Abstract
Alterations in metabolism influence lifespan in experimental models, but data in humans are lacking. Here we use liquid chromatography/mass spectrometry to quantify 217 plasma metabolites and examine their relation to longevity in a large cohort of men and women. In 647 individuals followed for up to 20 years, higher concentrations of the citric acid cycle intermediate, isocitrate, and the bile acid, taurocholate, are associated with lower odds of longevity, defined as attaining 80 years of age. In a larger cohort of 2,327 individuals with metabolite data available, higher concentrations of isocitrate but not taurocholate are also associated with worse cardiovascular health at baseline, as well as risk of future cardiovascular disease and death. None of the metabolites identified are associated with cancer risk. Our findings suggest that some, but not all, metabolic pathways to human longevity are dependent on modifying risk for the two most common causes of death.
The mechanisms underlying a person’s ability to achieve longevity remain poorly understood. Much work has been done to identify the biological pathways that regulate lifespan in several model organisms including C. elegans, Drosophila, and mice.1, 2 However, translating these findings to humans has been difficult. Identifying the mechanistic contributors to lifespan in humans is intrinsically challenging – largely due to the fact that numerous exposures, accumulating over the life course, can serve to modulate the influence of extant genetic determinants.3, 4 Despite this complex challenge, both the experimental literature and work to date in human studies point to alterations in metabolism as being the central and likely highest-yield targets for investigation.1, 5, 6, 7, 8 This recognition has focused attention on the possibility that metabolomics technologies may shed light on pathways to longevity in humans.9, 10, 11 By monitoring a large panel of purportedly related and unrelated small molecules in a given tissue (e.g. circulating plasma), metabolite profiling can provide ‘snapshot’ data reflecting the extent to which the biochemical pathways may be up-regulated or down-regulated in one individual compared to the next – thus integrating the influence of both endogenous and exogenous factors (i.e. providing data on both genetic and environmental influences at a given point in time). Studies to date have analyzed the cross-sectional associations of metabolites with clinical traits in aged adults.9, 10, 11, 12 By contrast, the extent to which distinct metabolite profiles captured earlier in life are prospectively associated with longevity in humans remains entirely unknown.
Because the human metabolome is dynamic and constantly in flux, there have been concerns at the outset that detectable variation at a single point in time may not necessarily be associated with a clinical outcome years later. However, recent investigations in large cohorts have now demonstrated that alterations in circulating concentrations of distinct polar and lipid metabolites can predict the development of a specific disease phenotype, such as cardiovascular disease or diabetes or cancer, even a decade or more after the metabolite profile was originally captured.13, 14, 15, 16, 17 These findings suggest that metabolite profiling could also help to uncover pathways related to overall survival and longevity.
To this end, we performed a high-throughput metabolomics-based investigation in two well-phenotyped cohorts of middle-aged to older adults. We sought to identify circulating metabolite profiles associated with the future ability to achieve longevity, and assessed whether the metabolites identified were related to cardiovascular or cancer risk. We discovered unique polar compounds and lipid analytes related to longevity, including citric acid cycle intermediates and the bile acid taurocholate. Notably, only the citric acid cycle intermediates were concomitantly associated with cardiovascular risk and no longevity related metabolites were associated with cancer risk, suggesting the possibility that some metabolic pathways to longevity in humans are distinct from those implicated in the development of common morbid diseases.
RESULTS
Longevity Outcomes
We performed metabolite profiling on plasma samples collected from 2,327 participants of the Framingham Offspring Study who underwent a routine examination between 1991 and 1995. Of this ‘source’ study sample, a subset of 647 individuals had the chance to reach age 80 years by the end of the follow-up period in December 31, 2010 (Supplementary Fig. 1). Approximately half of all study participants were women (Table 1). Of individuals with the chance to reach age 80 years, 79% (N=514) succeeded in this regard. Of those who achieved longevity, 73% (N=377) never developed cardiovascular disease and 25% (N=126) developed cardiovascular disease after age 70 (Supplementary Table 1). Overall, 73% (N=376) reached age ≥80 free of cancer, and 54% (N=275) lived to age ≥80 without ever developing either cardiovascular disease or cancer.
Table 1. Sample Characteristics.
| Clinical Characteristics | First Stage Study Sample N=647 |
Second Stage Study Sample N=2327 |
|---|---|---|
| Age, years | 66.9±3.9 | 54.9±9.8 |
| Female, % | 51 | 53 |
| Body mass index, kg/m2 | 27.7±4.6 | 27.4±5.0 |
| Systolic blood pressure, mmHg | 135±20 | 126±19 |
| Diastolic blood pressure, mmHg | 74±10 | 75±10 |
| Hypertension, % | 56 | 35 |
| Treatment for hypertension, % | 35 | 19 |
| Total cholesterol, mg/dL | 214±38 | 206±37 |
| HDL cholesterol, mg/dL | 49±15 | 50±15 |
| Total/HDL cholesterol | 4.7±1.5 | 4.5±1.6 |
| Triglycerides, mg/dL* | 135 (98,191) | 120 (85,178) |
| Treatment for cholesterol, % | 13 | 7 |
| Fasting glucose, mg/dL | 105±32 | 100±28 |
| Treatment for diabetes, % | 4 | 3 |
| Diabetes, % | 10 | 6 |
| Current smoker, % | 14 | 19 |
| Physical activity index | 33.8±5.5 | 34.7±6.3 |
Values are shown as means±standard deviation or percentages.
Triglyceride values are non-normally distributed and are thus shown as medians (25th, 75th percentiles).
Metabolite Markers of Longevity
In the first stage of analyses, we performed age- and sex-adjusted logistic regression models in the sample with the chance to reach age 80 (N=647) and identified multiple distinct metabolites associated with longevity (Table 2 and Supplementary Data 1). A Bonferroni corrected P value threshold of 0.001 was used to account for multiple testing across the 37 clusters of inter-correlated metabolites (0.05/37) (see Methods for additional details). In analyses additionally adjusting for previously reported correlates of attenuated lifespan, including smoking, the association of cotinine with longevity became non-significant, consistent with its generation as a by-product of nicotine. In these multivariable-adjusted models, isocitrate and taurocholate emerged as particularly significant correlates of longevity (Table 2). Each standard deviation increment in log-isocitrate was associated with a 37% lower odds of achieving longevity (adjusted odds ratio 0.63, 95% CI 0.49-0.81; P=3.9E-04). Addition of isocitrate to a multivariable model comprised of traditional risk factors modestly, but significantly improved the C statistic from 0.72 to 0.74 (P=0.0003). Similarly, each standard deviation increase in log-taurocholate was associated with a 37% lower odds of achieving longevity (adjusted odds ratio 0.63, 95% CI 0.48-0.83; P=7.4E-04), and inclusion of taurocholate in the model improved the C statistic from 0.72 to 0.75 (P=0.0003). With respect to these associations, there no significant effect modification by age or sex (P>0.20 for all interaction terms). In addition to isocitrate, two other citric acid cycle intermediaries, aconitate and malate, were also associated with lower odds of achieving longevity (P=0.002 and 0.001, respectively). Several other bile acids, including taurodeoxycholates (P=0.002) and glycodeoxycholates (P=0.007), also had nominal associations with longevity. The concordant associations of additional metabolites along these two metabolic pathways add further support to the primary findings. In secondary analyses performed using age thresholds of 85, 75, and 70 years to define longevity, results of the main analyses were similar (Supplementary Tables 2-4).
Table 2. Metabolites Associated with Attaining Longevity Defined as 80 Years of Age (First Stage Sample: N=647).
| Profiling Method |
Metabolite* | Age- and Sex-Adjusted | Multivariable-Adjusted† | ||
|---|---|---|---|---|---|
|
| |||||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Polar, positively charged |
cotinine | 0.65 (0.53-0.79) | 1.1E-05 | 0.79 (0.56-1.13) | 0.20 |
| histidine | 1.33 (1.10-1.61) | 0.004 | 1.28 (1.05-1.56) | 0.02 | |
| lysine | 1.36 (1.10-1.68) | 0.004 | 1.32 (1.06-1.65) | 0.01 | |
| threonine | 1.34 (1.09-1.64) | 0.006 | 1.30 (1.04-1.62) | 0.02 | |
|
| |||||
| Polar, negatively charged |
aconitate | 0.74 (0.60-0.92) | 0.007 | 0.73 (0.58-0.92) | 0.008 |
| beta-hydroxybutyrate | 0.71 (0.57-0.89) | 0.003 | 0.73 (0.58-0.92) | 0.008 | |
| isocitrate | 0.65 (0.52-0.82) | 2.5E-04 | 0.63 (0.49-0.81) | 3.9E-04 | |
| malate | 0.75 (0.62-0.91) | 0.003 | 0.75 (0.61-0.92) | 0.005 | |
| taurocholate | 0.65 (0.50-0.83) | 5.0E-04 | 0.63 (0.48-0.83) | 7.4E-04 | |
| uridine | 1.41 (1.14-1.76) | 0.002 | 1.27 (1.01-1.59) | 0.04 | |
|
| |||||
| Lipid | LPC 22:6 | 1.54 (1.23-1.94) | 2.1E-04 | 1.41 (1.11-1.79) | 0.005 |
| PC 38:6 | 1.62 (1.29-2.05) | 4.6E-05 | 1.52 (1.18-1.95) | 0.001 | |
LPC, lysophosphatidylcholine; PC, phosphatidylcholine.
Metabolites were natural log transformed and standardized (mean=0, sd=1).
All logistic regression analyses adjusted for age and sex; multivariable-adjusted models additionally adjusted for body mass index, systolic blood pressure, anti-hypertensive treatment, diabetes, smoking status, total cholesterol, HDL cholesterol, and log triglycerides.
Metabolite Markers of Morbid Disease and All-Cause Mortality
In the second stage of analyses, we examined whether metabolites associated with longevity were also associated with all-cause mortality, cardiovascular risk, and cancer risk in the full source sample (N=2,327). In these analyses, a Bonferroni corrected P threshold of 0.004 was used to account for multiple testing across the 12 metabolites found in association with longevity in the primary analyses (0.05/12). Over the total follow-up period of 13.6±6.2 years, there were a total of 439 deaths from all causes. In multivariable analyses adjusting for age, sex, body mass index, systolic blood pressure, anti-hypertensive treatment, diabetes, smoking status, and total/HDL cholesterol, we observed nominal to significant associations of aconitate (P=0.0007), isocitrate (P<0.0001), malate (P=0.003), and taurocholate (P<0.0001) with incidence of all-cause mortality (Table 3). We observed a significant association of uridine with all-cause mortality in the larger source sample, although the association of uridine with longevity did not meet the Bonferroni-corrected threshold of statistical significance in the subset longevity study sample (Table 2).
Table 3. Metabolites Associated with All-Cause Mortality (Second Stage Sample: N=2327).
| Profiling Method |
Metabolite* | Age- and Sex-Adjusted | Multivariable-Adjusted† | ||
|---|---|---|---|---|---|
|
| |||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Polar, positively charged |
cotinine | 1.39 (1.27-1.51) | 1.8E-13 | 1.21 (1.03-1.42) | 0.02 |
| histidine | 0.85 (0.77-0.93) | 0.0003 | 0.87 (0.79-0.95) | 0.003 | |
| lysine | 0.85 (0.77-0.93) | 0.0007 | 0.85 (0.77-0.94) | 0.002 | |
| threonine | 0.90 (0.82-0.996) | 0.04 | 0.93 (0.84-1.02) | 0.14 | |
|
| |||||
| Polar, negatively charged |
aconitate | 1.25 (1.12-1.40) | 5.0E-05 | 1.21 (1.09-1.36) | 0.0007 |
| beta-hydroxybutyrate | 1.21 (1.10-1.33) | 0.0002 | 1.17 (1.06-1.29) | 0.002 | |
| isocitrate | 1.34 (1.20-1.49) | 2.0E-07 | 1.26 (1.12-1.41) | 9.6E-05 | |
| malate | 1.17 (1.07-1.29) | 0.001 | 1.15 (1.05-1.27) | 0.003 | |
| taurocholate | 1.14 (1.02-1.28) | 0.02 | 1.14 (1.01-1.28) | 0.03 | |
| uridine | 0.77 (0.69-0.85) | 1.8E-07 | 0.80 (0.72-0.88) | 1.3E-05 | |
|
| |||||
| Lipid | LPC22:6 | 0.86 (0.77-0.95) | 0.003 | 0.92 (0.82-1.02) | 0.10 |
| PC38:6 | 0.83 (0.75-0.93) | 0.0007 | 0.88 (0.79-0.98) | 0.02 | |
No. deaths per persons at risk was 439/2327 for the sample with polar positive analyte data, 372/1937 for the sample with polar negative analyte data, and 373/1944 for the sample with lipid data.
LPC, lysophosphatidylcholine; PC, phosphatidylcholine.
Metabolites were natural log transformed and standardized (mean=0, sd=1).
All analyses adjusted for age and sex; multivariable-adjusted models additionally adjusted for body mass index, systolic blood pressure, anti-hypertensive treatment, diabetes, smoking status, and total/HDL cholesterol.
During the follow-up period, 358 of the total 2327 individuals at risk developed a new-onset cardiovascular event (coronary heart disease, stroke or transient ischemic attack, or heart failure). Of the metabolites associated with longevity (Table 2), only isocitrate was associated with incident cardiovascular disease (Table 4). For each 1-SD increase in log-isocitrate, the risk of incident cardiovascular disease increased 22% (P=0.002) in analyses adjusted for traditional cardiovascular risk factors. In multivariable-adjusted models, the citric acid cycle intermediate malate was similarly associated with greater cardiovascular risk (P=0.009; Table 4) as well as decreased longevity (Table 2). A total of 424 individuals developed a new diagnosis of cancer over the follow-up period. In multivariable analyses adjusting for age, sex, body mass index, and smoking status, none of the metabolites listed in Table 2 were significantly associated with incident cancer (P≥0.06 for all, data not shown).
Table 4. Metabolites Associated with Incident Cardiovascular Disease (Second Stage Sample: N=2327).
| Profiling Method |
Metabolite* | Age- and Sex-Adjusted | Multivariable-Adjusted† | ||
|---|---|---|---|---|---|
|
| |||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Polar, positively charged |
cotinine | 1.20 (1.08-1.32) | 0.0004 | 1.14 (0.95-1.36) | 0.16 |
| histidine | 0.87 (0.80-0.96) | 0.005 | 0.88 (0.80-0.97) | 0.01 | |
| lysine | 0.97 (0.88-1.07) | 0.55 | 0.95 (0.85-1.05) | 0.31 | |
| threonine | 0.91 (0.82-1.01) | 0.07 | 0.94 (0.85-1.04) | 0.20 | |
|
| |||||
| Polar, negatively charged |
aconitate | 1.18 (1.05-1.33) | 0.005 | 1.10 (0.97-1.24) | 0.14 |
| beta-hydroxybutyrate | 1.10 (0.99-1.23) | 0.07 | 1.10 (0.98-1.23) | 0.10 | |
| isocitrate | 1.35 (1.20-1.52) | 3.0E-07 | 1.22 (1.08-1.38) | 0.002 | |
| malate | 1.18 (1.05-1.32) | 0.004 | 1.16 (1.04-1.30) | 0.009 | |
| taurocholate | 1.06 (0.93-1.20) | 0.40 | 1.03 (0.90-1.17) | 0.69 | |
| uridine | 0.89 (0.80-0.995) | 0.04 | 0.93 (0.83-1.04) | 0.22 | |
|
| |||||
| Lipid | LPC22:6 | 0.92 (0.82-1.02) | 0.09 | 0.96 (0.87-1.07) | 0.50 |
| PC38:6 | 0.93 (0.83-1.04) | 0.18 | 0.95 (0.84-1.06) | 0.34 | |
No. events per persons at risk was 358/2327 for the sample with polar positive analyte data, 326/1937 for the sample with polar negative analyte data, and 326/1944 for the sample with lipid data.
LPC, lysophosphatidylcholine; PC, phosphatidylcholine.
Metabolites were natural log transformed and standardized (mean=0, sd=1).
All analyses adjusted for age and sex; multivariable-adjusted models additionally adjusted for body mass index, systolic blood pressure, anti-hypertensive treatment, diabetes, smoking status, and total/HDL cholesterol.
We sought to replicate findings from the second stage by performing analyses in the Malmö Diet and Cancer (MDC) cohort (N=325) with the same metabolites (polar positive, polar negative, and lipid compounds) assayed as in the Framingham cohort. We focused on all-cause mortality, because the smaller sample size did not provide adequate power to examine individual categories of events. In the MDC replication sample (Supplementary Table 4), there were a total of 36 deaths over a mean follow-up period of 15.8±2.4 years. In this smaller sample, and consistent with findings in the larger Framingham cohort, isocitrate was significantly associated with mortality in multivariable adjusted models with a hazards ratio (HR) of 1.42 (95% CI 1.02-1.98; P=0.04). Malate demonstrated a similar multivariable-adjusted association with mortality (HR 1.37 [95% CI 0.99-1.91); P=0.059) whereas aconitate (P=0.20) and taurocholate (P=0.43) were not significantly associated with mortality.
Metabolite Markers of Longevity and Morbidity Profiles
We also investigated the association of metabolites with pre-defined morbidity profiles (survivors, delayers, and escapers). Among individuals who lived to age 80, we compared the baseline values of select metabolites between those who ever developed cardiovascular disease or cancer and those who remained free of these disease conditions. Among individuals who achieved longevity, levels of the citric acid cycle intermediates were significantly different between those who ever developed cardiovascular disease compared with those who remained free of cardiovascular disease over the life course (Fig. 1): log aconitate (P=0.004), log isocitrate (P=0.0005), and log malate (P=0.012). Baseline levels of these metabolites did not differentiate between individuals who achieved longevity with or without ever developing cancer (Fig. 1; P>0.41 for all). Of the remaining metabolites identified in association with longevity in the main analyses (Table 2), none significantly differentiated between morbidity profiles among individuals who lived to at least age 80 (data not shown).
Figure 1. Metabolites associations with longevity and morbidity profiles.
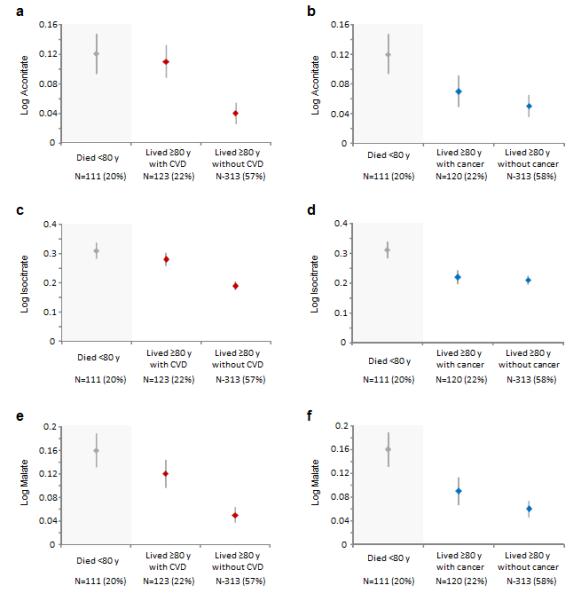
Mean and standard error values of selected log metabolite values are shown by morbidity profile in the first stage study sample (N=647). Among individuals who lived to age ≥80 years, log aconitate (P=0.004), log isocitrate (P=0.0005), and log malate (P=0.012) levels were significantly higher in those who ever developed cardiovascular disease compared to those who remained free of cardiovascular disease (a, c, and e). These metabolites did not differentiate between individuals based on cancer morbidity (b, d, and f). Comparisons were performed using t-tests.
Metabolite Markers of Longevity and Baseline Cardiovascular Health
To investigate the potential contribution of cardiovascular health to observed associations, we analyzed age- and sex-adjusted correlations of metabolites with the ideal cardiovascular health score at baseline in the source sample. The distribution of ideal cardiovascular health score and its components is shown in Supplementary Fig. 2. Of the metabolites associated with longevity (Table 2), those significantly related to the ideal cardiovascular health score at baseline included isocitrate as well as aconitate. Higher levels of these metabolites were associated not only with decreased longevity (Table 2), but also worse cardiovascular health score: age- and sex-adjusted Spearman’s rank correlations were −0.29 (P=6.6E-13) and ρ −0.16 (P=4.7E-05) for isocitrate and aconitate, respectively (Fig. 2 and Supplementary Data 2). Additionally, the lipophospholipid LPC 22:6 was associated not only with greater odds of longevity (Table 2), but also with an increased ideal cardiovascular health score (Spearman rank correlation, 0.14; P=9.6E-09) (Fig. 2 and Supplementary Data 2). Notably, ideal cardiovascular health score at baseline accounted for a modest proportion of variation in these metabolites (<7%), as shown in Supplementary Table 6. Associations between the full panel of metabolites and the ideal cardiovascular health score and its components are displayed in Supplementary Figs 3a, 3b, and 3c.
Figure 2. Metabolite associations with ideal cardiovascular health.
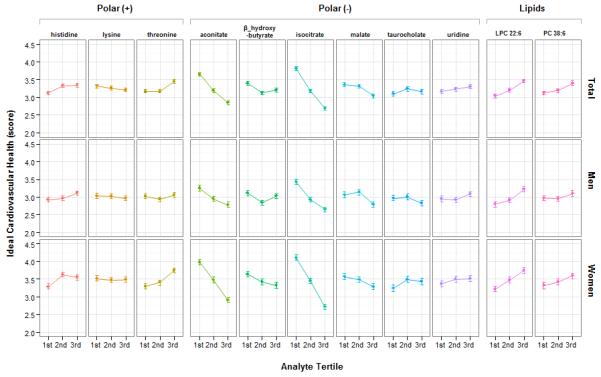
Mean and standard error cardiovascular health scores are shown by tertile of selected analytes in the second stage study sample (N=2327) and by sex, where higher score denotes more ideal cardiovascular health. Analytes were selected based on their associations with longevity in the main study sample (N=647).
Pathway Analyses
Our overall results identified metabolites associated with longevity through potentially distinct pathways (Fig. 3a). Therefore, we used metabolomic pathway topology analysis to estimate the relative importance of metabolites associated with longevity and the pathways they represent (MetPA, Edmonton, Canada).18 Based on existing Homo sapiens data available from the KEGG database, results of these analyses are detailed in Supplementary Table 7 and highlight the potential relative importance of several distinct pathways (Fig. 3b).
Figure 3. Metabolite pathways observed in association with human longevity.
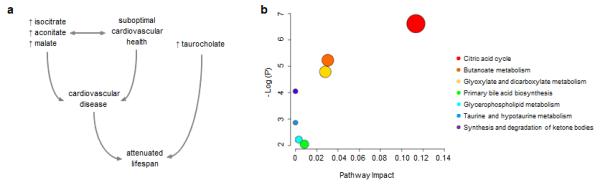
Aggregate results of the current study suggest that higher circulating levels of certain citric acid cycle intermediates (isocitrate, aconitate, and malate) are associated with shortened lifespan by modulating cardiovascular risk, whereas increased taurocholate is related to shorter lifespan independent of any concurrent associations with major morbid disease (a). Metabolomic pathway topology analysis highlights the potential importance of distinct pathways that comprise the human metabolome that are represented by metabolites associated with longevity in the current study (b).
DISCUSSION
Recent studies have sought to identify genetic as well as environmental factors that impact human lifespan.3, 4 Extending this prior work, we used high-throughput plasma metabolite profiling to identify ‘signatures’ of biochemical activity that may reflect the combined influence of endogenous and exogenous determinants – and offer a view of the potential pathways contributing to longevity among ambulatory adults living in the community. Using this approach, we identified metabolites not previously associated with lifespan in humans. Because cardiovascular diseases remain by far the leading cause of death worldwide,19 maintenance of cardiovascular health over the life course would appear to strongly favor longevity.20 Therefore, we also assessed associations between plasma metabolites and the ability to maintain cardiovascular health.
In our study, select citric acid cycle intermediates were associated with both worse cardiovascular health and decreased longevity. Although data in humans are scant, a substantial body of experimental data across several model systems has implicated citric acid cycle function in the regulation of lifespan in several model organisms. In yeast, citric acid cycle gene expression and activity are increased in the setting of increased longevity.21, 22 In Drosophila, reduced gene expression of Indy (the plasma membrane transporter for citric acid cycle intermediates) extends lifespan.23, 24 Knocked down homologs of Indy have also been related to extended lifespan in C. elegans and dietary restriction phenotypes mice.25, 26, 27 Furthermore, citric acid cycle genes are upregulated in long-lived Ames dwarf and Little mice,28 and citric acid cycle function is preserved rather than reduced with aging in long-lived compared to short-lived strains of Brown-Norway rats.29
Experimental studies have also reported on potential links between the specific citric acid cycle intermediates identified in our study (aconitate, isocitrate, and malate) and longevity as well as cardiovascular phenotypes. Citrate is known to undergo stereospecific isomerization to isocitrate via cis-aconitate in the presence of aconitase; in turn, isocitrate undergoes oxidative decarboxylation in the presence of isocitrate dehydrogenase (IDH). Interestingly, decreased expression of IDH has been shown to increase generation of reactive oxidation species, DNA fragmentation, lipid peroxidation, and concurrent mitochondrial damage.30 Accordingly, in C. elegans, inactivation of IDH as well as aconitase genes via RNA interference has been shown to extend lifespan.31 Addition of malate has also been reported to extend lifespan in C. elegans while increasing the NAD to NADPH ratio.32 In non-malignant cells, cytosolic IDH1 is known to be involved in lipid metabolism and glucose sensing, and mitochondrial IDH2 contributes to regulation of redox homeostasis.30, 33 Notably, mitochondrial IDH2 expression is particularly abundant in the heart.34 In fact, experimental data have implicated mitochondrial IDH35, 36, 37 as well as aconitase35, 38 and malate dehydrogenase,35, 39 in the development of cardiac hypertrophy. The extent to which activity of these enzymes affects variation in levels of peripherally circulating substrate metabolites is not yet known. In human malignant cells, mutations in the IDH1 and IDH2 isoforms appear to promote tumor survival.40, 41, 42 Our data showed no association between isocitrate levels and cancer, though the extent to which circulating plasma levels relate to oncologic risk merits further investigation.
Additional metabolites related to both better cardiovascular health and increased longevity included LPC 22:6. This docosahexaenoic acid-containing lipophospholipid has been related to increased fish intake43 and decreased risk for developing diabetes,14 but not previously associated with longevity. Our study also identified taurocholate as a marker of human longevity. For each 1-SD increase in log taurocholate, the odds of achieving longevity decreased by almost 40%. Taurocholate is a bile acid conjugate of cholic acid and taurine, and experimental studies have suggested that taurine lowers risk for hypertension, stroke, and other atherosclerotic diseases.44 Interestingly, increased taurocholate was associated with reduced lifespan in the absence of any relation to risk for cardiovascular disease or for cancer. This finding suggests that certain bile acids may represent a pathway to longevity unrelated to cardiovascular or cancer risk per se.
Bile acids are now recognized as signaling molecules that regulate not only cholesterol homeostasis but also glucose and energy metabolism.45, 46 Bile acids have been shown to induce energy expenditure, decrease body weight, and enhance glucose tolerance by activating TGR5, a G protein-coupled receptor expressed in brown fat and muscle tissue.47, 48 Additionally, intestinal infusion of taurocholate in an animal model was observed to substantially increase activation of the Akt signaling pathway,49 a reported determinant of cellular senescence.50, 51 Because bile acids are actively involved in regulating their own synthesis through enterohepatic circulation, the degree to which higher or lower circulating plasma levels of taurocholate reflect overall bile acid activity is not well understood. Further research is needed to better understand relationship between taurocholate metabolism and human longevity.
Several limitations of the present study merit consideration. Given the age range of our study cohort at baseline, few individuals in our study had the chance to live to age 90 or 100 years, which are definitions of human longevity that have been studied previously. Therefore, we considered longevity as living to age 80, and inclusion in our longevity study sample (as derived from the larger source sample) was dependent on a minimum age requirement at the baseline examination. However, the metabolites identified in the current study are distinct from those previously associated with older age,52 and all reported associations in our study were significant in analyses adjusting for age as well as sex. Cause of death could not be precisely adjudicated in our study because detailed clinical information around the time of death was not available for all participants. It is important to note that our liquid chromatography mass spectrometry platform identifies a large number of metabolites that have not been assayed in other large study samples with similarly long-lived individuals, precluding the ability for complete validation of our main findings in a separate cohort. Thus, our findings should be considered hypothesis-generating, while providing interesting results that are consistent with data from experimental literature. Because our study samples included only adults of European ancestry, the generalizability of our findings to individuals with broader demographic representation is unknown. Further investigations of metabolomic traits in more diverse cohorts with longitudinal follow-up data on adequately aged individuals appear warranted. Although we observed concordance in directionality of metabolite associations in both the primary FHS sample and the smaller-sized MDC replication sample, future studies in cohorts larger are also needed to further investigate possible additional metabolite associations with longevity that our samples were not adequately powered to detect.
In summary, several polar compounds and lipid analytes were associated with longevity in our large, community-based cohort. The citric acid cycle intermediate, isocitrate, and the bile acid, taurocholate, demonstrated particularly significant associations with longevity. Lower circulating levels of isocitrate and other citric acid cycle intermediates were not only associated with longevity but were also associated with ideal cardiovascular health at baseline, with lower risk for developing cardiovascular events over the follow-up period, and with greater likelihood of achieving longevity free of any cardiovascular disease. These findings suggest that alterations in citric acid cycle activity are related to longevity at least partly through modulating cardiovascular risk. In contrast, taurocholate demonstrated a significant association with longevity in the absence of any relations to either cardiovascular or cancer risk. Thus, our data indicate that some but not all metabolic pathways to longevity may be dependent on modulating risk for two of the most common causes of death.
METHODS
Study Samples
In 1971, the Framingham Offspring Study enrolled 5,124 individuals in a prospective community-based cohort study, as described previously.53 All study participants have been followed longitudinally with quadrennial examinations. Plasma samples from a total of 2,526 participants, from the fifth examination (1991-1995), were analyzed by metabolite profiling. After excluding 90 individuals with prevalent cardiovascular disease and 109 with missing clinical covariates, a total of 2,327 participants had complete information available, based on data collected from a physician-administered medical history, physical examination, and routine laboratory tests. These individuals comprised the ‘source’ study sample. Of this source sample, 647 participants attended their fifth examination on a date that allowed the possibility of reaching age 80.0 years by the end of the follow-up period (December 31, 2010). These individuals comprised the study sample for the first metabolite discovery stage. All participants provided written informed consent. All study protocols were approved by the Boston University Medical Center Institutional Review Board.
Selected findings from Framingham were examined for replication in the cardiovascular cohort of the Malmö Diet and Cancer (MDC) study, an investigation of 6,103 individuals originally enrolled between 1991 and 1996 as part of a longitudinal population-based epidemiologic cohort. All participants attending the first study visit underwent a standardized physical examination and medical history, and routine laboratory tests. A total of 791 participants provided fasting plasma specimens that underwent metabolite profiling using at least 1 of the 3 main profiling methods described herein (for identifying polar positive, polar negative, or lipid compounds).54 Of this sample, after excluding individuals with a history of cardiovascular disease or missing covariate data, plasma specimens from a total of 325 individuals were identified as having underwent metabolite profiling using all 3 of the exact same profiling methods as used in the Framingham analyses; these individuals were originally selected from the larger MDC study as part of a previously reported diabetes case-control study.13 All study protocols were approved by the Institutional Review Board of Lund University, Sweden. All participants provided written informed consent.
Assessment of Longevity and Related Outcomes
For the first stage of analyses in Framingham, we defined longevity as achieving age ≥80.0 years during the follow-up period. We selected age 80 years as the pre-specified threshold for longevity to maximize the total number of participants available for analyses given the baseline age and life expectancy in our cohort (Supplementary Table 8). Thus, the primary longevity analyses were restricted to the individuals who had the ability to reach 80 years old during the follow-up period, as determined by their age at baseline and the length of follow up. To further assess potential contributors to longevity, we categorized individuals who achieved longevity as ‘survivors’ (developed either of the 2 major morbidities, cardiovascular disease or cancer, before age 70), ‘delayers’ (developed cardiovascular disease or cancer after age 70) and ‘escapers’ (never developed cardiovascular disease or cancer). All study participants were under longitudinal surveillance for incidence of cardiovascular disease, cancer, and death; all events were adjudicated by a panel of 3 investigators based on a review of medical records.55 Incident cardiovascular disease was defined as new-onset coronary heart disease, heart failure, or stroke. Information on cancer outcomes was collected from the medical history at routine Framingham examinations, and all reports of cancer were verified by a review of medical records including submitted pathology, surgery, and autopsy reports.56 Incident cancer was defined as a new diagnosis of malignant neoplasm, not including non-melanoma skin cancer.57 Cause of death was determined based on review of medical records, death certificates, and interviews with next of kin.
Assessment of Cardiovascular Health
We further assessed the role of cardiovascular comorbities in the longevity experience by constructing a cardiovascular health score based on the 7 metrics identified by the American Heart Association (AHA) as ideal goals for risk reduction in the general population.58 These parameters of ‘ideal’ cardiovascular health have been associated, in aggregate, with lower risk for both cardiovascular death and all-cause mortality.20 Therefore, we calculated the ideal cardiovascular health score based on the following AHA criteria: untreated total cholesterol <200 mg/dL, untreated blood pressure <120/80 mmHg, non-smoker within the prior 12 months, untreated fasting blood glucose <100 mg/dL, body mass index <25 kg/m2, meeting ≥2 of the AHA diet goals based on data collected from a validated food frequency questionnaire,59 and optimal physical activity defined as being in the upper quartile of the physical activity index (PAI).60 Ideal diet was defined as meeting ≥2 dietary goals given the very low frequency of individuals meeting ≥4 dietary goals (2.1%); similar observations have been made in the NHANES cohorts.20 The assessment of ideal diet involves assigning 1 point for each of the following:58 daily intake of ≥4.5 cups of fruits and vegetables; at least twice weekly intake of 3.5-ounce serving of fish; at least thrice daily 1-ounce services of fiber-rich whole grains; less than 1500 mg daily of sodium; and, no more than 36 ounces weekly of sugar sweet beverages. For data regarding fruit and vegetables intake that was not reported in cup units in the Framingham food frequency questionnaire, conversions were performed based on USDA guidelines.61 Data regarding whole grains intake was converted from servings to ounces, based on recommendations of the US Whole Grains Council.62 For estimating sugar sweet beverage intake, 1 can of soda was considered equivalent to 12 ounces. It should be noted that because the questionnaire did not capture data on added salt, assessment of salt intake was derived exclusively from the food and beverage survey. Because leisure-time physical activity duration was not assessed, we used the PAI assessment which is known to be correlated with metabolic equivalent tasks (METs).63 Presence of any of these variables was assigned 1 point as part of a clinical score ranging from 0 to 7.
Metabolite Profiling
Metabolite profiling was performed on plasma samples that were collected from study participants following an overnight fast and then stored at −80°C prior to the metabolite profiling assays. Plasma metabolites were analyzed using liquid chromatography-tandem mass spectrometry (LC-MS). Metabolites were extracted using organic solvents (75%acetonitrile/25%methanol for positively charged polar compounds, 80% methanol for negatively charged polar compounds, and isopropanol for lipids) prior to separation on an LC column. LC-MS data were acquired using either an AB SCIEX 4000 QTRAP triple quadrupole mass spectrometer (positively charged polar compounds and lipids) or an AB SCIEX 5500 QTRAP triple quadrupole mass spectrometer (negatively charged polar compounds). Polar, positively charged metabolites were separated using hydrophobic interaction liquid chromatography (HILIC) and analyzed using multiple reaction monitoring (MRM) in the positive ion mode.13 Polar, negatively charged compounds, including central and polar phosphorylated metabolites, were separated using a Luna NH2 column (150 × 2 mm, Luna NH2, Phenomenex) and analyzed using MRM in the negative ion mode.64 Lipids were separated on a Prosphere C4 HPLC column and underwent full scan MS analysis in the positive ion mode.14 Collectively, >200 endogenous metabolites were reproducibly measured for comparative analyses. MultiQuant software (Version 1.2, AB SCIEX) was used for automated peak integration and manual review of data quality prior to statistical analysis.14 We prepared calibration curves for each analytical queue using internal reference standards (including isotope-labeled leucine-13C, 15N, isoleucine-13C6, 15N, alanine-13C, glutamic acid-13C5, 15N, taurine-13C2, trimethylamine-N-oxide-d9, and valine-d8 and the non-mammalian lipid 1-dodecanoyl-2-tridecanoyl-sn-glycero-3-phosphocholine), as previously described.13, 14, 65 For all 3 profiling platforms, a pooled plasma sample was also run following every 20 samples, and the peak areas in samples were normalized to metabolite peak areas in the nearest pooled plasma.
Statistical Analysis
All metabolite variables were natural log transformed due to skewed distributions. In the first stage study sample (N=647), we used age- and sex-adjusted logistic regression models to examine the associations of metabolites with longevity. We then repeated logistic regression analyses with additional adjustment for body mass index, systolic blood pressure, anti-hypertensive treatment, diabetes, smoking status, total cholesterol, HDL cholesterol, and log triglycerides. We used logistic regression analyses to examine associations with ability to achieve longevity given the age range of participants at baseline, precluding a time to event analysis. In the second stage, we examined the all individuals with metabolite profiling in Framingham (N=2327). For metabolites observed to demonstrate significant associations with longevity, we performed Cox proportional hazards models to analyze their associations with incidence of all-cause mortality, cardiovascular disease, and cancer. Covariates included in all multivariable-adjusted models were selected based on reported associations with longevity phenotypes in community-based studies.66, 67, 68 We also calculated Spearman rank correlation coefficients to assess the relation of each metabolite with the cardiovascular health score, with adjustment for age and sex.
To account for multiple testing, we used a Bonferroni corrected P value threshold for the primary analyses of longevity as the main outcome. The majority of metabolites studied are known to be correlated within biological groups (e.g. amino acids, bile acids, urea cycle metabolites, lipids). Therefore, for each profiling method (polar positive, polar negative, and lipid), we performed 3 separate cluster analyses to identify groups of closely correlated metabolites in the total sample of individuals who attended the fifth Framingham Offspring examination and had metabolite data available (N=2,526). In this total sample, batch-adjusted residuals were generated for each metabolite (PROC MIXED) and these residuals were rank normalized using the Bloomberg method; we used cluster analysis (PROC VARCLUS) to classify correlated values into hierarchical groupings. Metabolite clusters were then formed on the basis of principal components (using eigenvalue thresholds of 1). In total, we identified 37 clusters of inter-correlated metabolites, and these clusters were noted to contain metabolites associated with distinct biological pathways (Supplementary Tables 9 to 11). Thus, based on previously described methods for accounting for multiple comparisons,69 a P value threshold of 0.05/37=0.001 was selected for the primary statistical analyses of longevity as the outcome.
To examine the extent to which analytes associated with longevity in the main analyses were also associated with outcomes related to longevity (i.e. all-cause mortality, incident cardiovascular disease, and cancer), we used a Bonferroni corrected P value threshold of 0.004 (0.05/12) given the number of analytes (N=12) found in association with longevity in the primary analyses.
All analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC). Graphical displays were created using R v2.15.2 (R Development Core Team, Vienna, Austria).
Supplementary Material
Acknowledgements
This work was supported by NIH contract N01-HC-25195 and grants R00-HL-107642 (S.C.), R01-DK-HL-081572 (R.E.G. and T.J.W.), R01-HL-098280 (R.E.G.), U01-HL-107440 (R.E.G.), K08-DK-090142 (E.P.R.), K23-HL-116780 (J.E.H.), as well as the Ellison Foundation (S.C.), Leducq Foundation (R.E.G.), and American Heart Association grant 12IRG9130006 (R.E.G.).
Footnotes
Author contributions
S.C., M.G.L., J.M.M., R.S.V., R.E.G., and T.J.W. conceived and designed the study. J.M.M, E.P.P., P.F.J., M.M., A.L.S., A.A.D., K.A.P., K.B., O.M., C.B.C., R.S.V., R.E.G., and T.J.W. acquired the data for analyses. S.C., M.G.L., E.L.M., A.G., M.M. R.E.G., and T.J.W. analyzed and interpreted the data. S.C., R.E.G., and T.J.W. drafted the manuscript. J.M.M., E.P.P., J.E.H., P.F.J., A.G., M.M., A.L.S., A.A.D., K.A.P., K.B., C.O.D., M.M., C.B.C., R.S.V. performed critical revisions of the manuscript for important intellectual content. All authors have discussed the results and reviewed the final manuscript.
Competing financial interests
None.
Accession codes
Data on the interaction between genotype and phenotype have been deposited in the database of Genotypes and Phenotypes (dbGaP) under accession codes pht002234.v3.p7, pht002566.v2.p7, pht002343.v2.p7, pht002567.v2.p7, pht002565.v2.p7 and pht002894.v1.p7.
REFERENCES
- 1.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin GM. The biology of aging: 1985-2010 and beyond. FASEB J. 2011;25:3756–3762. doi: 10.1096/fj.11-1102.ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebastiani P, Perls TT. The genetics of extreme longevity: lessons from the new England centenarian study. Frontiers in genetics. 2012;3:277. doi: 10.3389/fgene.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzuto D, Orsini N, Qiu C, Wang HX, Fratiglioni L. Lifestyle, social factors, and survival after age 75: population based study. BMJ. 2012;345:e5568. doi: 10.1136/bmj.e5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miquel J, Lundgren PR, Bensch KG, Atlan H. Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech Ageing Dev. 1976;5:347–370. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- 6.Anson RM, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- 8.Ogg S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 9.Montoliu I, et al. Serum profiling of healthy aging identifies phospho- and sphingolipid species as markers of human longevity. Aging (Albany NY) 2014;6:9–25. doi: 10.18632/aging.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collino S, et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013;8:e56564. doi: 10.1371/journal.pone.0056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Covarrubias V, et al. Lipidomics of familial longevity. Aging cell. 2013;12:426–434. doi: 10.1111/acel.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auro K, et al. A metabolic view on menopause and ageing. Nature communications. 2014;5:4708. doi: 10.1038/ncomms5708. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee EP, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121:1402–1411. doi: 10.1172/JCI44442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah SH, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. 2012;163:844–850. e841. doi: 10.1016/j.ahj.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Wang TJ, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123:4309–4317. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayers JR, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193–1198. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010;26:2342–2344. doi: 10.1093/bioinformatics/btq418. [DOI] [PubMed] [Google Scholar]
- 19. [Accessed March 1, 2014];The top 10 causes of death, World Health Organization. http://www.who.int/mediacentre/factsheets/fs310/en/index.html
- 20.Yang Q, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Jiang JC, Jazwinski SM. Gene regulatory changes in yeast during life extension by nutrient limitation. Experimental gerontology. 2010;45:621–631. doi: 10.1016/j.exger.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamei Y, Tamura T, Yoshida R, Ohta S, Fukusaki E, Mukai Y. GABA metabolism pathway genes, UGA1 and GAD1, regulate replicative lifespan in Saccharomyces cerevisiae. Biochemical and biophysical research communications. 2011;407:185–190. doi: 10.1016/j.bbrc.2011.02.136. [DOI] [PubMed] [Google Scholar]
- 23.Knauf F, Rogina B, Jiang Z, Aronson PS, Helfand SL. Functional characterization and immunolocalization of the transporter encoded by the life-extending gene Indy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14315–14319. doi: 10.1073/pnas.222531899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogina B, Helfand SL. Indy mutations and Drosophila longevity. Frontiers in genetics. 2013;4:47. doi: 10.3389/fgene.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fei YJ, Inoue K, Ganapathy V. Structural and functional characteristics of two sodium-coupled dicarboxylate transporters (ceNaDC1 and ceNaDC2) from Caenorhabditis elegans and their relevance to life span. J Biol Chem. 2003;278:6136–6144. doi: 10.1074/jbc.M208763200. [DOI] [PubMed] [Google Scholar]
- 26.Fei YJ, et al. Relevance of NAC-2, an Na+-coupled citrate transporter, to life span, body size and fat content in Caenorhabditis elegans. The Biochemical journal. 2004;379:191–198. doi: 10.1042/BJ20031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birkenfeld AL, et al. Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab. 2011;14:184–195. doi: 10.1016/j.cmet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging cell. 2004;3:423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 29.Perron JT, Tyson RL, Sutherland GR. Maintenance of tricarboxylic acid cycle kinetics in Brown-Norway Fischer 344 rats may translate to longevity. Neuroscience letters. 2000;281:91–94. doi: 10.1016/s0304-3940(00)00825-9. [DOI] [PubMed] [Google Scholar]
- 30.Jo SH, et al. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem. 2001;276:16168–16176. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton B, et al. A systematic RNAi screen for longevity genes in C. elegans. Genes & development. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards CB, Copes N, Brito AG, Canfield J, Bradshaw PC. Malate and fumarate extend lifespan in Caenorhabditis elegans. PLoS One. 2013;8:e58345. doi: 10.1371/journal.pone.0058345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smolkova K, Jezek P. The Role of Mitochondrial NADPH-Dependent Isocitrate Dehydrogenase in Cancer Cells. International journal of cell biology. 2012;2012:273947. doi: 10.1155/2012/273947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haraguchi CM, Mabuchi T, Yokota S. Localization of a mitochondrial type of NADP-dependent isocitrate dehydrogenase in kidney and heart of rat: an immunocytochemical and biochemical study. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2003;51:215–226. doi: 10.1177/002215540305100210. [DOI] [PubMed] [Google Scholar]
- 35.Tokheim AM, Martin BL. Association of calcineurin with mitochondrial proteins. Proteins. 2006;64:28–33. doi: 10.1002/prot.20996. [DOI] [PubMed] [Google Scholar]
- 36.Benderdour M, et al. Decreased cardiac mitochondrial NADP+-isocitrate dehydrogenase activity and expression: a marker of oxidative stress in hypertrophy development. American journal of physiology Heart and circulatory physiology. 2004;287:H2122–2131. doi: 10.1152/ajpheart.00378.2004. [DOI] [PubMed] [Google Scholar]
- 37.Benderdour M, Charron G, DeBlois D, Comte B, Des Rosiers C. Cardiac mitochondrial NADP+-isocitrate dehydrogenase is inactivated through 4-hydroxynonenal adduct formation: an event that precedes hypertrophy development. J Biol Chem. 2003;278:45154–45159. doi: 10.1074/jbc.M306285200. [DOI] [PubMed] [Google Scholar]
- 38.Koga K, Kenessey A, Ojamaa K. Macrophage migration inhibitory factor antagonizes pressure overload-induced cardiac hypertrophy. American journal of physiology Heart and circulatory physiology. 2013;304:H282–293. doi: 10.1152/ajpheart.00595.2012. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka T, Morita H, Koide H, Kawamura K, Takatsu T. Biochemical and morphological study of cardiac hypertrophy. Effects of thyroxine on enzyme activities in the rat myocardium. Basic research in cardiology. 1985;80:165–174. doi: 10.1007/BF01910464. [DOI] [PubMed] [Google Scholar]
- 40.Turcan S, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mardis ER, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 43.Lankinen M, et al. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: the Sysdimet study. PLoS One. 2011;6:e22646. doi: 10.1371/journal.pone.0022646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. Journal of biomedical science. 2010;17(Suppl 1):S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Fabiani E, Mitro N, Gilardi F, Caruso D, Galli G, Crestani M. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem. 2003;278:39124–39132. doi: 10.1074/jbc.M305079200. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen A, Bouscarel B. Bile acids and signal transduction: role in glucose homeostasis. Cellular signalling. 2008;20:2180–2197. doi: 10.1016/j.cellsig.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe M, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 48.Thomas C, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao R, et al. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(alpha)i-protein-coupled receptors and the AKT pathway. Journal of lipid research. 2010;51:2234–2244. doi: 10.1194/jlr.M004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minamino T, Miyauchi H, Tateno K, Kunieda T, Komuro I. Akt-induced cellular senescence: implication for human disease. Cell Cycle. 2004;3:449–451. [PubMed] [Google Scholar]
- 51.Nogueira V, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu Z, et al. Human serum metabolic profiles are age dependent. Aging cell. 2012;11:960–967. doi: 10.1111/j.1474-9726.2012.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 54.Magnusson M, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs424. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kannel WB, Wolf PA, Garrison RJ. The Framingham Study: an epidemiological investigation of cardiovascular disease. Section 34. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Heart Study, 30-year follow-up. National Heart, Lung, and Blood Institute; 1987. NIH publication no. 87-2703. [Google Scholar]
- 56.Kreger BE, Splansky GL, Schatzkin A. The cancer experience in the Framingham Heart Study cohort. Cancer. 1991;67:1–6. doi: 10.1002/1097-0142(19910101)67:1<1::aid-cncr2820670102>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 57.Driver JA, et al. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ. 2012;344:e1442. doi: 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lloyd-Jones DM, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 59.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127-1136. [DOI] [PubMed] [Google Scholar]
- 60.Wilson PW, Paffenbarger RS, Jr., Morris JN, Havlik RJ. Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am Heart J. 1986;111:1177–1192. doi: 10.1016/0002-8703(86)90022-0. [DOI] [PubMed] [Google Scholar]
- 61.U.S. Department of Agriculture, Agricultural Research Service [Accessed December 12, 2012];USDA National Nutrient Database for Standard Reference, Release 25. Nutrient Data Laboratory Home Page. 2012 http://www.ars.usda.gov/ba/bhnrc/ndl
- 62. [Accessed December 12, 2012];What is an Ounce Equivalent? http://wholegrainscouncil.org/whole-grains-101/what-is-an-ounce-equivalent
- 63.Garcia-Palmieri MR, Costas R, Jr., Cruz-Vidal M, Sorlie PD, Havlik RJ. Increased physical activity: a protective factor against heart attacks in Puerto Rico. Am J Cardiol. 1982;50:749–755. doi: 10.1016/0002-9149(82)91229-2. [DOI] [PubMed] [Google Scholar]
- 64.Rhee EP, et al. A genome-wide association study of the human metabolome in a community-based cohort. Cell Metab. 2013;18:130–143. doi: 10.1016/j.cmet.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhee EP, et al. A Combined Epidemiologic and Metabolomic Approach Improves CKD Prediction. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2012101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasmussen-Torvik LJ, et al. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terry DF, et al. Characteristics of Framingham offspring participants with long-lived parents. Arch Intern Med. 2007;167:438–444. doi: 10.1001/archinte.167.5.438. [DOI] [PubMed] [Google Scholar]
- 68.Pencina MJ, D’Agostino RB, Sr., Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson RC, et al. Accounting for multiple comparisons in a genome-wide association study (GWAS) BMC genomics. 2010;11:724. doi: 10.1186/1471-2164-11-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


