Abstract
Background
The objective of the study was to analyze the relationship between interaction of polymorphisms in the estrogen receptor alpha gene (Erα) and estradiol (E2), and the occurrence of selected atherosclerosis risk factors in postmenopausal women without the diagnosis of a cardiovascular disease.
Material/Methods
The study covered 210 women, a minimum of 2 years after menopause, with FSH >30 mlU/ml, aged 50–60 years, with no chronic diseases diagnosed. In the women examined, the levels of estradiol, total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides were determined, as well as height, waist circumference (W), hip circumference (R), and arterial hypertension. The BMI and W/H ratio were calculated.
Genotyping of the ER-α polymorphism was performed using a polymerase chain reaction and restriction enzymes (PCR-RFLP). The alleles of the XbaI polymorphism were defined as A and G: heterozygote AG, wild type GG and homozygote AA. The alleles of PvuII polymorphism were defined as T and C: heterozygote TC, homozygote TT, and wild type CC.
Results
The concentration of endogenous estradiol and ERα XbaI and PuvII polymorphisms as independent parameters did not significantly affect the BMI, waist circumference, W/H ratio, levels of CHOL, HDL, LDL, TG, or LDL/HDL, nor the systole and diastole in the postmenopausal women in the study.
Conclusions
The presented study suggests that ERα XbaI AA polymorphism may intensify the beneficial effect of estradiol on the distribution of fatty tissue after menopause; ERα XbaI GG and PuvII TC genotypes may intensify the beneficial effect of estradiol on HDL level; ERα PuvII TT genotype unfavorably modifies the relation between concentration of estradiol and systolic pressure after menopause.
MeSH Keywords: Atherosclerosis, Estradiol, Estrogen Receptor alpha, Menopause
Background
Within the last decade, a decrease in mortality has been observed due to diseases developing on the background of atherosclerosis; however, despite this tendency, these diseases are still the main cause of death in many countries [1]. Hence, it is necessary to apply prophylaxis, including, among other things, the control of risk factors. Many studies confirm that the main risk factors of atherosclerosis are obesity and lipid disorders [2]. Based on the results of studies, it was confirmed that the intensification of atherosclerotic changes is positively correlated with obesity and the levels of LDL and VLDL cholesterol, and negatively correlated with the level of HDL cholesterol, irrespective of race, age, and gender [3].
Population studies show that among young women, morbidity due to ischemic heart disease is approximately 6 times lower compared to males in analogous age groups [4]. Nevertheless, this gender-related difference decreases after menopause, when the frequency of occurrence of atherosclerosis and arterial hypertension in females drastically increases [5,6]. These observations suggest that the reduction in the level of endogenous estrogens after menopause may be a critical risk factor in these diseases. Studies of the genetic risk factors of cardiovascular diseases, including atherosclerosis, are still being continued. Although many candidate genes of cardiovascular diseases have been found, the role of genes participating in the impact of sex hormones still remains unclear. Estrogens exert an effect on the endothelium and smooth muscle cells in the vascular wall, where they inhibit cell proliferation and induce vasodilatation [7]. Estrogens also affect the hepatic cells, resulting in decreased LDL cholesterol fraction [8,9] and increase in HDL cholesterol fraction in blood [7,8]. Two estrogen receptors participate in the genome effect of estrogens (ER): alpha receptor (ERα, ESR1) and beta receptor (ERβ, ESR2) [10]. Both receptors are located on the endothelial cells and in the vascular smooth muscle cells, with the main protective role of the cardiovascular system ascribed to ERα [11]. The gene for this receptor is located on the long arm of chromosome 6, and contains 8 exons encoding receptor protein of 595 amino acids in length and a molecular weight of 66 kDa. Among many polymorphic variants of estrogen receptor gene, the most clinically important are 2 single-nucleotide polymorphisms (SNPs) detected by using restriction enzymes PvuII and XbaI. The PvuII and XbaI restriction fragment length polymorphisms of ERα gene are caused by changes in the sequence of DNA of single nucleotides in intron, located within a distance of 397 and 351 alkaline pairs (ap) from exon 2 [12]. Many studies have indicated that there is a relationship between XbaI and PvuII ERα polymorphism variants, and an increased risk of atherosclerosis in adults [13–16]. It is suggested that PvuII polymorphism plays a key role in the regulation of ERα protein expression, and its intensified transcription encoded by allele C leads to the stronger expression of receptor, stronger estradiol binding of ERα in CC homozygotes, and a stronger effect of estradiol on a cell. The ERα polymorphisms may affect estrogen receptor function, and thus the tissue response to the stimulation with estrogen.
The objective of the study was analysis of an interaction between ERα polymorphism and estradiol, with the occurrence of selected risk factors of atherosclerosis in postmenopausal women without the diagnosis of a cardiovascular disease.
Material and Methods
The study was conducted during 2011–2013 at the Institute of Rural Health in Lublin. The study group consisted of women from south-eastern Poland. The inclusion criteria were: age 50–65 years, good general health (lack of chronic diseases in medical history, including cardiovascular diseases), and education level at least completed elementary. The women were qualified to the study group also based on clinical symptoms (minimum 2 years from the last menstrual period) and based on the criterion of FSH level (FSH >30 mlU/ml). The exclusion criteria were: active cancerous disease within the period of 5 years after recruitment; addiction to drugs or alcohol; and diagnosed disease entity with the symptoms of dementia. We examined 210 postmenopausal women.
Prior to the collection of blood for laboratory examinations and genetic tests, the women were weighed and their height, waist circumference (W), and hip circumference (R) measured, as well as arterial hypertension. Based on the measurements obtained, the BMI (kg/height in m2) and W/H ratio were calculated.
The women under examination had their blood collected to measure the level of estradiol and samples were instantly taken to the laboratory. The determinations were performed by an accredited laboratory; the laboratory standard for estradiol is 0–44.5 pg/ml.
Lipid profile was determined using an automatic biochemistry analyser, Express Plus (Chiron Diagnostics, USA), with reagents by Siemens (Siemens Healthcare Diagnostics, Tarrytown, NY, USA), according to the procedure provided by the manufacturer. The following were determined in blood plasma: triglycerides, and total cholesterol and its fractions HDL cholesterol and LDL cholesterol.
Based on data from the literature, the values of the analyzed parameters that could create risk of cardiovascular diseases were determined: BMI ≥25 kg/m2, waist circumference >88 cm, W/H ratio ≥0.85, total cholesterol ≥191 mg/dl, HDL cholesterol ≤64 mg/dl, LDL cholesterol ≥115 mg/dl, triglycerides ≥150 mg/dl, LDL/HDL ratio >4, systole >140 mmHg, and diastole >90 mmHg.
Genomic DNA was isolated using the QIAmp DNA Blood Mini Kit (Qiagen, USA) according to the producer’s instructions. Genotyping of the ER-α polymorphism was performed using a polymerase chain reaction and restriction enzymes (PCR-RFLP). PCR reaction was performed in a total amount of 50 μl containing: 1 U (1 μl) of DNA polymerase (Biotools), 1·PCR buffer (5 μl) containing 15 mM MgCl2 (Biotools), 2.5 μl 2 mM dNTPs (final concentration 0.1 mM) (Fermentas, Vilnius, Lithuania), 1 μl of 10 μM of each primer, 34.5 μl nuclease-free water (Applied Biosystems Inc., USA) and 5 μl of genomic DNA. The polymerase chain reactions (PCR) were carried out with the following primers [17]:
Forward: 5′-CTG CCA CCC TAT CTG TAT CTT TTC CTA TTC TCC-3′
Reverse: 5′-TCT TTC TCT GCC ACC CTG GCG TCG ATT ATC TGA-3′.
The reactions were performed in a C1000 Thermal Cycler (BioRad) and consisted of the initial denaturation (3 min at 95oC) and 30 cycles, each of which included the proper denaturation (30 s at 95oC), primers annealing (50 s at 62oC), elongation (50 s at 72oC), and the final elongation (7 min at 72oC). Electrophoresis was performed in 2% agarose gel in standard conditions. The products of PCR (1372 bp) were digested overnight at 37ºC using 2 separate restriction enzymes for determining the polymorphisms: PvuII (c.454-397 T>C) and XbaI (c.454-351 A>G). The products of restriction were electrophoresed in 2.5% agarose gel.
The alleles of the XbaI polymorphism were defined as A and G: heterozygote AG (fragments: 1372 bp, 936 bp, and 436 bp), wild type GG (fragment: 1372 bp), and homozygote AA (fragments: 936 bp and 436 bp). The alleles of PvuII polymorphism were defined as T and C: heterozygote TC (fragments: 1372 bp, 982 bp, and 390 bp), homozygote TT (982 bp and 930 bp), and wild type CC (1372 bp).
Statistical analysis was performed and graphs were created using the software STATISTICA. We estimated numbers (n) and frequency distributions (%) for categorical variables and for continuous variables we used means (M), reflecting the average level, as well as standard deviations (SD), measuring the degree of measurements variation around the arithmetic mean.
The analysis of variance F test was used to investigate whether the age at last menstruation period and lipid parameters depended on XbaI and PvuII polymorphisms. The χ2 test for stochastic independence was applied to examine whether the frequencies of occurrence of cardiovascular risk factors depended on XbaI and PvuII polymorphisms.
Pearson’s correlation coefficient was estimated to check the correlation between the level of estradiol and lipid parameters in the total group examined, and in the groups with XbaI and PvuII polymorphisms.
Considering the large size of the sample (N=210), border normal distributions of parameters’ estimators were assumed. In statistical tests, the level of significance was set at p=0.05.
Informed consent for participation in the study was obtained from the women. The study was approved by the institute’s Ethics Committee.
Results
The study included 210 postmenopausal women. Table 1 presents their characteristics. Mean age of the women examined was 56.5±3.5 years. Their mean age at last menstruation was 50.3±2.7 years and the mean BMI was 26.8±4.04 kg/m2. Nearly a half of the women had secondary school education (47.62%), and 40% had university education, while 9.05% had basic vocational, and 3.33% had primary educational level. In the study group, 71 women (33.81%) had the genotype ERα XbaI AA, 104 (49.52%) were carriers of ERα XbaI AG, and 35 (16.67%) had the genotype ERα XbaI GG. With respect to ERα PvuII polymorphism, 57 (27.14%) of women had genotype TT, 97 (46.19%) had genotype TC, and 56 (26.67%) had genotype CC. Age at last menstruation was not significantly dependent on the possessed ERα XbaI polymorphism (F=0.853, p=0.428) or PvuII polymorphism (F=0.492, p=0.612).
Table 1.
Characteristics of postmenopausal women in the study (N=210).
| Variable | Parameter | Estimate |
|---|---|---|
| Age (years) | M ±SD* | 56.5±3.5 |
| Age at last menstruation (years) | M ±SD | 50.3±2.7 |
| BMI (kg/m2) | M ±SD | 26.38±4.04 |
| Educational level | n (%) | |
| Primary | 7 (3.33) | |
| Basic vocational | 19 (9.05) | |
| Secondary | 100 (47.62) | |
| University | 84 (40.00) | |
| XbaI | n (%) | |
| AA | 71 (33.81) | |
| AG | 104 (49.52) | |
| GG | 35 (16.67) | |
| PvuII | n (%) | |
| TT | 57 (27.14) | |
| TC | 97 (46.19) | |
| CC | 56 (26.67) | |
Mean ± standard deviation.
Table 2 presents risk factors of cardiovascular diseases and estradiol level in the total examined group and according to Erα polymorphism. The values of analyzed parameters were not significantly dependent on ERα XbaI or PvuII polymorphisms possessed (p>0.05). The mean waist circumference was 1.54±12.34 cm, and the W/H ratio 0.79±0.09. The mean value of total cholesterol was 229.09±42.58 mg/dl, HDL cholesterol 53.45±12.22 mg/dl, LDL cholesterol 145.84±43.70 mg/dl, and triglycerides 149.00±64.13 mg/dl. The mean LDL/HDL ratio was 2.91±1.15. The mean value of systolic pressure in the group examined was 129.11±18.52 mmHg, and diastolic pressure 79.35±10.46 mmHg. The mean level of estradiol in the women examined was 23.53±20.45 pg/ml.
Table 2.
Values of analyzed risk factors of cardiovascular diseases and estradiol in postmnenopausal women in general, and according to ERα XbaI and PvuII polymorphisms. M ±SD*.
| Cardiovascular diseases risk factors | Total (N=210) | XbaI | PvuII | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA (N=71) | AG (N=104) | GG (N=35) | F** | p*** | TT (N=57) | TC (N=97) | CC (N=56) | F** | p*** | ||
| BMI (kg/m2) | 26.38±4.04 | 26.46±4.33 | 26.29±3.72 | 26.48±4.43 | 0.047 | 0.954 | 26.40±4.64 | 26.71±3.56 | 25.79±4.17 | 0.929 | 0.396 |
| Waist circumference (cm) | 81.54±12.34 | 82.01±13.15 | 81.46±11.83 | 80.80±12.43 | 0.116 | 0.890 | 81.86±13.77 | 82.38±11.71 | 79.75±11.90 | 0.833 | 0.436 |
| W/H ratio | 0.79±0.09 | 0.79±0.09 | 0.79±0.09 | 0.80±0.10 | 0.120 | 0.887 | 0.80±0.09 | 0.79±0.09 | 0.78±0.09 | 0.299 | 0.742 |
| CHOL (mg/dl) | 229.09±42.58 | 225.20±41.43 | 228.52±41.94 | 238.69±46.43 | 1.197 | 0.304 | 227.09±41.55 | 227.35±42.39 | 234.14±44.27 | 0.536 | 0.586 |
| HDL (mg/dl) | 53.45±12.22 | 52.79±13.56 | 54.08±11.95 | 52.94±10.27 | 0.269 | 0.765 | 52.74±13.08 | 53.85±12.94 | 53.50±10.04 | 0.147 | 0.863 |
| LDL (mg/dl) | 145.84±43.70 | 142.77±43.60 | 145.92±43.32 | 151.82±45.67 | 0.501 | 0.606 | 144.09±43.93 | 145.09±42.44 | 148.90±46.23 | 0.196 | 0.822 |
| TG (mg/dl) | 149.00±64.13 | 148.21±62.93 | 142.62±58.22 | 169.60±79.33 | 2.357 | 0.097 | 151.30±66.53 | 142.06±59.22 | 158.70±69.38 | 1.247 | 0.289 |
| LDL/HDL ratio | 2.91±1.15 | 2.93±1.27 | 2.86±1.08 | 3.01±1.13 | 0.226 | 0.798 | 2.96±1.30 | 2.87±1.09 | 2.92±1.11 | 0.111 | 0.895 |
| Systole (mmHg) | 129.11±18.52 | 129.48±19.49 | 131.04±18.59 | 122.63±15.01 | 2.767 | 0.065 | 128.16±18.50 | 131.89±19.40 | 125.27±16.39 | 2.402 | 0.093 |
| Diastole (mmHg) | 79.35±10.46 | 78.77±10.59 | 80.52±10.24 | 77.03±10.69 | 1.627 | 0.199 | 79.23±10.07 | 80.44±11.02 | 77.57±9.77 | 1.347 | 0.262 |
| E2 (pg/ml) | 23.53±20.45 | 22.90±20.76 | 25.41±21.36 | 19.20±16.42 | 1.262 | 0.285 | 23.37±21.09 | 23.70±20.94 | 23.39±19.27 | 0.006 | 0.994 |
Mean ± standard deviation;
F-test;
significance level.
Table 3 presents analysis of the occurrence of cardiovascular risk factors according to ERα XbaI and PvuII polymorphisms in the postmenopausal women in the study. There were 125 women (59.52%) with BMI ≥25 kg/m2, 71 women (33.81%) with waist circumference of more than 88 cm, and 61 (29.05%) women with W/H ratio ≥0.85. There were 174 (82.86%) women with CHOL ≥191 mg/dl, 181 (86.19%) with HDL ≤64 mg/dl, 166 (79.05%) with LDL ≥115 mg/dl, and 87 (41.43%) with TG ≥150 mg/dl. The LDL/HDL ratio >4 was observed in 34 (16.19%) women. The values of systolic arterial blood pressure higher than 140 mmHg were found in 49 (23.33%) of the women in the study, and the values of diastolic pressure higher than 90 mmHg were observed in 30 women (14.29%). The occurrence of the analyzed risk factors of cardiovascular diseases was not significantly dependent to the possessed ERα XbaI and PvuII polymorphisms (p>0.05).
Table 3.
Frequency of occurrence of risk factors of cardiovascular diseases in postmenopausal women in general, and according to ERα XbaI and PvuII polymorphisms n (%).
| Cardiovascular diseases risk factors | Total (N=210) | XbaI | PvuII | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA (N=71) | AG (N=104) | GG (N=35) | χ2* | p** | TT (N=57) | TC (N=97) | CC (N=56) | χ2* | p** | ||
| BMI ≥25 kg/m2 | 125 (59.52) | 40 (56.34) | 63 (60.58) | 22 (62.86) | 0.508 | 0.776 | 30 (52.63) | 66 (68.04) | 29 (51.79) | 5.436 | 0.066 |
| Waistline >88 cm | 71 (33.81) | 24 (33.80) | 38 (36.54) | 9 (25.71) | 1.371 | 0.504 | 18 (31.58) | 39 (40.21) | 14 (25.00) | 3.842 | 0.146 |
| W/H ratio ≥0.85 | 61 (29.05) | 16 (22.54) | 33 (31.73) | 12 (34.29) | 2.290 | 0.318 | 12 (21.05) | 32 (32.99) | 17 (30.36) | 2.546 | 0.280 |
| CHOL ≥191 mg/dl | 181 (86.19) | 60 (84.51) | 90 (86.54) | 31 (88.57) | 0.346 | 0.841 | 50 (87.72) | 81 (83.51) | 50 (89.29) | 1.150 | 0.563 |
| HDL ≤64 mg/dl | 174 (82.86) | 58 (81.69) | 84 (80.77) | 32 (91.43) | 2.198 | 0.333 | 45 (78.95) | 79 (81.44) | 50 (89.29) | 2.379 | 0.304 |
| LDL ≥115 mg/dl | 166 (79.05) | 54 (76.06) | 82 (78.85) | 30 (85.71) | 1.325 | 0.515 | 45 (78.95) | 77 (79.38) | 44 (78.57) | 0.015 | 0.993 |
| TG ≥150 mg/dl | 87 (41.43) | 29 (40.85) | 41 (39.42) | 17 (48.57) | 0.918 | 0.632 | 25 (43.86) | 37 (38.14) | 25 (44.64) | 0.808 | 0.668 |
| LDL/HDL ratio >4 | 34 (16.19) | 13 (18.31) | 13 (12.50) | 8 (22.86) | 2.425 | 0.297 | 11 (19.30) | 13 (13.40) | 10 (17.86) | 1.076 | 0.584 |
| Systole >140 mmHg | 49 (23.33) | 16 (22.54) | 27 (25.96) | 6 (17.14) | 1.177 | 0.555 | 13 (22.81) | 27 (27.84) | 9 (16.07) | 2.759 | 0.252 |
| Diastole >90 mmHg | 30 (14.29) | 8 (11.27) | 17 (16.35) | 5 (14.29) | 0.889 | 0.641 | 5 (8.77) | 19 (19.59) | 6 (10.71) | 4.225 | 0.121 |
Chi-squared test;
significance level.
Subsequently, the correlation was investigated between the level of estradiol and the values of analyzed risk factors of cardiovascular diseases in the total examined group, and according to the ERα XbaI and PvuII polymorphisms possessed by the women in the study (Table 4).
Table 4.
Correlation coefficients between estradiol (pg/ml) and risk factors of cardiovascular diseases in general, and according to ERα XbaI and PvuII polymorphisms.
| Cardiovascular diseases risk factors | Total (N=210) | XbaI | PvuII | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA (N=71) | AG (N=104) | GG (N=35) | TT (N=57) | TC (N=97) | CC (N=56) | |||||||||
| r* | p** | r | p | r | p | r | p | r | p | r | p | r | p | |
| BMI (kg/m2) | −0.066 | 0.345 | −0.051 | 0.673 | −0.117 | 0.236 | 0.183 | 0.292 | 0.004 | 0.978 | −0.120 | 0.242 | −0.154 | 0.256 |
| Waist circumference (cm) | −0.128 | 0.064 | −0.251 | 0.035 | −0.071 | 0.474 | 0.157 | 0.369 | −0.165 | 0.220 | −0.070 | 0.497 | −0.195 | 0.150 |
| W/H ratio | −0.077 | 0.269 | −0.283 | 0.017 | 0.073 | 0.459 | −0.128 | 0.465 | −0.202 | 0.131 | 0.038 | 0.712 | −0.147 | 0.279 |
| CHOL (mg/dl) | −0.067 | 0.331 | −0.073 | 0.544 | −0.058 | 0.561 | −0.045 | 0.799 | −0.040 | 0.766 | −0.141 | 0.170 | 0.037 | 0.788 |
| HDL (mg/dl) | 0.060 | 0.384 | −0.109 | 0.364 | 0.089 | 0.368 | 0.477 | 0.004 | −0.253 | 0.058 | 0.224 | 0.027 | 0.114 | 0.404 |
| LDL (mg/dl) | −0.045 | 0.521 | −0.015 | 0.905 | −0.038 | 0.704 | −0.120 | 0.492 | 0.048 | 0.725 | −0.162 | 0.112 | 0.063 | 0.645 |
| TG (mg/dl) | −0.130 | 0.061 | −0.073 | 0.545 | −0.159 | 0.107 | −0.094 | 0.592 | −0.035 | 0.797 | −0.166 | 0.104 | −0.175 | 0.198 |
| LDL/HDL ratio | −0.066 | 0.342 | 0.071 | 0.556 | −0.092 | 0.351 | −0.333 | 0.050 | 0.164 | 0.224 | −0.237 | 0.020 | −0.046 | 0.737 |
| Systole (mmHg) | 0.084 | 0.224 | 0.130 | 0.281 | −0.002 | 0.982 | 0.208 | 0.230 | 0.314 | 0.017 | −0.009 | 0.933 | 0.004 | 0.977 |
| Diastole (mmHg) | 0.050 | 0.475 | −0.057 | 0.635 | 0.059 | 0.554 | 0.196 | 0.259 | 0.005 | 0.968 | 0.127 | 0.216 | −0.067 | 0.626 |
Correlation coefficient;
significance level.
None of the analyzed parameters significantly correlated with the level of estradiol in the total group of examined women. However, the analysis of correlations according genotype ERα polymorphism proved some of them to be significant. The description below concerns only these significant ones.
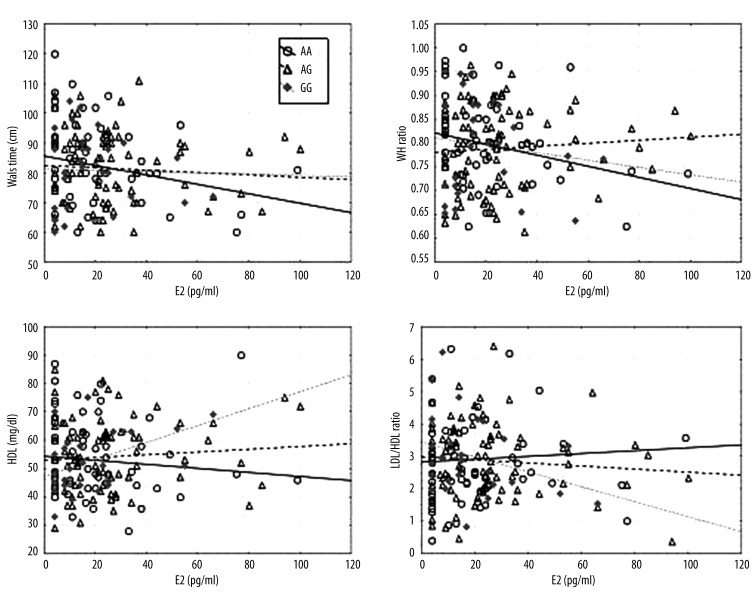
The level of estradiol correlated significantly negatively to waist circumference (r=−0.251; p=0.035) and the W/H ratio (r=−0.283; p=0.017) in women with the genotype ERα XbaI AA. Women with lower estradiol levels had higher average waist circumference and W/H ratio. Figure 1 shows the regression line between estradiol and waistline goes up, as did the line between estradiol and W/H ratio.
Figure 1.
Scatter diagrams comparing estradiol and waist circumference, W/H, HDL, and LDL/HDL ratio, according to XbaI polymorphism.
In women with ERα XbaI GG genotype, the level of estradiol correlated positively to HDL concentration (r=0.477; p=0.004) and negatively to LDL/HDL ratio (r=−0.333; p=0.050). The sub-group of women with higher estradiol levels had higher average HDL level and lower average LDL/HDL ratio. Figure 1 shows that the regression line between estradiol and HDL goes up while the line between estradiol and LDL/HDL goes down among that group of women.
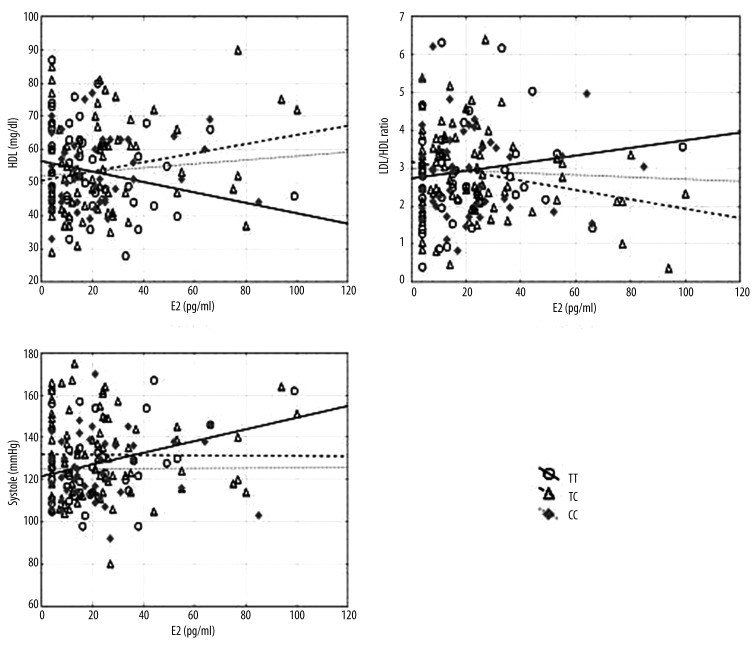
The results obtained also indicated a significant positive correlation between the level of estradiol and systolic blood pressure in women who possessed PvuII ERα TT genotype (r=0.314; p=0.017). In this sub-group, an increase in the level of estradiol was accompanied by an increase in the value of systolic blood pressure on average. In women with PvuII genotype TC, a positive correlation was noted between the level of estradiol and the level of HDL (r=0.224; p=0.027), and a negative correlation between the level of estradiol and the LDL/HDL ratio (r=−0.237; p=0.020). Higher levels of estradiol in this sub-group were associated with higher average HDL level and average LDL/HDL ratio. Figure 2 shows that the regression line between estradiol and systole for TT as well as estradiol and HDL for TC goes up, but goes down for estradiol and LDL/HDL.
Figure 2.
Scatter diagrams comparing estradiol and HDL, LDL/HDL ratio, and systole, according to PvuII polymorphism.
The following correlations were calculated: age, age at last menstruation, BMI, risk factors of cardiovascular diseases, and estradiol in the total group of examined women (Table 5). Only the statistically significant correlations are described below.
Table 5.
Correlation coefficients between age, age at last menstruation, BMI and risk factors of cardiovascular diseases, estradiol in total group of examined women.
| Cardiovascular diseases risk factors | Age (years) | Age at last menstruation (years) | BMI (kg/m2) | |||
|---|---|---|---|---|---|---|
| r* | p** | r | p | r | p | |
| Waist circumference (cm) | −0.157 | 0.023 | −0.091 | 0.190 | 0.607 | <0.001 |
| W/H ratio | −0.104 | 0.134 | −0.112 | 0.105 | 0.635 | <0.001 |
| CHOL (mg/dl) | 0.041 | 0.559 | 0.094 | 0.173 | −0.004 | 0.950 |
| HDL (mg/dl) | −0.040 | 0.566 | −0.106 | 0.124 | −0.067 | 0.334 |
| LDL (mg/dl) | 0.019 | 0.781 | 0.102 | 0.141 | −0.016 | 0.817 |
| TG (mg/dl) | 0.107 | 0.123 | 0.067 | 0.334 | 0.104 | 0.133 |
| LDL/HDL ratio | 0.060 | 0.390 | 0.159 | 0.021 | 0.003 | 0.963 |
| Systole (mmHg) | 0.054 | 0.440 | 0.155 | 0.025 | 0.165 | 0.017 |
| Diastole (mmHg) | −0.041 | 0.552 | 0.078 | 0.258 | 0.119 | 0.084 |
| E2 (pg/ml) | −0.122 | 0.078 | −0.048 | 0.490 | −0.088 | 0.207 |
Correlation coefficient;
significance level.
We found that BMI was positively correlated with waist circumference (r=0.607; p<0.001) and W/H ratio (r=0.635; p<0.001) was positively correlated with systolic blood pressure (r=0.165; p=0.017). Higher levels of BMI were associated with larger waist circumference and higher waist W/H ratio and systolic blood pressure on average.
Age at last menstruation had a positive correlation with LDL/HDL ratio (r=0.159; p=0.021) and systolic blood pressure (r=0.155; p=0.025). Women who began menopause later had higher average level of LDL/HDL ratio and systolic blood pressure during menopause.
We found a negative correlation of waist circumference and age of women during a menopause (r=−0.157; p=0.023). Younger women had larger average waist circumference during menopause.
Discussion
Many studies show that the frequency of occurrence of cardiovascular diseases increases after menopause. It is considered that a decrease in endogenous estrogens is responsible for this unfavorable effect [18,19]. A few studies suggest that there is no reliable evidence for a rapid increase in morbidity due to CVD at postmenopausal age; however, a constant, proportional increase in the occurrence of these diseases is observed with age, caused by the co-existence of risk factors such as obesity, hypertension, lipid disorders, and glucose intolerance [20]. Thus, the question of whether estrogens exert a direct effect on the development of cardiovascular diseases or whether this effect is related to their impact on lipidogram, arterial hypertension, and body weight, remains to be answered. Also, it is not clear in what way the possession of particular ERα genotypes is related to increased risk of these diseases.
In the group of postmenopausal women in the presented study, the values of the parameters analyzed – BMI, BMI, WHR, waist circumference, levels of CHOL, LDL, HDL, TG, LDL/HDL ratio, systole, and diastole – were not significantly dependent to the possessed ERα XbaI and PvuII polymorphisms. Also, the occurrence of cardiovascular risk factors in the group examined, specified in the methodology, was not significantly dependent on the possessed ERα XbaI and PvuII polymorphisms.
Matsubara et al. also did not confirm any relationships between XbaI and PvuII polymorphisms, and the levels of total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides [21]. Contrary to the results of the presented study, Kikichi et al. [22] found that XbaI polymorphism exerted a significant effect on the level of LDL-cholesterol. Studies by Denga et al. [14] show that ERα gene PvuII and XbaI polymorphisms are important for the amount of fatty tissue and the BMI in postmenopausal women.
Clinical studies indicate that estrogens greatly affect the amount of fatty tissue. A decrease in their level is related to an increase in the amount of fatty tissue in postmenopausal women [23]. It is noteworthy that estrogens may increase the level of HDL-cholesterol, which explains the lower frequency of occurrence of cardiovascular diseases in pre-menopausal women. This observation has not been confirmed based on the presented material. In the group of women in this study, none of the analyzed parameters were significantly correlated with the level of estradiol.
Studies on the effect of ERα polymorphism and the level of estradiol indirectly showed that women with the ERα PuvII CC genotype more effectively respond to the effect of estradiol than those possessing the T allele. In addition, it was discovered that after menopause the level of endogenous estradiol is lowest in women possessing the T allele, whereas this level is the highest in women with the CC genotype, which has not been confirmed in the presented study [24].
Attempts were undertaken to take advantage of the beneficial effect of estrogens on the cardiovascular system in women by applying hormone replacement therapy (HRT). It was observed that the benefits resulting from the implementation of this therapy may depend on the type of estrogen receptor alpha polymorphism. Herrington et al. [12] published results of studies concerning the level of HDL-cholesterol during the use of HRT, which clearly showed its increase in women with the CC genotype. It is interesting that as early as before the implementation of HRT, the level of HDL cholesterol in homozygotes CC and GG was significantly higher than in women with TT and AA genotypes.
In the presented study, significant negative correlations were found between waist circumference and W/H ratio, and the level of estradiol in women with the ERα XbaI AA genotype. In women possessing the ERα XbaI GG genotype, a positive correlation was observed between the levels of estradiol and HDL, and a negative correlation between estradiol level and LDL/HDL ratio. This suggests that the AA genotype is related to the strongest effect of estradiol on the distribution of fatty tissue in postmenopausal women, whereas the GG genotype intensifies the beneficial effect of estradiol on the level of HDL. Similarly, a beneficial interaction between the level of estradiol and the level of HDL was discovered in women with the ERα PvuII TC genotype. The results of this study concerning the level of endogenous estradiol are partly consistent with the results presented by Herrington et al. [12]; however, in the current study we found that the AA genotype was important with respect to the effect of endogenous estradiol on the distribution of fatty tissue after menopause. In addition, we noted that in the examined women who possessed the ERα PvuII TT genotype, the value of systolic blood pressure increased together with the level of estradiol. This suggests a stronger unfavorable effect of estradiol on systolic blood pressure in carriers of the TT allele.
The Rotterdam Study, which covered approximately 4000 women, revealed that those with the TT genotype were more exposed to cardiovascular diseases, which took a more severe and complicated course. The risk of cardiovascular diseases found in the Rotterdam Study occurred irrespective of the classic cardiovascular risk factors, such as BMI, level of lipids, hypertension, and diabetes [25–27]. A meta-analysis performed by Ding et al. shows interesting results, in which the researchers, after analyzing 21 studies, found that PuvII polymorphism plays an important role in the susceptibility to atherosclerosis. They also confirmed that susceptibility related to PuvII polymorphism is clearly observed in Asian countries but not significantly in Western countries. The differences in the gene expression and epigenetic effects observed between Eastern and Western populations may lead to differences in the susceptibility to ischemic disease [28,29]. Moreover, the pathomechanism of ischemic disease is related to various factors – both genetic and environmental – with lifestyle, geographic conditions, or climate. These conditionings may affect the gene-environment interactions, and differentiate gene response (e.g., of the ERα genes) related to susceptibility to cardiovascular diseases [30–32].
Conclusions
The level of endogenous estradiol and ERα XbaI and PuvII polymorphisms as independent parameters did not significantly correlate to BMI, waist circumference, W/H ratio, CHOL, HDL, LDL, TG, LDL/HDL, systole, or diastole of the examined postmenopausal women.
-
The polymorphism of α estrogen receptor affected correlations between estradiol concentration and the analyzed atherosclerosis risk factors among healthy postmenopausal women:
A negative correlation of estradiol concentration with waist circumference and W/H ratio was observed in women with genotype AA of ERα XbaI polymorphism.
A positive correlation between estradiol concentration and HDL concentration was observed in women with AA genotype of ERα XbaI polymorphism.
A positive correlation between estradiol concentration and systolic blood pressure after a menopause was observed in women with TT genotype of ERα PuvII polymorphism.
Footnotes
Source of support: This study was sponsored by the Institute of Rural Health in Lublin, Poland
References
- 1.Islami F, Mańczuk M, Vedanthan R, et al. A cross-sectional study of cardiovascular disease and associated factors. Ann Agric Environ Med. 2011;18(2):255–59. [PubMed] [Google Scholar]
- 2.Matyjaszczyk P, Hoffmann K, Bryl W. Epidemiology of selected risk factors of cardiovascular diseases. Cardio-Diabetological Review. 2011;6(4):255–62. [Google Scholar]
- 3.Berenson GS. Cardiovascular health promotion for elementary school children. Risk Factors. 1998;(Supl.5):11–13. [Google Scholar]
- 4.Gerhard M, Ganz P. How do we explain the clinical benefits of estrogen? From bedside to bench. Circulation. 1995;92:5–8. doi: 10.1161/01.cir.92.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–66. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 6.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 7.Lundeen SG, Carver JM, McKean ML, Winneker RC. Characterization of the ovariectomized rat model for the evaluation of estrogen effects on plasma cholesterol levels. Endocrinology. 1997;138:1552–58. doi: 10.1210/endo.138.4.5083. [DOI] [PubMed] [Google Scholar]
- 8.Campos H, Walsh BW, Judge H, Sacks FM. Effect of estrogen on very low density lipoprotein and low density lipoprotein subclass metabolism in postmenopausal women. J Clin Endocrinol Metab. 1997;82:3955–63. doi: 10.1210/jcem.82.12.4437. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn ME, Karas RH. Estrogen and the blood vessel wall. Curr Opin Cardiol. 1994;9:619–26. doi: 10.1097/00001573-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Couse JF, Curtis SW, Washburn TF, et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–54. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen SB, Børglum JD, Møller-Pedersen T, Richelsen B. Effects of in vivo estrogen treatment on adipose tissue metabolism and nuclear estrogen receptor binding in isolated rat adipocytes. Mol Cell Endocrinol. 1992;85:13–19. doi: 10.1016/0303-7207(92)90120-u. [DOI] [PubMed] [Google Scholar]
- 12.Herrington DM, Howard TD, Hawkins GA, et al. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med. 2002;346:967–74. doi: 10.1056/NEJMoa012952. [DOI] [PubMed] [Google Scholar]
- 13.Schuit SC, Oei HH, Witteman JC, et al. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. JAMA. 2004;291:2969–77. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 14.Deng HW, Li J, Li JL, et al. Association of estrogen receptor-alpha genotypes with Body mass index in normal healthy postmenopausal Caucasian women. J Clin Endocrinol Metab. 2000;85:2748–51. doi: 10.1210/jcem.85.8.6728. [DOI] [PubMed] [Google Scholar]
- 15.Fox CS, Yang Q, Cupples LA, et al. Sex-specific association between estrogen receptor-alpha gene variation and measures of adiposity: the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90:6257–62. doi: 10.1210/jc.2005-0670. [DOI] [PubMed] [Google Scholar]
- 16.Speer G, Cseh K, Winkler G, et al. Vitamin D and estrogen receptor gene polymorphisms in type 2 diabetes mellitus and in android type obesity. Eur J Endocrinol. 2001;144:385–89. doi: 10.1530/eje.0.1440385. [DOI] [PubMed] [Google Scholar]
- 17.Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Estrogen receptors alpha (rs2234693 and rs9340799), and beta (rs4986938 and rs1256049) genes polymorphism in prostate cancer: evidence for association with risk and histopathological tumor characteristics in Iranian men. Mol Carcinog. 2012;51(Suppl 1):E104–17. doi: 10.1002/mc.21870. [DOI] [PubMed] [Google Scholar]
- 18.Kozakiewicz K, Wycisk A. [Hormonal Replacement Therapy and Selective Estrogen Receptor Modulators in Prevention of Cardiovascular Disease]. Medical News. 2006;59(5–6):377–82. [in Polish] [PubMed] [Google Scholar]
- 19.Ilow R, Regulska-Ilow B, Różańska D, et al. Prevalence of metabolic syndrome among 40- and 50-year-old inhabitants of Wroclaw, Poland. Ann Agric Environ Med. 2012;19(3):551–56. [PubMed] [Google Scholar]
- 20.Rossouw JE. Hormones, genetic factors, and gender differences in cardiovascular disease. Cardiovasc Res. 2002;53:550–57. doi: 10.1016/s0008-6363(01)00478-3. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara Y, Murata M, Kawano K, et al. Genotype distribution of estrogen receptor polymorphisms in men and postmenopausal women from healthy and coronary populations and its relation to serum lipid levels. Arterioscler Thromb Vasc Biol. 1997;17(11):3006–12. doi: 10.1161/01.atv.17.11.3006. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi T, Kawasaki T, Uchiyama M. Association of serum low-density lipoprotein metabolism with oestrogen receptor gene polymorphisms in healthy children. Acta Paediatr. 2000;89:42–45. doi: 10.1080/080352500750029040. [DOI] [PubMed] [Google Scholar]
- 23.Shen C, Chen J, Fan S, et al. Association between the polymorphism of estrogen receptor α and coronary artery disease in a Chinese population. Eur J Intern Med. 2012;23(2):175–78. doi: 10.1016/j.ejim.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Schuit SCE, de Jong FH, Stolk L, et al. Estrogen receptor alpha gene polymorphisms are associated with estradiol levels in postmenopausal women. Eur J Endocrinol. 2005;153:327–34. doi: 10.1530/eje.1.01973. [DOI] [PubMed] [Google Scholar]
- 25.Schuit SC, Oei HH, Witteman JC, et al. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. JAMA. 2004;291:2969–77. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 26.Ferrero V, Ribichini F, Matullo G, et al. Estrogen receptor-alpha polymorphisms and angiographic outcome after coronary artery stenting. Arterioscler Thromb Vasc Biol. 2003;23:2223–28. doi: 10.1161/01.ATV.0000101181.81022.BF. [DOI] [PubMed] [Google Scholar]
- 27.Nordström P, Glader CA, Dahlén G, et al. Oestrogen receptor a gene polymorphism is related to aortic valve sclerosis in postmenopausal women. J Int Med. 2003;254:140–46. doi: 10.1046/j.1365-2796.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 28.Thomas KL, Honeycutt E, Shaw LK, et al. Racial differences in long-term survival among patients with coronary artery disease. Am Heart J. 2010;160(4):744–51. doi: 10.1016/j.ahj.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Batchelor WB, Ellis SG, Ormiston JA, et al. Racial differences in long-term outcomes after percutaneous coronary intervention with paclitaxel-eluting coronary stents. J Interv Cardiol. 2013;26(1):49–57. doi: 10.1111/j.1540-8183.2012.00760.x. [DOI] [PubMed] [Google Scholar]
- 30.Ioana M, Ferwerda B, Plantinga TS, et al. Different patterns of Toll-like receptor 2 polymorphisms in populations of various ethnic and geographic origins. Infect Immun. 2012;80(5):1917–22. doi: 10.1128/IAI.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labonté B, Suderman M, Maussion G, et al. Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry. 2012;69(7):722–31. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Q, Liu EQ, Zhao SH, et al. Association between TaqIB polymorphism of cholesteryl ester transfer protein and coronary artery disease in the Chinese population. J Zhejiang Univ Sci B. 2012;13(5):342–47. doi: 10.1631/jzus.B1100264. [DOI] [PMC free article] [PubMed] [Google Scholar]