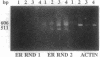
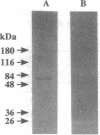
Abstract
The decrease in estrogen levels that follows the onset of menopause results in rapid bone loss and osteoporosis. The major effect of estrogen deficiency on bone metabolism is an increase in the rate of bone resorption, but the precise mechanism by which this occurs remains unresolved. A recently developed technique for the isolation of avian osteoclasts has been modified to obtain highly purified multinucleated cells from human giant cell tumors. These osteoclast-like cells have been examined for evidence of estrogen receptors (ERs) and responses to 17 beta-estradiol (17 beta-E2). Analysis of giant-cell RNA demonstrated expression of ER mRNA. Furthermore, immunoblot analysis revealed that the giant cells contained a 66-kDa protein that was recognized by a monoclonal antibody specific for the human ER. When isolated multinucleated cells were cultured on slices of bone, there was a dose-dependent decrease in resorption in response to treatment detectable at 10 pM 17 beta-E2. Treatment with 10 nM 17 alpha-estradiol or vehicle (control) did not inhibit resorption. Moreover, the multinucleated cells isolated from these tumors had decreased mRNA levels for cathepsin B, cathepsin D, and tartrate-resistant acid phosphatase (TRAP) as well as secreted cathepsin B and TRAP enzyme activity in response to treatment with 10 nM 17 beta-E2. In contrast to these data, no change in gene expression was detected in mononuclear cells from these tumors in response to 17 beta-E2 treatment. These data support the proposition that human osteoclasts are target cells for estrogen and that estrogen can inhibit bone resorption by human osteoclasts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avioli L. V. Calcium and osteoporosis. Annu Rev Nutr. 1984;4:471–491. doi: 10.1146/annurev.nu.04.070184.002351. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. Molecular mechanisms of bone resorption by the osteoclast. Anat Rec. 1989 Jun;224(2):317–324. doi: 10.1002/ar.1092240220. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Winchester R. J., Dimitriu-Bona A., Klein M., Steiner G., Sissons H. A. Delineation of four cell types comprising the giant cell tumor of bone. Expression of Ia and monocyte-macrophage lineage antigens. J Clin Invest. 1983 Jun;71(6):1633–1648. doi: 10.1172/JCI110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collin-Osdoby P., Oursler M. J., Webber D., Osdoby P. Osteoclast-specific monoclonal antibodies coupled to magnetic beads provide a rapid and efficient method of purifying avian osteoclasts. J Bone Miner Res. 1991 Dec;6(12):1353–1365. doi: 10.1002/jbmr.5650061213. [DOI] [PubMed] [Google Scholar]
- Everts V., Beertsen W., Schröder R. Effects of the proteinase inhibitors leupeptin and E-64 on osteoclastic bone resorption. Calcif Tissue Int. 1988 Sep;43(3):172–178. doi: 10.1007/BF02571316. [DOI] [PubMed] [Google Scholar]
- Flanagan A. M., Nui B., Tinkler S. M., Horton M. A., Williams D. M., Chambers T. J. The multinucleate cells in giant cell granulomas of the jaw are osteoclasts. Cancer. 1988 Sep 15;62(6):1139–1145. doi: 10.1002/1097-0142(19880915)62:6<1139::aid-cncr2820620617>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Dayer J. M., Krane S. M. Regulation of hormone-induced cyclic AMP response to parathyroid hormone and prostaglandin E2 in cells cultured from human giant cell tumors of bone. Calcif Tissue Int. 1979;29(3):193–200. doi: 10.1007/BF02408080. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Nolan C., Engler J. P., Jensen E. V. Monoclonal antibodies to human estrogen receptor. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5115–5119. doi: 10.1073/pnas.77.9.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFSTEE B. H. Direct and continuous spectrophotometric assay of phosphomonoesterases. Arch Biochem Biophys. 1954 Jul;51(1):139–146. doi: 10.1016/0003-9861(54)90461-0. [DOI] [PubMed] [Google Scholar]
- Hanaoka H., Friedman B., Mack R. P. Ultrastructure and histogenesis of giant-cell tumor of bone. Cancer. 1970 Jun;25(6):1408–1423. doi: 10.1002/1097-0142(197006)25:6<1408::aid-cncr2820250622>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Joyner C. J., Quinn J. M., Triffitt J. T., Owen M. E., Athanasou N. A. Phenotypic characterisation of mononuclear and multinucleated cells of giant cell tumour of bone. Bone Miner. 1992 Jan;16(1):37–48. doi: 10.1016/0169-6009(92)90820-4. [DOI] [PubMed] [Google Scholar]
- Kasahara K., Yamamuro T., Kasahara A. Giant-cell tumour of bone: cytological studies. Br J Cancer. 1979 Aug;40(2):201–209. doi: 10.1038/bjc.1979.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks S. C., Jr, Grolman M. L. Tartrate-resistant acid phosphatase in mononuclear and multinuclear cells during the bone resorption of tooth eruption. J Histochem Cytochem. 1987 Nov;35(11):1227–1230. doi: 10.1177/35.11.3655324. [DOI] [PubMed] [Google Scholar]
- Oursler M. J., Osdoby P., Pyfferoen J., Riggs B. L., Spelsberg T. C. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6613–6617. doi: 10.1073/pnas.88.15.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oursler M. J., Pederson L., Pyfferoen J., Osdoby P., Fitzpatrick L., Spelsberg T. C. Estrogen modulation of avian osteoclast lysosomal gene expression. Endocrinology. 1993 Mar;132(3):1373–1380. doi: 10.1210/endo.132.3.8440193. [DOI] [PubMed] [Google Scholar]
- Pensler J. M., Radosevich J. A., Higbee R., Langman C. B. Osteoclasts isolated from membranous bone in children exhibit nuclear estrogen and progesterone receptors. J Bone Miner Res. 1990 Aug;5(8):797–802. doi: 10.1002/jbmr.5650050802. [DOI] [PubMed] [Google Scholar]
- Pilbeam C. C., Klein-Nulend J., Raisz L. G. Inhibition by 17 beta-estradiol of PTH stimulated resorption and prostaglandin production in cultured neonatal mouse calvariae. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1319–1324. doi: 10.1016/0006-291x(89)91122-4. [DOI] [PubMed] [Google Scholar]
- Rifkin B. R., Vernillo A. T., Kleckner A. P., Auszmann J. M., Rosenberg L. R., Zimmerman M. Cathepsin B and L activities in isolated osteoclasts. Biochem Biophys Res Commun. 1991 Aug 30;179(1):63–69. doi: 10.1016/0006-291x(91)91334-9. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Steiner G. C., Ghosh L., Dorfman H. D. Ultrastructure of giant cell tumors of bone. Hum Pathol. 1972 Dec;3(4):569–586. doi: 10.1016/s0046-8177(72)80007-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G. Cellular biology and biochemical mechanism of bone resorption. A review of recent developments on the formation, activation, and mode of action of osteoclasts. Clin Orthop Relat Res. 1988 Jun;(231):239–271. [PubMed] [Google Scholar]
- Wood G. S., Beckstead J. H., Medeiros L. J., Kempson R. L., Warnke R. A. The cells of giant cell tumor of tendon sheath resemble osteoclasts. Am J Surg Pathol. 1988 Jun;12(6):444–452. doi: 10.1097/00000478-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Moonga B., Moss D. W., MacIntyre I. Inhibition of osteoclastic acid phosphatase abolishes bone resorption. Biochem Biophys Res Commun. 1989 Feb 28;159(1):68–71. doi: 10.1016/0006-291x(89)92405-4. [DOI] [PubMed] [Google Scholar]