Abstract
Objectives
Subjects with/without temporomandibular joint disorders (TMJD) were tested for differences in muscle forces.
Setting and Sample Population
School of Dental Medicine, University at Buffalo. Ninety-one subjects were classified in 4 groups based on presence/absence (+/-) of chronic myofascial and/or TMJ pain (P) and bilateral disc displacement (DD).
Material & Methods
Validated numerical models employed an organizational objective and subjects’ anatomy to calculate masticatory muscle forces during static biting. ANOVA and Holm step-down procedure post-hoc tests assessed group differences. Theoretical geometries, representing the range of subjects’ muscle orientations, were surveyed via numerical models to identify key combinations resulting in high muscle forces. Effect-size (Cohen’s d) and ANOVA/post-hoc tests assessed group differences in key muscle orientations.
Results
+P-DD subjects had significantly higher muscle forces, especially for lateral pterygoid muscles, compared to the other groups (P<0.01) for bite-forces that were directed posteromedially or posterolaterally on mandibular molars and posteriorly and slightly medially on mandibular incisors. Key muscle orientations for peak lateral pterygoid muscle forces were identified and group comparisons showed mean orientation in +P-DD compared to other diagnostic groups was ≥5° more upright for masseter and ≥3° more posteriorly-directed for temporalis muscles (all Cohen’s d ≥0.8).
Conclusion
Predicted lateral pterygoid muscle forces were significantly higher in +P-DD compared to other groups for specific biting conditions and were attributable, in part, to differences in masseter and temporalis muscle orientations.
Keywords: Human, TMJ, masticatory muscles, biomechanics, computer models
Introduction
Computer-assisted numerical models of human craniomandibular biomechanics (1), using central nervous system (CNS) organizational objectives of minimization of joint loads (MJL) and muscle effort (MME), have proven to be useful tools. For example, these models were employed to elucidate temporomandibular joint (TMJ) eminence growth (2) and shape (3,4), as well as inter-individual differences in TMJ loads (5) and disc mechanics (6). Furthermore, static biting experiments based on data from numerical modeling showed that constants of proportionality between masticatory muscle activities and bite-forces varied with bite-force direction (7,8). These findings suggest simplifying assumptions applied to mathematical modeling such as assignment of muscle forces based on muscle cross-sectional areas and averaged electromyographic data from groups of individuals (9,10) are unlikely to yield predictions that can be tested by comparing to individual-specific outcomes. In contrast, results from MJL- and MME-based numerical models (1) have been validated for individuals by comparison with in vivo measurements of TMJ eminence shapes (4) and muscle forces during static biting tasks (11). A next step is to apply these validated numerical models to address clinical questions such as: Do individuals with/without TMJD generate different masticatory muscle forces during biting due to differences in CNS organization and craniomandibular biomechanics? The application of numerical modeling, thus, may translate to predicting groups of individuals who are susceptible to increased jaw muscle activity and joint loading during routine daily function.
Variability amongst diagnostic groups in the biomechanics of biting has been reported (5,6,11). However, it is unknown if, during static biting, ratios of masticatory muscle forces: bite-forces are higher in some clinically defined groups compared to others. Furthermore, it is unknown which anatomical relationships and mandibular loading conditions critically determine biomechanical differences amongst diagnostic groups. Employment of the previously validated numerical models can investigate these unknowns. Outcomes may improve understanding of human susceptibility for development or maintenance of different categories of TMD, and, thus, suggest candidate preventative and treatment approaches.
This project tested the hypothesis that mean predicted masticatory muscle forces during standardized static biting tasks were higher in individuals with (+P), compared to those without, myofascial and/or TMJ pain (-P). Then, using numerical models, anatomical and jaw loading conditions were surveyed to identify those that accounted for the highest masticatory muscle forces. Finally, a second hypothesis was tested to see if these anatomical relations were more common in +P compared to –P individuals.
Material and Methods
Subjects
Institutional Review Boards at University at Buffalo and University of Missouri-Kansas City approved study protocols. The 91 informed, consenting, and qualified subjects (47 women, 44 men) were previously described for the model-validation study (11). Four diagnostic groups were represented according to presence or absence of chronic myofascial and/or TMJ pain (+/-P) and bilateral TMJ disc displacement (+/-DD) determined by a calibrated examiner and radiologist, respectively using Research Diagnostic Criteria for TMD (12) and computed-tomography and magnetic resonance images (13). Gender was approximately balanced within each group (+P+DD: 13 women, 13 men; +P-DD: 8 women, 8 men; -P+DD: 16 women, 13 men; -P-DD: 10 women, 10 men).
Modeling Protocol and Analyses
Overview of Model Validation
As previously reported (11), individual-specific muscle activation patterns during biting tasks, predicted by computer-assisted numerical models, were tested for accuracy by comparison with masseter and anterior temporalis muscle activities measured in vivo via surface electromyography when the same individual performed similar biting tasks on a bite-force transducer. The transducer was positioned between custom acrylic crowns on maxillary and mandibular right and left central incisors and first molars. The vestubulolingual direction and magnitude of a mechanical moment produced by the bite-force were controlled by orientation of the transducer relative to the center of resistance of each mandibular tooth. For each of four biting positions (left or right, incisors or molars), each subject was asked to produce a range of comfortable bite-forces. For each subject, biting position, moment, and muscle, analyzed data were plotted, slopes were calculated for muscle activity versus bite-force (root mean square mV/N), and normalized to peak slope. Within subjects, normalized results from two laboratory sessions were compared with numerical model-predicted muscle activities during simulation of the in vivo biting tasks. Customized programs calculated differences between measured and predicted data for similar tasks which defined the errors in model results. The model-predicted and in vivo-measured data with minimum error was identified as the “best-match” result for a given biting task. Average absolute errors between best numerical model-predicted and in vivo muscle activities were ≤15% overall and similar amongst diagnostic groups. Errors ranged from 11–13% and 8–15% for incisor and molar biting, respectively. Average coefficients of determination demonstrated that predicted and measured data generally matched well and similarly amongst diagnostic groups, ranging from 0.70–0.74 and 0.68–0.74 for incisor and molar biting, respectively.
Modeling of Muscle Forces during Biting in Diagnostic Groups
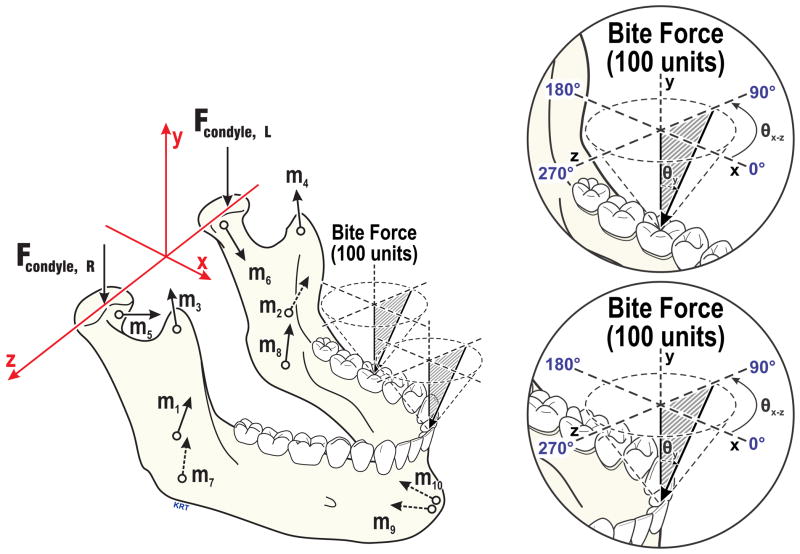
Each model employed the subject’s anatomical data and an organizational objective to produce unique solutions for static equilibrium. Anatomical data were determined using established methods (4,5,11) and consisted of the subject’s three-dimensional craniomandibular geometry (Fig. 1) developed from standardized lateral and postero-anterior cephalometric radiographs and bilateral sagittal effective eminence shapes measured via jaw tracking (4). Objectives were: 1) minimization and equalization of right and left TMJ loads (MJL); or 2) minimization of muscle effort (MME), defined as minimization of the sum of muscle forces squared. Overall, predictions from the MME model fit best with in vivo data from incisor and molar biting tasks for ≥53% of men and women (Table 1). However, for each subject and biting location, the model that predicted muscles forces which matched best with the subject’s electromyographic data was used to calculate muscle forces (% applied bite-force) for a spectrum of molar and incisor bite-force angles. Specifically, 324 biting angles were modeled on each of right and left mandibular first molars and central incisors in each subject according to (Fig. 1): 0–350° in the occlusal plane (θxz) in 10°increments, and at each θxz, 0–40° relative to vertical (normal to the occlusal plane, θy) in 5° increments.
Figure 1.
Axis system and force vectors based on an individual’s anatomy used in numerical models (left) including: Applied bite-force (100%, shown at left molar and incisor), joint (Fcondyle), and muscles (m1,2=masseter, m3,4=anterior temporalis, m5,6=lateral pterygoid, m7,8=medial pterygoid, m9,10=anterior digastric muscles). Enlargements (right) show modeled bite-forces characterized by angles in the occlusal plane (θxz, 0–350°, illustrated above occlusal plane for left biting angles; right biting angles were mirror images) and relative to vertical (θy, 0–40°where 0° is normal to the occlusal plane). Modified from (11).
Table 1.
Frequency distribution of “best model” for predicting muscle activities during molar and incisor biting in men and women. The data from all groups are combined, where MME (minimization of muscle effort) or MJL (minimization of joint load) were the objective functions used by the computer models to predict in vivo muscle activities. Model predicted data were compared with in vivo data. On average, model accuracy was 86–91% for predicting these in vivo muscle activities (11).
| Gender | Biting Position | MME (%) | MJL (%) |
|---|---|---|---|
| Men | Molar | 81 | 19 |
| Women | Molar | 72 | 28 |
| Men | Incisor | 72 | 28 |
| Women | Incisor | 53 | 47 |
Model-predicted forces for each muscle: masseter, temporalis, lateral pterygoid, medial pterygoid, and anterior digastric, relative to the applied bite-force at each biting angle were determined for each subject. Initially, seven variables (gender, diagnostic group, biting location, θxz, θy, muscle, side) were considered. Then, each biting location (molar, incisor) and muscle were considered separately, so omnibus tests of five-factor ANOVAs were conducted using between-subject variables of gender and group (+PP/+DD, +PP/-DD, -PP/+DD, -PP/-DD) and within-subject variables of θxz (36 angles), θy (9 angles) and side (ipsilateral, contralateral to applied bite-force). All interactions up through four-way were examined (five-way was not significant) and where significant, further comparisons with family-wise error testing were used (α=0.05).
Analysis of Geometry Effects on Muscle Forces
Geometries for all subjects were averaged to determine mean positions of mandibular anatomical components important to biting (Fig. 1). Model predictions showed anterior digastric muscles were relatively inactive during biting tasks, as supported by previous in vivo data (8,14) and could, thus be excluded from the analyses. Inspection of the sample population’s mean geometry file showed muscle orientations varied most in the sagittal plane (x-y in Fig. 1). Thus, muscle orientations were characterized using mean y-coordinates for each muscle vector, and varying x-coordinates to depict the extremes which ranged from 0 to 45 mm for masseter, −40 to 12 mm for temporalis, 14 to 33 mm for lateral pterygoid, and −4 to 21 mm for medial pterygoid muscles. Each range was represented in the theoretical geometry files by 10 equal steps. A systematic method was applied via customized software (MatLab R2013a, MathWorks, Natick MA), where individual masseter, temporalis, lateral pterygoid, and medial pterygoid muscle orientations were incrementally changed while holding other muscle orientations constant. Through this method, 14,641 theoretical geometries were assembled to reflect the mean geometry with all possible combinations of these ranges of 4 muscle orientations. Theoretical geometry files were used in the two models to identify, through regression analyses, key combinations of muscle orientations that produced the highest predicted muscle forces during biting. These key muscle orientations were then compared amongst groups using effect-size (Cohen’s d) and ANOVA with post-hoc tests (α=0.05).
Results
Intergroup Differences in Predicted Muscle Forces during Biting
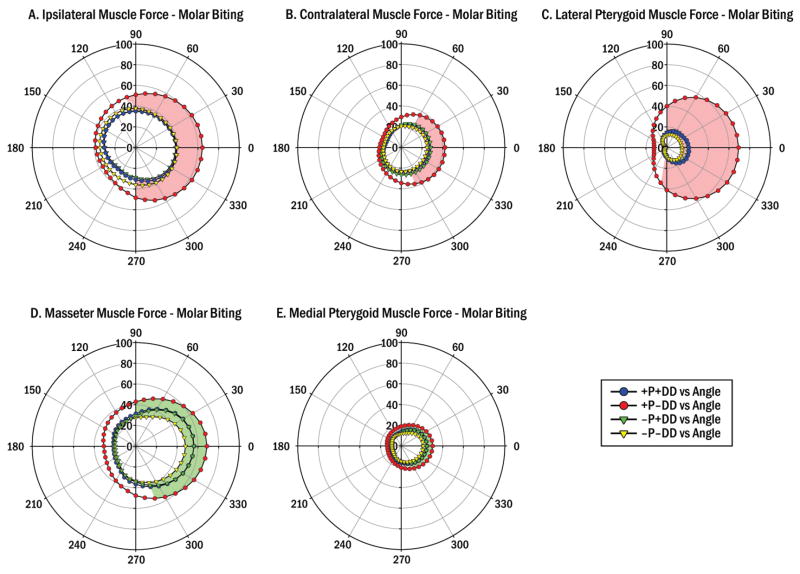
Polar plots depicted group means in muscle forces for a range of biting angles, where at each θxz results from θy (0–40°) were averaged. Asymmetric shapes of group data sets suggested that bite-force direction, θxz, was important to muscle forces during biting (Figs. 2 and 3). Generally, +P-DD subjects showed higher predicted forces for all biting conditions tested for ipsilateral and contralateral muscles, as well as masseter, lateral pterygoid and medial pterygoid muscles compared to the other groups (Figs. 2 and 3).
Figure 2.
Mean predicted muscle forces during molar biting in 4 diagnostic groups with/without (+/-) pain (P) and TMJ disc displacement (DD). Circumferential scale indicates biting angle in occlusal plane (θxz, Fig. 1). Radial scales indicate muscle force magnitude (%) relative to applied bite-force (100%). The +P-DD group showed significantly higher: (A) Ipsilateral muscle forces than other groups (all P<0.01, red area): θxz=0–100°and 270–350°; (B) Contralateral muscle forces than other groups (all P<0.01, red area): θxz=0–60°and 290–350°; (C) Lateral pterygoid muscle forces than other groups (all P<0.01, red area): θxz=0–90° and 260–350°; (D) Masseter muscle forces than the –P-DD group (P<0.05, green area): θxz=0–90° and 290–350°; and (E) Medial pterygoid muscle than the –P-DD group (P<0.05, green area): θxz=0–90° and 290–350;
Figure 3.
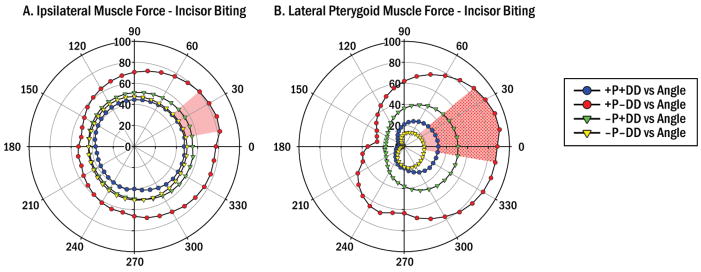
Mean predicted muscle forces during incisor biting in 4 diagnostic groups with/without (+/-) pain (P) and TMJ disc displacement (DD). Circumferential scale indicates biting angle in occlusal plane (θxz, Fig. 1). Radial scales indicate muscle force magnitude (%) relative to applied bite-force (100%). The +P-DD group showed significantly higher (A) Ipsilateral muscle forces than other groups (all P<0.01, red area) for: θxz=10–40°; and (B) Lateral pterygoid muscle forces than the –P-DD (P<0.01, red area) and +P+DD (P<0.05, stippled area) groups for: θxz=0–40° and ≥350°.
ANOVA showed a substantial number of three-way interactions were significant so paired comparisons with Holm step-down procedures were applied. For posteromedially- or posterolaterally-directed molar bite-forces, predicted muscle forces from individually validated numerical models were significantly higher in the +P-DD compared to the other groups (P<0.01) by: >11% for ipsilateral, >10% for contralateral, and >25% for lateral pterygoid muscles (Fig. 2A–C) and compared to the –P-DD group (P<0.05) by: >15% for masseter (Fig. 2D) and >6% for medial pterygoid (Fig. 2E) muscles. Predicted temporalis muscle forces showed no significant differences between groups for molar biting (data not shown).
Similar comparisons demonstrated that for incisor biting which directed the jaw posteriorly and slightly medially, predicted muscle forces in the +P-DD were significantly higher compared to the other groups (P<0.01) by >26% for ipsilateral muscles (Fig. 3A) and compared to the –P-DD group (P<0.01) by >68% and the +P+DD group (P<0.05) by >55% for lateral pterygoid muscles (Fig. 3B). Predicted contralateral, masseter, medial pterygoid, and temporalis muscle forces showed no significant differences between groups for incisor biting (data not shown).
Survey of Geometry Effects on Muscle Forces during Biting
Geometries which produced the highest predicted lateral pterygoid muscle forces for molar and incisor biting at θxz=30° and θy=20° were used to identify key muscle orientations because this muscle and these biting conditions showed the largest between-group differences (Figs. 2 and 3).
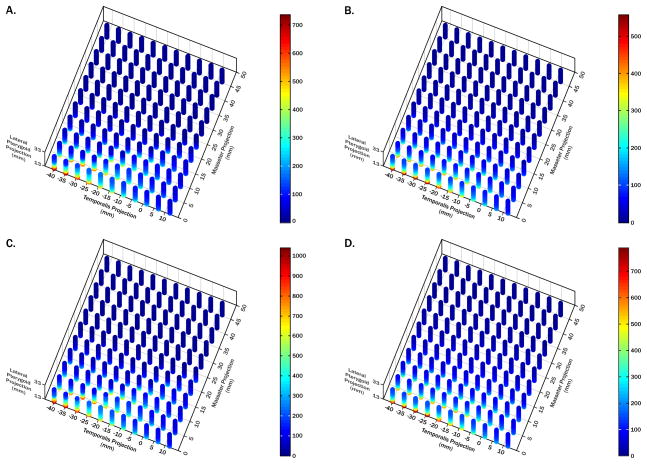
The survey showed that peak lateral pterygoid muscle forces for molar and incisor biting predicted by the MJL model were 7.4- and 10.4-fold the applied bite-force, respectively, and by the MME model were 5.6- and 7.9-fold the applied bite-force, respectively (Fig. 4A–D). These peak forces occurred where masseter (x=0mm) and lateral pterygoid (x=14mm) muscles were relatively upright, where temporalis muscles were more posteriorly directed (x=−40, −30mm for MJL, MME models respectively), and were the same for the range of medial pterygoid muscle orientations tested. Step-wise regression analyses confirmed that x-coordinates of masseter, temporalis, and lateral pterygoid muscles accounted for ≥60% of the variability in predicted lateral pterygoid muscle forces for molar and incisor biting in both models.
Figure 4.
Theoretical analysis of muscle orientation effects on lateral pterygoid muscle forces. 3D plots of MJL (A,C) and MME (B,D) predicted lateral pterygoid muscle forces for molar (A,B) and incisor (C,D) biting, each with a unique color scale. Orientation ranges for each muscle found in the sample were used in two models to identify key combinations of muscle orientations resulting in the highest predicted muscle forces during biting. Lateral pterygoid muscle forces are expressed relative to a 100 unit bite-force (%). Step-wise regression analyses confirmed that x-coordinates of masseter, temporalis, and lateral pterygoid muscles accounted for ≥60% of predicted lateral pterygoid muscle force variability for molar and incisor biting in both models. Lateral pterygoid muscle forces increased when: i) masseter muscles were more upright; ii) temporalis muscles were more posteriorly directed; and iii) lateral pterygoid muscles were less anteriorly directed.
Group Differences in Key Muscle Orientations
Key muscle orientations overall were not significantly different (P=0.09) between groups. However, mean masseter muscle orientations were significantly different between +P-DD subjects and the other groups (Table 2; all P≤0.05). In +P-DD subjects the masseter muscle was more upright by ≥5° (Table 3), whereas the temporalis muscle was more posteriorly directed by ≥3°. Large effect sizes (Table 2, all Cohen’s d ≥0.8) were found for both muscles.
Table 2.
Comparisons of mean muscle x-coordinates for +P-DD group with other groups. Cohen’s d effect size and ANOVA/post-hoc tests were used to characterize differences.
| Muscle | Cohen’s d effect size (P-value, unadjusted post-hoc test): | ||
|---|---|---|---|
| +P-DD vs +P+DD | +PP-DD vs -P+DD | +PP-DD vs -P-DD | |
| Masseter | 2.2 (0.02) | 2.0 (0.02) | 2.2 (0.05) |
| Temporalis | 1.1 (0.23) | 0.8 (0.32) | 1.6 (0.07) |
| Lateral Pterygoid | 1.4 (0.09) | 0.4 (0.72) | 0.4 (0.69) |
| Medial Pterygoid | 0.5 (0.64) | 0.2 (0.82) | 0.3 (0.74) |
Table 3.
Mean (standard deviation) of x-coordinates (mm) for muscles in diagnostic groups.
| Diagnostic Group | Masseter | Temporalis | Lateral Ptyergoid | Medial Pterygoid |
|---|---|---|---|---|
| +P+DD (n=26) | 27.8 (6.8) | −0.1 (5.6) | 24.2 (4.0) | 8.5 (5.2) |
| +P-DD (n=16) | 22.3 (9.0) | −3.4 (12.8) | 22.4 (4.0) | 9.3 (5.2) |
| -P+DD (n=29) | 27.8 (8.9) | −0.7 (9.3) | 22.0 (2.5) | 9.7 (5.8) |
| -P-DD (n=20) | 27.4 (4.5) | 2.0 (6.2) | 22.8 (2.7) | 8.7 (5.4) |
Discussion
+P-DD subjects showed significantly higher predicted muscle forces, especially for lateral pterygoid muscles, compared to other groups for bite-forces directed posteromedially or posterolaterally on mandibular molars and posteriorly and slightly medially on mandibular incisors. These results suggest future tests to see if +P-DD individuals demonstrate higher levels of lateral pterygoid muscle activities and pain compared to others when doing specific biting tasks. In vivo data from the lateral pterygoid muscles would also permit an expanded comparison of model-predicted and electromyographic data to validate model results for lateral pterygoid as well as masseter and temporalis muscles. Nevertheless, +P-DD subjects exhibited anatomical differences compared to other groups that could account for differences in jaw biomechanics. Although effect sizes were large, group differences in key muscle orientations overall did not quite reach significant levels (P=0.09), suggesting larger and more balanced sample sizes are indicated in future studies.
Modeling of muscles by single vectors simplifies the complex three-dimensional architecture and localized regional innervation of motor areas. Indeed, some experiments have demonstrated heterogeneity within human masseter (23,24), temporalis (25,26) and lateral pterygoid (27) muscles during jaw loading. However, this occurs with to access to visual feedback during the experiments and/or when teeth with an arch are splinted together. Similar influence of visual feedback on muscle recruitment patterns has been demonstrated in spinal and appendicular muscles (28–30). In contrast, within muscle heterogeneity during jaw loading, does not occur when subjects are without visual feedback and when teeth are not splinted together (31,32). Hence, currently no unequivocal evidence contradicts the use of single vectors to define masticatory muscle orientations for modeling static tasks.
The modeling of muscle forces during static biting was limited to molar and incisor positions. Future work should investigate other jaw loading positions that reflect both functional and non-functional behaviors. Notably, significant group differences occurred at molar biting angles causing distolingual (θxz=0–60°) and distobuccal (θxz=290–350°) mandibular crown tipping moments where regional periodontal mechanoreceptor activation provides specific CNS feedback (15). It remains unknown if this range of bite-force directions normally occurs and if certain positions and directions of jaw loading are characteristic for a particular group.
The Pain Adaptation Model (16) suggests that the presence of muscular pain results in inhibition of jaw loading behaviors. However, in a clinical study (11) where subjects with/without chronic pain performed the same biting tasks, there were no significant differences in magnitudes of voluntary bite-forces, and hence no evidence that pain altered bite-force production. Furthermore, no significant differences were found in high intensity masticatory muscle behavior amongst women with/without myofascial pain during polysomnographic recording (17), whereas low intensity chronic muscle activities were significantly elevated in women with myofascial pain compared to healthy women (18). The more recent Integrated Pain Adaptation Model (19) hypothesizes that individuals will alter neuromuscular organization in particular muscles because of pain. To this end, during in vivo biting in baseline compared to experimentally induced pain conditions by otherwise asymptomatic subjects, patterns of single motor unit firing in the masseter muscle were quickly altered in different ways between different subjects (20). Furthermore, the validated numerical models showed masseter and temporalis muscle activities during biting matched best overall with MME predictions for +/-P and +/-DD groups (11). However, right-left asymmetry in muscle organization was different. For molar biting, asymmetry was common in both TMD (61%) and healthy (53%) subjects, which could reflect habitual sidedness in biting. In contrast, incisor biting showed greater asymmetry in TMD (40%) versus healthy (11%) subjects, which could reflect the phenomenon of altered neuromuscular organization during pain.
From the perspective of a biopsychosocial model of TMD (21,22), biomechanics may be a contributing factor associated with specific TMD diagnostic subgroups. Despite recognized limitations (1), numerical modeling has been validated using in vivo data and shown to be a relatively accurate way to study jaw biomechanics in living humans non-invasively (4,11). In addition to the current study, previous work has shown other group differences, such as, in –P+DD subjects, predicted TMJ loads were up to 69% higher compared to healthy subjects (5). Considering this, a next step for application of numerical modeling is the identification of -P-DD individuals with relatively high and low predicted muscle and/or TMJ forces that are linked to high and low TMJ energy densities (6) for long-term follow-up to investigate differences in development of TMD. Further to this, future comparisons of individuals should include additional diagnostic criteria, for example: distinguishing myofascial or TMJ pain or both, TMJ disc position, muscle loading behavior, and heritable traits that affect inflammation and pain sensitivity to increase the likelihood of identifying more homogenous TMD subgroups.
Conclusions
Predicted lateral pterygoid muscle forces were significantly higher in the +P-DD group compared to +P+DD, -P+DD, and –P-DD groups for specific biting conditions and were attributable, in part, to differences in masseter and temporalis muscle orientations.
Clinical Relevance.
Validated computer-assisted numerical models of human jaw mechanics were used to compare muscle forces between people for a comprehensive survey of biting directions on molars and incisors. For the same model, differences in muscle forces during biting between people depend on anatomical differences. Hence, differences in jaw muscle orientation were compared between groups of people with/without TMJDs. Subjects with jaw muscle and/or TMJ pain had anatomical differences that could explain relatively higher muscle forces for the same biting tasks compared to others.
Acknowledgments
Support was provided in part by NIDCR (R01 DE016417). The authors thank the study participants, former dental students and research staff who assisted, and Kim Theesen, graphic artist, who helped produce the figures.
References
- 1.Trainor PG, McLachlan KR, McCall WD. Modelling of forces in the human masticatory system with optimization of the angulations of the joint loads. J Biomech. 1995;28:829–43. doi: 10.1016/0021-9290(94)00128-q. [DOI] [PubMed] [Google Scholar]
- 2.Nickel JC, McLachlan KR, Smith DM. Eminence development of the postnatal human temporomandibular joint. J Dent Res. 1988;67:896–902. doi: 10.1177/00220345880670060201. [DOI] [PubMed] [Google Scholar]
- 3.de Zee M, Cattaneo PM, Svensson P, Pedersen TK, Melsen B, Rasmussen J, et al. Prediction of the articular eminence shape in a patient with unilateral hypoplasia of the right mandibular ramus before and after distraction osteogenesis-A simulation study. J Biomech. 2009;42:1049–53. doi: 10.1016/j.jbiomech.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki LR, Crosby MJ, Marx DB, Gonzalez Y, McCall WD, Jr, Ohrbach R, et al. Human temporomandibular joint eminence shape and load minimization. J Dent Res. 2010;89:722–7. doi: 10.1177/0022034510364492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwasaki LR, Crosby MJ, Gonzalez Y, McCall WD, Marx DB, Ohrbach R, et al. Temporomandibular joint loads in subjects with and without disc displacement. Orthop Rev (Pavia) 2009;1:90–93. doi: 10.4081/or.2009.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickel JC, Iwasaki LR, Gallo LM, Palla S, Marx DB. Tractional forces, work, and energy densities in the human TMJ. In: Kapila SD, McNamara JA, editors. Temporomandibular Disorders and Orofacial Pain – Separating Controversy from Consensus. Ann Arbor, MI: Needham Press; 2009. pp. 427–50. [Google Scholar]
- 7.Uchida S, Iwasaki LR, Marx DB, Yotsui Y, Inoue H, Nickel JC. Variations in activities of human jaw muscles depend on tooth-tipping moments. Arch Oral Biol. 2008;53:199–205. doi: 10.1016/j.archoralbio.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki LR, Petsche PE, McCall WD, Jr, Marx D, Nickel JC. Neuromuscular objectives of the human masticatory apparatus during static biting. Arch Oral Biol. 2003;48:767–77. doi: 10.1016/s0003-9969(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka E, Hirose M, Koolstra JH, van Eijden TM, Iwabuchi Y, Fujita R, et al. Modeling of the effect of friction in the temporomandibular joint on displacement of its disc during prolonged clenching. J Oral Maxillofac Surg. 2008;66:462–8. doi: 10.1016/j.joms.2007.06.640. [DOI] [PubMed] [Google Scholar]
- 10.Tuijt M, Koolstra JH, Lobbezoo F, Naeije M. Differences in loading of the temporomandibular joint during opening and closing of the jaw. J Biomech. 2010;43:1048–54. doi: 10.1016/j.jbiomech.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Nickel JC, Gonzalez YM, McCall WD, Ohrbach R, Marx DB, Liu H, et al. Muscle Organization in Individuals with and without Pain and Joint Dysfunction. J Dent Res. 2012;91:568–73. doi: 10.1177/0022034512445909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- 13.Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2009;107:844–60. doi: 10.1016/j.tripleo.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohrbach R, Markiewicz MR, McCall WD., Jr Waking-state oral parafunctional behaviors: specificity and validity as assessed by electromyography. Eur J Oral Sci. 2008;116:438–44. doi: 10.1111/j.1600-0722.2008.00560.x. [DOI] [PubMed] [Google Scholar]
- 15.Trulsson M, Johansson RS. Orofacial mechanoreceptors in humans: encoding characteristics and responses during natural orofacial behaviors. Behav Brain Res. 2002;135:27–33. doi: 10.1016/s0166-4328(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 16.Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–94. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 17.Raphael KG, Sirois DA, Janal MN, Wigren PE, Dubrovsky B, Nemelivsky LV, et al. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012;143:1223–31. doi: 10.14219/jada.archive.2012.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raphael KG, Janal MN, Sirois DA, Dubrovsky B, Wigren PE, Klausner JJ, et al. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J Oral Rehabil. 2013;40:883–91. doi: 10.1111/joor.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peck CC, Murray GM, Gerzina TM. How does pain affect jaw muscle activity? The Integrated Pain Adaptation Model. Aust Dent J. 2008;53:201–7. doi: 10.1111/j.1834-7819.2008.00050.x. [DOI] [PubMed] [Google Scholar]
- 20.Minami I, Akhter R, Albersen I, Burger C, Whittle T, Lobbezoo F, et al. Masseter Motor Unit Recruitment is Altered in Experimental Jaw Muscle Pain. J Dent Res. 2013;92:143–8. doi: 10.1177/0022034512470832. [DOI] [PubMed] [Google Scholar]
- 21.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12:T27–45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dougall AL, Jimenez CA, Haggard RA, Stowell AW, Riggs RR, Gatchel RJ. Biopsychosocial factors associated with the subcategories of acute temporomandibular joint disorders. J Orofac Pain. 2012;26:7–16. [PMC free article] [PubMed] [Google Scholar]
- 23.McMillan AS, Hannam AG. Task-related behavior of motor units in different regions of the human masseter muscle. Arch Oral Biol. 1992;37:849–57. doi: 10.1016/0003-9969(92)90119-s. [DOI] [PubMed] [Google Scholar]
- 24.Blanksma NG, Van Eijden TM, Weijs WA. Electromyographic heterogeneity in the human masseter muscle. J Dent Res. 1992;71:47–52. doi: 10.1177/00220345920710010801. [DOI] [PubMed] [Google Scholar]
- 25.McMillan AS. Task-related behaviour of motor units in the human temporalis muscle. Exp Brain Res. 1993;94:336–42. doi: 10.1007/BF00230303. [DOI] [PubMed] [Google Scholar]
- 26.Arima T, Tomonaga A, Yachida W, Tanosoto T, Haugland M, Ohata N, et al. Site-to-site variation of muscle activity and sensitivity in the human anterior temporalis muscle: implications for contingent stimulation. Acta odontologica Scandinavica. 2012;70:89–95. doi: 10.3109/00016357.2011.597778. [DOI] [PubMed] [Google Scholar]
- 27.Murray GM. The lateral pterygoid muscle: Function and dysfunction. Sem Orthod. 2012;18:44–50. [Google Scholar]
- 28.Safavynia SA, Ting LH. Task-level feedback can explain temporal recruitment of spatially fixed muscle synergies throughout postural perturbations. J Neurophysiol. 2012;107:159–77. doi: 10.1152/jn.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jilk DJ, Safavynia SA, Ting LH. Contribution of vision to postural behaviors during continuous support-surface translations. Exp Brain Res. 2014;232:169–80. doi: 10.1007/s00221-013-3729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baweja HS, Kwon M, Christou EA. Magnified visual feedback exacerbates positional variability in older adults due to altered modulation of the primary agonist muscle. Exp Brain Res. 2012;222:355–64. doi: 10.1007/s00221-012-3219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobbezoo F, van der Glas HW, van Kampen FM, Bosman F. The effect of an occlusal stabilization splint and the mode of visual feedback on the activity balance between jaw-elevator muscles during isometric contraction. J Dent Res. 1993;72:876–82. doi: 10.1177/00220345930720050801. [DOI] [PubMed] [Google Scholar]
- 32.Blanksma NG, van Eijden TM. Electromyographic heterogeneity in the human temporalis and masseter muscles during static biting, open/close excursions, and chewing. J Dent Res. 1995;74:1318–27. doi: 10.1177/00220345950740061201. [DOI] [PubMed] [Google Scholar]