the Val/Val genotype, the plasma MHPG levels increased to the same degree as in the healthy control subjects with the Val/Val genotype after 8 weeks of duloxetine treatment.
Conclusion
The relationship among the COMT Val158Met polymorphism, plasma levels of catecholamine metabolites, and responses to duloxetine is complex. Nevertheless, our results suggest that patients with MDD and the Val/Val genotype are more sensitive to the influence of noradrenergic neurons by duloxetine treatment.
Keywords: major depressive disorder, duloxetine, catechol-O-methyltransferase, 3-methoxy-4-hydroxyphenylglycol, homovanillic acid
Introduction
Duloxetine, a serotonin–noradrenaline reuptake inhibitor (SNRI), is an effective first-line treatment for patients with major depressive disorder (MDD). Duloxetine is also useful for the treatment of diabetic neuropathic pain and fibromyalgia.1 Duloxetine is generally safe and well tolerated, although it can lead to nausea, headache, dry mouth, insomnia, general fatigue, constipation, diarrhea, dizziness, sweating, sexual dysfunction, and loss of appetite in patients with MDD.1 We previously reported that milnacipran, another SNRI, increased the plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) (a major metabolite of noradrenaline) in patients with MDD; this increase was related to the milnacipran-associated clinical improvement in patients with MDD.2 Because duloxetine potently inhibits both serotonin and noradrenaline transporters, we hypothesized that duloxetine (similar to milnacipran) may also increase plasma MHPG levels in patients with MDD. We recently reported that duloxetine treatment for 8 weeks significantly increased MHPG plasma levels in patients with MDD.3 To the best of our knowledge, this is the first study examining the influence of duloxetine on plasma MHPG levels in patients with MDD. Monoamines play an important role in the pathogenesis of MDD.4 Our previous studies demonstrated that the response to antidepressants was associated with the plasma levels of catecholamine metabolites.2,5 Specifically, the plasma levels of catecholamine metabolites, such as MHPG and homovanillic acid (HVA), predicted the response to selective serotonin reuptake inhibitors (SSRIs) and SNRIs.2,5 Patients with MDD and lower plasma levels of MHPG responded better to milnacipran, and patients with higher plasma levels of MHPG responded better to paroxetine. Considering these findings, an increase in MHPG levels may play an important role in improving depressive symptoms.
Catechol-O-methyltransferase (COMT) is a methylation enzyme that participates in the degradation of noradrenaline and dopamine by catalyzing the transfer of a methyl group from S-adenosylmethionine. Biochemical studies have established that the enzyme activities of patients with MDD differ from those of non-depressed subjects.6 The COMT gene is located at 22q 11.21. Val158Met (G324A) (rs 4680), functional polymorphism in the COMT gene, was found to be associated with MDD in a multicenter European study.7 Furthermore, the valine (Val) allele has been reported to result in three- to fourfold higher COMT activity than the methionine (Met) allele.8 One recent report suggested an association between the higher activity of the Val/Val-type COMT gene and poor antidepressant treatment response.9 Furthermore, the Met variants of the COMT gene Val158Met polymorphism was shown to increase the risk of depressed mood and low motivation in Swedish males with MDD.10
Considering these findings, we hypothesized that 1) patients with the COMT Val/Val genotype would respond better to duloxetine than COMT Met carriers due to catecholamine deficiencies; 2) patients with the COMT Val/Val genotype would have lower plasma levels of MHPG and HVA than COMT Met carriers; and 3) patients with MDD and the COMT Val/Val genotype would exhibit increased plasma levels of MHPG and HVA compared to COMT Met carriers. To address these hypotheses, we investigated the plasma levels of catecholamine metabolites and the clinical responses to duloxetine treatment in patients with MDD and the COMT Val/Val genotype and in patients with MDD who were COMT Met carriers. To the best of our knowledge, this is the first study to investigate the COMT Val158Met polymorphism, plasma catecholamine metabolite levels, and clinical improvement in response to duloxetine treatment in Japanese patients with MDD.
Subjects
Sixty-four in/outpatients and 30 healthy control subjects were recruited. Major depressive episodes were diagnosed using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria. Patients whose 17-item Hamilton Rating Scale for Depression (HAMD17) scores were 15 or greater were enrolled in the current study. The exclusion criteria included any history of neurological disease or other physical diseases and comorbidities with other disorders such as bipolar disorder or Axis II disorders (ie, personality disorders or mental retardation diagnosed using the Structured Clinical Interview DSM-IV Axis II instrument). We enrolled 30 sex- and age-matched healthy control subjects without any psychiatric disorders (diagnosed by KA using the Structured Clinical Interview for DSM-IV Axis I Disorders, Non-patient Edition [SCID-I/NP]). All of the subjects were provided with information about the procedures. Written informed consent was obtained via forms approved by the local Ethics Committee of the University of Occupational and Environmental Health, Kitakyushu, Japan.
Methods
The present study utilized an open-label and non-fixed dose design. All of the patients were administered duloxetine monotherapy for 8 weeks. Benzodiazepines were used, if necessary, during the treatment period. A 1-week washout period occurred before duloxetine treatment began. The severity of depressive symptoms was evaluated (by KA) each week using the HAMD17. We defined responders as patients whose HAMD17 scores decreased by 50% or more. Blood sampling and clinical evaluations were performed at week 0 and week 8. Forty-five of the 64 patients (70%) finished the study. The demographic data of the subjects who finished the study are shown in Table 1. The persistence rate is illustrated in Figure 1.
Table 1.
The demographic data for the patients with major depressive disorder and the healthy control subjects
| Patients (N=45) | Controls (N=30) | P-value | |
|---|---|---|---|
| Age (years) | 50.6± 15.3 | 46.5±10.6 | 0.251* |
| Sex (male/female) (n) | 23/22 | 14/16 | 0.706** |
Notes: P-values calculated using
Student’s t-test,
χ2 test. Age is presented as mean ± standard deviation.
Figure 1.
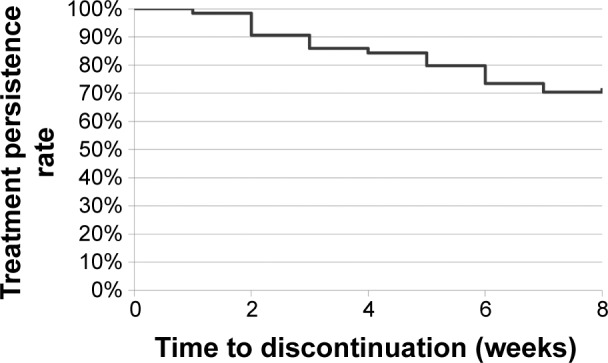
Treatment persistence rate.
Note: The persistence rate was calculated using the Kaplan–Meier method.
HVA and MHPG plasma level assays
Plasma HVA levels were analyzed using high-performance liquid chromatography with electrochemical detection (HPLC-ECD) according to the method of Yeung et al11 with slight modifications. In short, each cyano-bonded, solid-phase extraction cartridge was preconditioned with methanol followed by glass-distilled water. The plasma sample or standard solution and internal standard solution (5-hydroxyindolecarboxylic acid) were added to each cartridge. The samples were then deproteinized with acetonitrile. After vortexing and centrifugation, an aliquot of the supernatant was slowly drawn through the cartridge under a mild vacuum. The cartridge was washed twice with distilled water and extracted once with ethyl acetate. The aliquot was then evaporated with N2. After being dissolved in the mobile phase (phosphate buffer), 10 μL of this solution was injected into the HPLC-ECD. The intra- and inter-assay coefficients of variation were 6% and 8%, respectively. The recovery rate was >80%.
The plasma MHPG levels were also analyzed by HPLC-ECD, according to the method of Minegishi and Ishizaki.12 Briefly, after the plasma was separated by centrifugation, extraction was performed under a vacuum using Bond-Elut columns (Varian, Palo Alto, CA, USA) pre-packed with C18-bonded silica in a capacity disposable syringe. The columns, which were inserted into a vacuum chamber connected to an aspirator, were prepared by washing with 1 mL of methanol followed by 1 mL of water. After the addition of the internal standard (vanillyl alcohol) to the plasma, the samples were passed through the columns, followed by two rinses with distilled water to wash off any residual samples and easily eluted hydrophilic compounds. The adsorbed materials were eluted with methanol in a phosphate buffer mixture. A 20 μL portion of this solution was then injected into the HPLC-ECD. The intra- and inter-assay coefficients of variation were 6% and 8%, respectively. The recovery rate was >80%.
COMT Val158Met genotyping
Genomic DNA was extracted from peripheral leukocytes using a QIP DNA blood kit (Qiagen NV, Venlo, the Netherlands). The presence of the COMT Val158Met genotype was determined by sequencing of the genomic region.
Statistical analysis
The persistence rate was calculated using the Kaplan– Meier method. Non-paired t-tests were used to compare age, maximal daily duloxetine doses, number of major depressive episodes, HAMD17 scores, and plasma levels of catecholamine metabolites between the Val/Val group and the Met-carrier group among patients with MDD and healthy control subjects. Paired t-tests were performed to compare the plasma levels of catecholamine metabolites before (ie, at baseline) and after duloxetine treatment (8 weeks) between the Val/Val group and the Met-carrier group. P-values <0.05 were considered significant. All analyses were performed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA).
Results
The baseline plasma levels of HVA and MHPG in the patients with MDD were significantly lower than those in the healthy control subjects (Figure 2). Thirty of 45 patients (67%) responded to duloxetine treatment during the 8 weeks of treatment. However, there was no significant correlation between the changes in the total HAMD17 scores and the changes in MHPG or HVA plasma levels. In addition, there was no correlation between the changes in MHPG or HVA plasma levels and the changes in the sub-item scores on the HAMD17. Table 2 provides the COMT Val158Met genotype distributions and the χ2 and P-values for Hardy–Weinberg equilibrium. The demographic data for the patients with MDD did not differ between those with the Val/Val genotype and the Met carriers (Table 3). The baseline plasma levels of MHPG, but not HVA, were lower in the patients with MDD who had the Val/Val genotype than in patients with MDD who were Met carriers (Figure 3). We also compared the MDD patients’ baseline scores on the HAMD17 subcategories according to the method of Seretti et al.13 No differences were detected between the Val/Val group and the Met-carrier group (Table 4).
Figure 2.
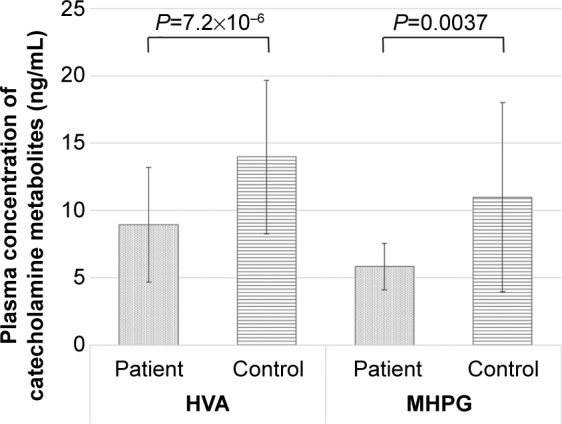
Baseline plasma levels of catecholamine metabolites in the patients with major depressive disorder and the healthy control subjects.
Note: Data are presented as mean ± standard deviation.
Abbreviations: HVA, homovanillic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol.
Table 2.
Hardy–Weinberg equilibrium of the patients with major depressive disorder and the healthy control subjects
| Genotypes
|
χ2 | P-value | Allele frequency
|
||||
|---|---|---|---|---|---|---|---|
| Val/Val | VaI/Met | Met/Met | Val | Met | |||
| Patients | 19 (42.2%) | 25 (55.6%) | 1 (2.2%) | 0.104 | 0.949 | 63 (70.0%) | 27 (30.0%) |
| Controls | 15 (50.0%) | 11 (36.6%) | 4 (13.3%) | 0.076 | 0.963 | 41 (68.3%) | 19 (31.7%) |
Abbreviations: Val, valine; Met, methionine.
Table 3.
The demographic data of all patients with major depressive disorder and respective COMT Val158Met genotypes
| All patients (N=45) | Val/Val (N=19) | Met carrier (N=26) | P-value | |
|---|---|---|---|---|
| Age (years) | 50.6±15.3 | 48.6±15.7 | 52.1±15.1 | 0.455* |
| Sex (male/female) (n) | 23/22 | 10/9 | 13/13 | 0.861** |
| DLX max dose (mg/day) | 49.3±12.3 | 50.0±12.9 | 48.9±12.1 | 0.760* |
| Depressive episodes before DLX treatment | 1.09±1.70 | 0.68±1.25 | 1.39±1.94 | 0.176* |
| Baseline HAMD17 | 21.2±4.9 | 21.9±5.5 | 20.7±4.4 | 0.437* |
| After 8 weeks HAMD17 score | 9.0±5.4 | 8.3±4.4 | 9.5±6.1 | 0.489* |
| Delta HAMD17 score (0w–8w) | 12.2±6.1 | 13.6±6.2 | 11.3±5.9 | 0.212* |
| Response rate (%) | 66.7 | 68.4 | 65.4 | 0.831** |
Notes: P-values calculated using
Student’s t-test,
χ2 test. Data are presented as mean ± standard deviation unless otherwise stated.
Abbreviations: DLX, duloxetine; HAMD17, 17-item Hamilton Rating Scale for Depression; COMT, catechol-O-methyltransferase; Val, valine; Met, methionine; w, weeks.
Figure 3.
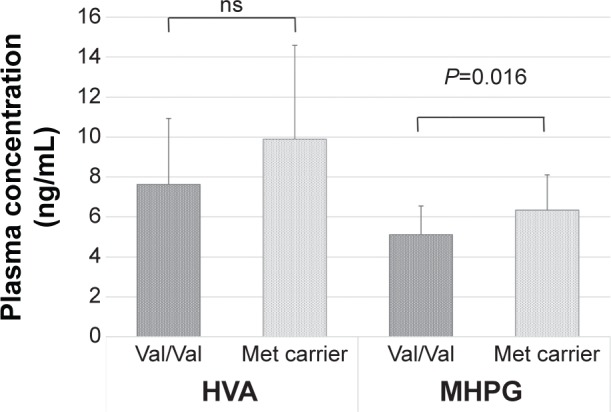
Baseline plasma levels of HVA and MHPG according to the COMT Val158Met genotype in the patients with major depressive disorder.
Note: Data are presented as mean ± standard deviation.
Abbreviations: HVA, homovanillic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol; COMT, catechol-O-methyltransferase; Val, valine; Met, methionine; ns, not significant.
Table 4.
Comparison of the COMT Val158Met genotype with the subcategories of HAMD17 scores according to the method of Seretti et al13 at baseline for the patients with major depressive disorder
| Seretti et al’s category (modified) | Subcategories of HAMD17 | Val/Val | Met carrier | P-value |
|---|---|---|---|---|
| Core symptom | 1. depressed mood, 2. feelings of guilt, 7. work and activities, 8. retardation: psychomotor, 10. anxiety (psychological), 13. somatic symptom general | 11.32±2.83 | 10.58±2.14 | 0.323 |
| Sleep | 4. insomnia early, 5. insomnia middle, 6. insomnia late | 2.89±0.46 | 2.96±1.59 | 0.840 |
| Activity | 7. work and activities, 8. retardation: psychomotor | 4.05±1.39 | 4.27±1.58 | 0.637 |
| Psychic anxiety | 9. agitation, 10. anxiety (psychological) | 2.68±1.34 | 2.31±1.16 | 0.318 |
| Somatic anxiety | 11. anxiety somatic, 12. somatic symptoms (gastrointestinal), 13. somatic symptoms general | 3.74±1.59 | 3.81±1.16 | 0.866 |
| Delusion | 2. feelings of guilt, 15. hypochondriasis | 2.68±1.34 | 2.35±1.06 | 0.348 |
Notes: P-values calculated using Students t-test. Data are presented as mean ± standard deviation.
Abbreviation: HAMD17, 17-item Hamilton Rating Scale for Depression; COMT, catechol-O-methyltransferase; Val, valine; Met, methionine.
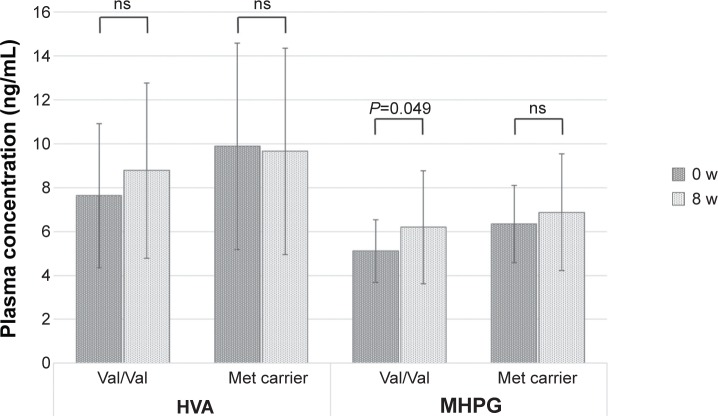
Next, we investigated the changes in plasma catecholamine metabolites between the two MDD groups. Only patients with MDD and the Val/Val genotype (not Met carriers) demonstrated increased MHPG plasma levels, which were similar to the healthy control subjects after 8 weeks of duloxetine treatment. The HVA plasma levels did not change after 8 weeks of duloxetine treatment for either the Val/Val group or the Met-carrier group (Figure 4).
Figure 4.
The plasma levels of HVA and MHPG according to the COMT Val158Met genotype at baseline and after 8 weeks of duloxetine treatment.
Note: Data are presented as mean ± standard deviation.
Abbreviations: HVA, homovanillic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol; COMT, catechol-O-methyltransferase; Val, valine; Met, methionine; w, weeks; ns, not significant.
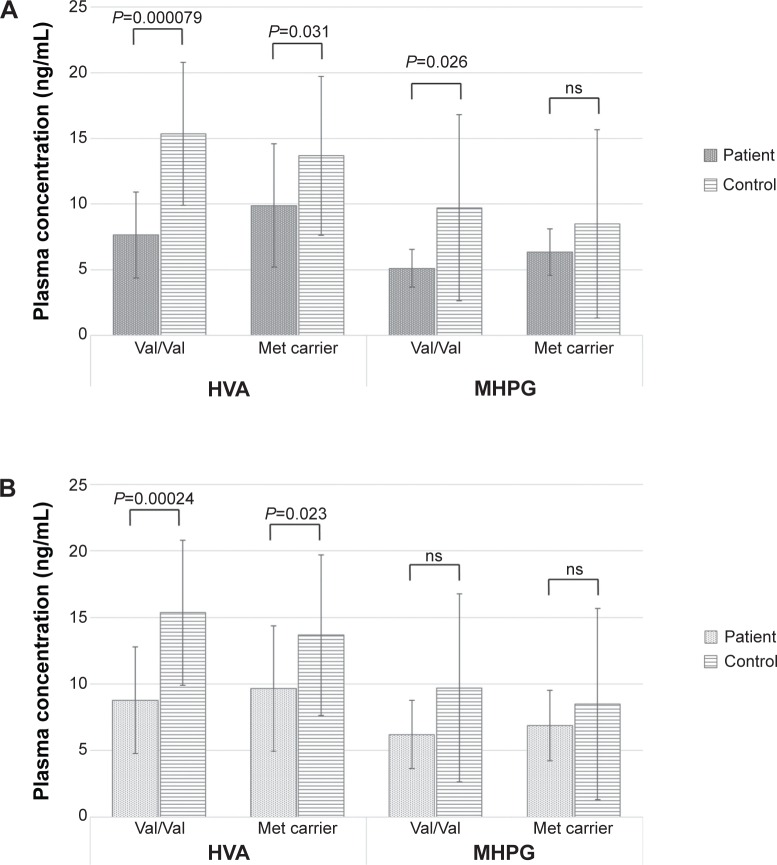
The baseline plasma HVA levels were significantly higher in the healthy control subjects than in the MDD group, regardless of the COMT Val158Met genotype (Val/Val versus Met carriers). Conversely, the baseline plasma MHPG levels in the healthy control subjects were significantly higher than those in the MDD patients only among subjects with the Val/Val genotype. For Met carriers, the baseline plasma MHPG levels did not differ between healthy control subjects and the patients with MDD (Figure 5A). The plasma MHPG levels increased among the subjects in the MDD group with the Val/Val genotype after 8 weeks of duloxetine treatment and were similar to those of the healthy control subjects with the Val/Val genotype. No other groups demonstrated changes in the levels of plasma catecholamine metabolites between baseline and the end of duloxetine treatment (8 weeks) (Figure 5B). However, there was no significant correlation between the changes in the total HAMD17 scores and the changes in MHPG or HVA plasma levels in the patients with MDD. In addition, there was no correlation between the changes in MHPG or HVA plasma levels and the changes in the HAMD17 sub-item scores in the patients with MDD.
Figure 5.
The plasma levels of HVA and MHPG according to the COMT Val158Met genotype in the patients with major depressive disorder and the healthy control subjects.
Notes: (A) At baseline. (B) After 8 weeks of treatment. Data are presented as mean ± standard deviation.
Abbreviations: HVA, homovanillic acid; MHPG, 3-methoxy-4-hydroxyphenylglycol; COMT, catechol-O-methyltransferase; Val, valine; Met, methionine; ns, not significant.
Discussion
This is the first study to investigate the response to duloxetine, the plasma levels of catecholamine metabolites, and the COMT Val158Met polymorphism in Japanese patients with MDD. There was no difference in the response to 8 weeks of duloxetine treatment between the Val/Val group and the Met-carrier group, which suggests that the COMT Val158Met polymorphism is not associated with duloxetine treatment response.
Depression is a common and disabling psychiatric disorder with a complex etiology that includes predisposing risk genes and environmental stressors. Variations in the COMT gene, particularly the Val158Met polymorphism, have been extensively investigated for associations with the clinical phenotypes of depression. Similarly, neurocognitive processes have been examined to evaluate the impact of COMT variants on phenotypes relevant to depression. We observed that clinical phenotypes, such as depression severity and diagnosis, or behavioral endpoints, were less reliably associated with COMT genetic variations. We recently reported that duloxetine increased the plasma levels of MHPG among responders (but not nonresponders) in a sample of Japanese patients with MDD.3 The potent inhibition of the noradrenaline transporter (NAT) by duloxetine is consistent with the increase in plasma MHPG levels in patients with MDD. MDD is a heterogeneous disorder; thus, patients’ plasma levels of MHPG and HVA vary widely.2 Indeed, some studies have reported the plasma levels of MHPG and HVA to be higher in depressed patients than in normal controls, whereas others have shown these levels to be lower in depressed patients than in normal controls.14
Considering these findings, it is possible that duloxetine strongly affects noradrenergic neurons in patients with MDD. Noradrenaline is mainly associated with attention, activity, concentration, and pain. Duloxetine might improve MDD symptoms associated with these factors by enhancing noradrenergic neuronal activity. However, no correlation was observed between the changes in plasma MHPG levels and the changes in the HAMD17 sub-item scores. In contrast, Chalon et al15 reported that duloxetine administration in healthy volunteers for 5 or 6 days significantly decreased urinary MHPG levels. The source of the discrepancy between the results of our study and the results of Chalon et al15 remains unknown, although differences in subjects and/or sampling points could be major reasons. Although a 1-week washout period was utilized in the current study, some of the patients had been administered SSRIs before duloxetine treatment was initiated. However, duloxetine did not alter the plasma HVA levels of the patients in our study.
Responses to venlafaxine, another SNRI, in patients with MDD were stratified by COMT genotype (Val158Met) in a previous randomized, double-blind, placebo-controlled clinical trial. Improvements in the depression scores of subjects with the Val/Val genotype were larger than those of patients with the Met/Met genotype, suggesting that venlafaxine may alter noradrenergic flux differentially according to COMT activity.16 Several previous biochemical, pharmacological, and genetic studies have implicated the COMT gene in the pathogenesis and pharmacological treatment of affective disorders. According to a review by Antypa et al17 the majority of studies have reported that Met carriers exhibit a better response to SSRIs, SNRIs, tricyclic antidepressants, or mirtazapine than Val/Val carriers. In the present study, no difference in the response to duloxetine was found between the Val/Val group and the Met-carrier group. However, it is not yet possible to form definitive conclusions regarding the COMT Val158Met polymorphism and the response to antidepressants. It is possible that COMT plays a different role in the response to each category of antidepressant (eg, SSRIs, SNRIs, tricyclic antidepressants).
The results of the current study should be interpreted with caution. We cautiously conclude that the plasma MHPG levels of MDD patients after duloxetine treatment are higher in those with the Val/Val genotype than in Met carriers. However, this finding is independent of improvements in clinical symptoms.
There are several limitations to the current study. First, this study was an open-label trial with a 1-week washout period. Second, the sample was small and heterogeneous, and it included subjects with both moderate and severe depression. Third, the dropout rate was high. Fourth, the plasma levels of MHPG and HVA are considered to reflect only 30%–50% and 10%–20% of the brain dynamics, respectively.18 Fifth, the 1-week washout period (for former antidepressant therapy) was not sufficiently long. Thus, future studies should be performed to confirm the results of this preliminary study.
Not all of our hypotheses were confirmed. The only confirmed hypothesis was that patients with MDD and the Val/Val genotype had lower plasma MHPG levels than patients with MDD who were Met carriers. In other words, we found that 1) patients with MDD and the COMT Val/Val genotype did not respond better to duloxetine treatment than patients who were COMT Met carriers; 2) patients with MDD and the COMT Val/Val genotype had lower plasma MHPG levels than patients who were COMT Met carriers (Figure 3); and 3) patients with MDD and the COMT Val/Val genotype demonstrated significant increase in MHPG plasma levels compared to COMT Met carriers (Figure 4). The most important finding of the current study was that patients with MDD and the Val/Val genotype recovered to the same plasma MHPG levels as the healthy control subjects when treated with duloxetine. However, this improvement occurred independently of clinical improvement.
Conclusion
The plasma MHPG levels in patients with MDD and the Val/Val genotype were lower than those in patients with MDD who were Met carriers. The MHPG levels in the former group significantly increased (to healthy control levels) after duloxetine treatment. Our results suggest that patients with MDD and the Val/Val genotype are more sensitive to the influence of duloxetine treatment on noradrenergic neurons.
Acknowledgments
The findings of this study were presented at the 29th CINP World Congress, in Vancouver, Canada on June 23rd, 2014. Dr R Yoshimura received a Grant-in-Aid for Scientific Research Japan. We thank Ms Kazuko Shimizu for her technical advice.
Footnotes
Disclosure
Dr J Nakamura received research support or speaker’s honoraria from Tanabe Mitsubishi, Pfizer, GlaxoSmithKline, Otsuka, Astellas, Eli Lilly, and Dainihon Sumitomo. The authors report no other conflicts of interest in this work.
References
- 1.Pergolizzi JV, Jr, Raffa RB, Taylor R, Jr, Rodriguez G, Nalamachu S, Langley P. A review of duloxetine 60 mg once-daily dosing for the management of diabetic peripheral neuropathic pain, fibromyalgia, and chronic musculoskeletal pain due to chronic osteoarthritis pain and low back pain. Pain Pract. 2013;13(3):239–252. doi: 10.1111/j.1533-2500.2012.00578.x. [DOI] [PubMed] [Google Scholar]
- 2.Shinkai K, Yoshimura R, Ueda N, Okamoto K, Nakamura J. Associations between baseline plasma MHPG (3-methoxy-4-hydroxyphenylglycol) levels and clinical responses with respect to milnacipran versus paroxetine treatment. J Clin Psychopharmacol. 2004;24(1):11–17. doi: 10.1097/01.jcp.0000104904.75206.19. [DOI] [PubMed] [Google Scholar]
- 3.Atake K, Yoshimura R, Hori H, et al. Duloxetine, a selective noradrenaline reuptake inhibitor, increased plasma levels of 3-methoxy-4-hydroxyphenylglycol but not homovanillic acid in patients with major depressive disorder. Clin Psychopharmacol Neurosci. 2014;12(1):37–40. doi: 10.9758/cpn.2014.12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshimura R, Nakamura J, Shinkai K, Ueda N. Clinical response to antidepressant treatment and 3-methoxy-4-hydroxyphenylglycol levels: mini review. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):611–616. doi: 10.1016/j.pnpbp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Ueda N, Yoshimura R, Shinkai K, Nakamura J. Plasma levels of catecholamine metabolites predict the response to sulpiride or fluvoxamine in major depression. Pharmacopsychiatry. 2002;35(5):175–181. doi: 10.1055/s-2002-34116. [DOI] [PubMed] [Google Scholar]
- 6.Karege F, Bovier P, Gaillard JM, Tissot R. The decrease of erythrocyte catechol-O-methyltransferase activity in depressed patients and its diagnostic significance. Acta Psychiatr Scand. 1987;76(3):303–308. doi: 10.1111/j.1600-0447.1987.tb02899.x. [DOI] [PubMed] [Google Scholar]
- 7.Massat I, Souery D, Del-Favero J, et al. Association between COMT (Val158Met) functional polymorphism and early onset in patients with major depressive disorder in a European multicenter genetic association study. Mol Psychiatry. 2005;10(6):598–605. doi: 10.1038/sj.mp.4001615. [DOI] [PubMed] [Google Scholar]
- 8.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Baune BT, Hohoff C, Berger K, et al. Association of the COMT val158-met variant with antidepressant treatment response in major depression. Neuropsychopharmacology. 2008;33(4):924–932. doi: 10.1038/sj.npp.1301462. [DOI] [PubMed] [Google Scholar]
- 10.Åberg E, Fandiño-Losada A, Sjöholm LK, Forsell Y, Lavebratt C. The functional Val158Met polymorphism in catechol-O-methyltransferase (COMT) is associated with depression and motivation in men from a Swedish population-based study. J Affect Disord. 2011;129(1–3):158–166. doi: 10.1016/j.jad.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Yeung PK, Buckley SJ, Pedder SC, Dingemanse J. Determination of 3,4-dihydroxyphenylacetic acid and 5-hydroxyindoleacetic acid in human plasma by a simple and rapid high-performance liquid chromatography assay. J Pharm Sci. 1996;85(4):451–453. doi: 10.1021/js950361q. [DOI] [PubMed] [Google Scholar]
- 12.Minegishi A, Ishizaki T. Determination of free 3-methoxy-4-hydroxyphenylglycol with several other monoamine metabolites in plasma by high-performance liquid chromatography with amperometric detection. J Chromatogr. 1984;311(1):51–57. doi: 10.1016/s0378-4347(00)84690-3. [DOI] [PubMed] [Google Scholar]
- 13.Seretti A, Cusin C, Lattuada E, Di Bella D, Catalano M, Smeraldi E. Serotonin transporter gene (5-HTTLPR) is not associated with depressive symptomatology in mood disorders. Mol Psychiatry. 1999;4(3):280–283. doi: 10.1038/sj.mp.4000485. [DOI] [PubMed] [Google Scholar]
- 14.Heninger GR, Charney DS. Mechanism of action of antidepressant treatment implication for the etiology and treatment of depressive disorders. In: Meltzer HY, editor. Psychopharmacology The 3rd Generation of Progress. New York, NY: Raven Press; 1987. pp. 535–544. [Google Scholar]
- 15.Chalon SA, Granier LA, Vandenhende FR, et al. Duloxetine increases serotonin and norepinephrine availability in healthy subjects: a double-blind, controlled study. Neuropsychopharmacology. 2003;28(9):1685–1693. doi: 10.1038/sj.npp.1300209. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins SC, Reasner DS, Koblan KS. Catechol-O-methyltransferase genotype as modifier of superior responses to venlafaxine treatment in major depressive disorder. Psychiatry Res. 2013;208(3):285–287. doi: 10.1016/j.psychres.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Antypa N, Drago A, Serretti A. The role of COMT gene variants in depression: bridging neuropsychological, behavioral and clinical phenotypes. Neurosci Biobehav Rev. 2013;37(8):1597–1610. doi: 10.1016/j.neubiorev.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura R, Nakamura J, Ueda N, Terao T. Effect of risperidone on plasma free 3-methoxy-4-hydroxyphenylglycol (pMHPG) levels in schizophrenic patients: relationship among plasma concentrations of risperidone and 9-hydroxyrisperidone, pMHPG levels, and clinical improvement. Int Clin Psychopharmacol. 2000;15(3):175–180. doi: 10.1097/00004850-200015030-00007. [DOI] [PubMed] [Google Scholar]