Abstract
Objectives
Pain-related Temporomandibular disorders (TMD) are the most prevalent conditions among TMDs. There is contrasting evidence available for association of pain-related TMD and masticatory muscle activity (MMA). The present investigation assesses the associations between MMA levels of masseter and temporalis muscles during awake and sleep among pain-related TMD diagnostic groups.
Setting and Sample Population
The department of Oral Diagnostic Sciences, University at Buffalo. Twenty females and 6 males participated in this study.
Material & Methods
Using the Diagnostic Criteria for Temporomandibular Disorders (DC-TMD), participants were diagnostically categorized. Subjects used a custom monitoring system, which recorded in–field muscle activities. A factorial model tested for association between independent variable (muscle, time period, MMA level, diagnostic group) effects and the logarithm of MMA. Greenhouse–Geisser test was used to determine any statistically significant associations (p ≤ 0.003).
Results
No statistically significant association was found among four-way, three-way, and two-way analyses. However, among the main effects, range of magnitudes was the only variable to be statistically significant. Although the data suggest a trend of increased masseter MMA in the pain-related TMD diagnoses group both during awake and sleep time periods, such observation is not maintained for the temporalis muscle. In addition, temporalis MMA was found to be higher in the pain-related TMD diagnoses group only at extreme activity levels (<25% and ≥80% ranges).
Conclusion
This data support the association between masticatory muscle hyperactivity and painful-TMD conditions.
Keywords: Bruxism, Electromyography, Masticatory Muscles, Pain, Temporomandibular joint disorders
Introduction
Temporomandibular disorders (TMD) is a collective term, embracing a number of clinical problems that involve the masticatory muscles, the temporomandibular joint (TMJ) and the associated structures (1). The pain-related TMD consist of myalgia, arthralgia, and headaches attributed to TMD (2). The prime manifestations of pain-related TMD consist of pain of a persistent, recurring, or chronic nature in the masticatory muscles, TMJ, or in the adjacent structures (3, 4). The other major symptoms include limitation in the range of mandibular motion, and joint noises (1,3, 4).
The prevalence of pain-related TMD is about 10 % in the general population and the incidence in the general population of United States is about 3.9 % (5, 6). The etiology of pain-related TMD is considered multifactorial resulting from a complex interaction among biological, psychological, social, and environmental variables (7). Historically, two competitive models have been presented to explain the presence of pain among individuals diagnosed with TMDs, the stress-hyperactivity (8) and the pain adaptation models (9). The evidence for either of the two models has been inconsistent and poor, primarily due to the presence of multiple potential confounders, particularly at the diagnostic levels (10, 11). Recently through new data, based on methodologically solid designs, a better understanding of the multifactorial nature of the conditions is available leading into a peripheral/ central sensitization model (12, 13). Risk factors identified with pain-related TMD including gender, psychological characteristics, sustained parafunctional activity, non-specific orofacial symptoms, and various comorbid pre–existing pain conditions (6, 13).
The aim of this study was to evaluate the associations between masticatory muscle activity (MMA) levels of masseter and temporalis muscles during awake and sleep time-periods among groups of subjects with various pain-related TMD diagnoses. To test these associations a standardized Diagnostic Criteria for Temporomandibular Disorders (DC-TMD) examination protocol (2) and a calibrated ambulatory EMG monitoring system (14) to measure masticatory muscle activities were used.
Methods
Study Participants
Recruitment
All participants were recruited at the University at Buffalo, School of Dental Medicine. Participants were consecutively recruited from direct referrals from local health care providers and in response to community advertisements.
Inclusion and exclusion criteria
Adult males and females were included. Excluded were individuals who were pregnant; had evidence of degenerative joint diseases (determined by cone beam computer tomography [CBCT]); had unilateral disc displacement (determined by magnetic resonance imaging [MRI]); had diagnoses of systemic musculoskeletal or reheumatological diseases (e.g. fibromyalgia, muscular atrophy); had missing teeth or large restorations; were unable to read or follow tasks associated with the laboratory and field recordings.
This study was approved by the Institutional Review Boards of the State University of New York at Buffalo (HSIRB) and University of Missouri Kansas City (Adult IRB). Informed consent was obtained from each participant.
Appointments for study
To complete participation in the protocols of the study, each subject made a minimum of 5 visits. During an initial clinic visit, an examiner explained the study, obtained informed consent, reviewed the participant’s medical history, and performed a screening exam, particularly with reference to the exclusion and the inclusion criteria. During subsequent visits, temporomandibular joint MRI and CBCT images were obtained using standardized acquisition protocols for all study participants and interpreted by a calibrated radiologist. Those who qualified for participation underwent the standardized Diagnostic Criteria for Temporomandibular Disorders (DC-TMD) examination (2) by a calibrated examiner. In addition, maxillary and mandibular arch impressions, and face-bow recordings were obtained. Eligible participants presented for 2 laboratory visits during which bite forces along with electromyographic (EMG) activities of masseter and temporalis muscles were recorded bilaterally using surface electrodes during static and dynamic bites on molars and incisors. Participants were also trained to use portable EMG recorders and surface electrodes on one side to make in–field ambulatory EMG recordings during the first of the 2 laboratory visits.
Equipment
Bite force measurement
Custom bite force transducers made of electrically resistant film (Flexforce, Tekscan Inc., Boston MA) attached to a stainless steel wire handle, sandwiched by acrylic (Triad, TruTray, Dentsply Inc., York PA) and wrapped with clear plastic film were used (Figure 1). Each transducer was pre-calibrated so the electrical voltage output recorded (V) could be later converted to units of force in Newton (N).
Figure 1.
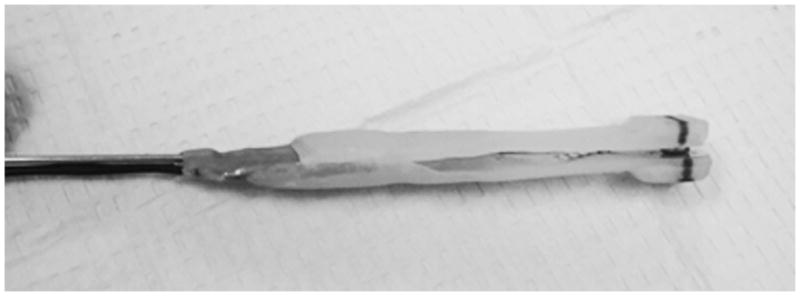
Bite – force transducer.
Bite – force transducer consisting of acrylic biting surfaces sandwiching sensor film (Tekscan Flexiforce®, Tekscan Inc., South Boston, MA, USA) attached to a wire handle. Shown without protective plastic film barrier.
(Photograph courtesy of Dr. Adam Reynolds.)
Laboratory EMG recordings during biting
Bipolar surface electrodes, amplifiers, and a digital recording system were used as previously described (15, 16).
Ambulatory EMG activity of masticatory muscles
The EMG surface electrode signals were band pass filtered (20 – 1000 Hz) and amplified (5000×) by means of the portable device with digital amplifier having an input impedance of 250 MΩ, noise level of 0.7 μVolt, and common mode rejection ratio of 100 dB.
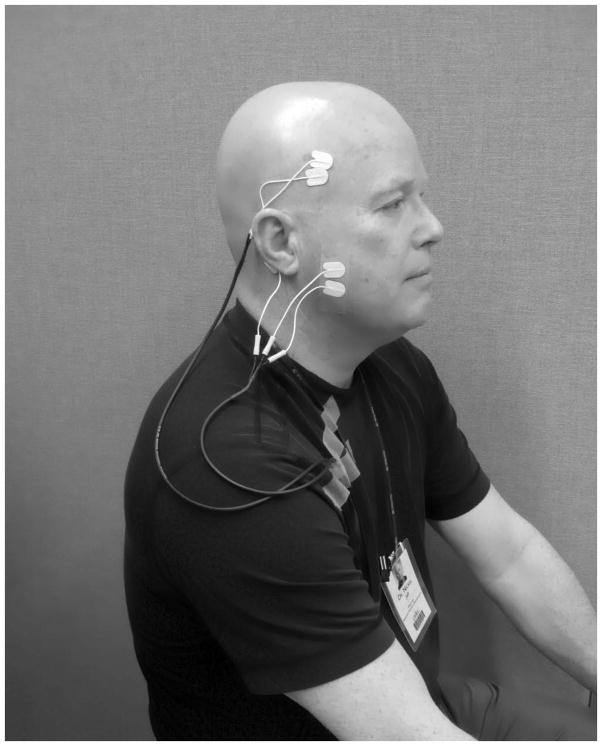
During in-field data collection, participants used the portable EMG recording equipment and supplies as instructed (Figure 2), to capture unilateral electromyographic signals from the masseter and temporalis muscles. Subjects were asked to record for periods of at least 6 total hours for each of 3 days and 3 nights.
Figure 2.
Ambulatory EMG setup.
Picture illustrating ambulatory EMG setup, the two surface electrodes are attached with an adhesive tape over the anterior temporalis muscle and the body of masseter muscle. Electrodes are attached to a portable EMG device.
Data Processing
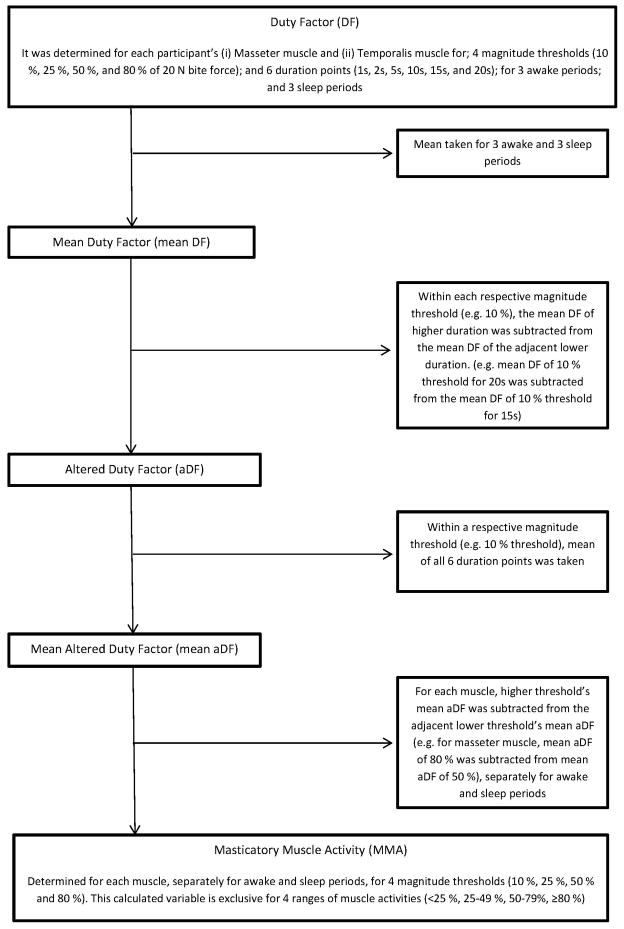
Duty factor (DF)
Duty factor is the percentage of time when a respective muscle has been active above a certain threshold out a total given time (duration of muscle activity/duration of recording period × 100 %). Thresholds used in the current study were (1) magnitude of muscle activity and (2) duration of muscle activity.
A custom software program (MATLAB, The MathWorks Inc., Natick, MA) was used with the calibration information for the specific transducer to identify and calculate force (N) and root-mean-square EMG (μV) outputs for each of the static and dynamic bite tasks over a 128 ms contiguous period. A total of 50 EMG:BF data points (5 static bites and 5 dynamic bites for 4 frequencies on right and left molars) were plotted for each muscle, subject, and visit and from the resulting regression relation (μV/N), a threshold EMG activity for a 20 N bite force (T20N, μV) was determined. T20N results from laboratory visits were then averaged (T20Nave) for masseter and temporalis muscles in each subject and used to set 4 arbitrary magnitude (10%, 25%, 50%, 80% • T20Nave) and 6 arbitrary duration (1s, 2s, 5s, 10s, 15s, and 20s) activity thresholds to calibrate in-field ambulatory EMG recordings for the same muscle type and subject.
Each in-field ambulatory EMG recording for each muscle type was viewed, noisy signals were identified and excluded, and then task-definers were applied to calculate DF for the desired thresholds using customizable software.
Masticatory Muscle Activity (MMA)
For each muscle type and subject, DF was determined for 4 magnitude and 6 duration thresholds in two different time periods (awake and sleep) and across 3 days. The combination of duty factors allowed estimating “Masticatory Muscle Activity” as outcome variable with mutually exclusive values (Figure 3).
Figure 3.
Flow chart explaining the derivation of Masticatory Muscle Activity (MMA)
Statistical Analysis
A multi–step analytic approach identified associations of masseter and temporalis muscle MMA during awake and sleep time periods with TMD–pain diagnoses. The first step examined the distribution of the data across the two diagnostic groups using the Shaprio–Wilk test of normality. If data showed non-parametric distribution, logarithmic transformation would be applied.
The second step involved using a generalized linear model for repeated measurements with Mauchly’s test of sphericity. The goal of this analysis was to determine any interaction among the main variables (Diagnostic group, Time period, Muscles, MMA ranges).
The third step involved evaluation of the trend of distribution of the dependent variables among the diagnostic groups. Since four-way analyses of variance provide 15 tests, a Bonferroni correction was used, with alpha of 0.003 determining significance.
Results
Evaluation of the data showed a non-normal distribution, using a Shapiro–Wilk normality test (p > .05). Therefore, logarithmic transformation was used. Study population had 26 participants, 77% females (n=20) with a mean age of 33.3 ± 12.3 years. Based on the DC/TMD diagnosis classification subjects were classified as pain-related TMD diagnoses (myalgia and arthralgia) and no pain diagnoses. Among the pain-related TMD diagnoses group the mean age was 30.6 ± 11 years and 84.6% were females (n=11), while among the control group the mean age was 36 ± 13.3 years and 69.2% were females (n=9). No statistical differences were observed between the groups for age (t – test, p =.272) or gender distribution (chi –square, p = .352).
A full factorial model was used initially with factors for muscle (2 levels, within subjects), time (2 levels, within subjects), MMA range (4 levels, within subjects), and diagnostic group (2 levels, between subjects). The dependent variable was the logarithm of MMA. Mauchly’s test for sphericity was found to be statistically significant (p < 0.001), therefore, it cannot be assumed that variances were equal. Consequently, Greenhouse–Geisser test to determine any statistically significant associations was used.
No significant interactions were observed among four-way, three-way, and two-way analyses. However, among the main effects, range of magnitudes was the only variable to be statistically significant (table 1). MMA range, paired t –tests demonstrated that the differences in MMA at <25% and the rest of the ranges (25–49%, 50–79%, and ≥80%) were statistically significant (p < .001).
Table 1.
Results of Generalized linear measurement test of repeated measurements, four way interaction among the study variables
| Factors | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Muscle | 0.008 | 1 | 0.008 | 0.03 | 0.864 |
| Time | 0.687 | 1 | 0.687 | 9.182 | 0.006 |
| MMA Range | 21.255 | 1.281 | 16.591 | 30.073 | >0.001‡ |
| Diagnostic Group | 1.225 | 1 | 1.225 | 1.217 | 0.281 |
| Muscle * Diagnostic Group | 0.788 | 1 | 0.788 | 3.089 | 0.092 |
| Time * Diagnostic Group | 0.005 | 1 | 0.005 | 0.064 | 0.803 |
| MMA Range * Diagnostic Group | 1.805 | 1.281 | 1.409 | 2.554 | 0.113 |
| Muscle * Time | 0.316 | 1 | 0.316 | 4.298 | 0.049 |
| Muscle * MMA Range | 0.091 | 1.558 | 0.058 | 0.462 | 0.585 |
| Time * MMA Range | 0.682 | 1.787 | 0.382 | 3.593 | 0.041 |
| Muscle * MMA Range * Diagnostic Group | 1.3 | 1.558 | 0.834 | 6.608 | 0.006 |
| Muscle * Time * Diagnostic Group | 0.249 | 1 | 0.249 | 3.393 | 0.078 |
| Time * MMA Range * Diagnostic Group | 0.042 | 1.787 | 0.024 | 0.222 | 0.777 |
| Muscle * Time * MMA Range | 0.631 | 1.448 | 0.436 | 2.96 | 0.08 |
| Muscle * Time * MMA Range *Diagnostic Group | 0.15 | 1.448 | 0.104 | 0.704 | 0.458 |
Statistically significant (p ≤ .003)
In order to illustrate the effect of logarithm of MMA for the masseter and temporalis muscles, Tables 2 and 3 present the data by diagnostic group within the awake and the sleep time periods. For the masseter muscle, during the awake time period, there was a linear pattern in which those with pain-related TMD diagnoses have higher activity than the no pain diagnoses group. This pattern is more perceptible at the <25% range. During the sleep time period, at extreme magnitude ranges (<25% and ≥80%) the masseter activity appears to be higher in the pain-related TMD diagnoses group.
Table 2.
Summary of logarithm masseter muscle MMA in participants of pain-related TMD diagnoses and no pain diagnoses group
| Awake Time Period | Sleep Time Period | |||||||
|---|---|---|---|---|---|---|---|---|
| Pain-related TMD diagnoses | No Pain Diagnoses | Pain-related TMD diagnoses | No Pain Diagnoses | |||||
| MMA Range (%) | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation |
| <25 | 1.101 | 0.792 | 0.487 | 0.540 | 0.751 | 0.903 | 0.170 | 0.459 |
| 25–49 | 0.406 | 0.481 | 0.194 | 0.504 | 0.100 | 0.128 | 0.072 | 0.255 |
| 50–79 | 0.133 | 0.201 | 0.040 | 0.128 | 0.019 | 0.032 | 0.132 | 0.475 |
| ≥80 | 0.112 | 0.153 | 0.026 | 0.070 | 0.113 | 0.231 | 0.050 | 0.179 |
Table 3.
Summary of logarithm temporalis muscle MMA in participants of pain-related TMD diagnoses and no pain diagnoses group
| Awake Time Period | Sleep Time Period | |||||||
|---|---|---|---|---|---|---|---|---|
| Pain-related TMD diagnoses | No Pain Diagnoses | Pain-related TMD diagnoses | No Pain Diagnoses | |||||
| MMA Range (%) | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation | Mean | Std. Deviation |
| <25 | 0.663 | 0.637 | 0.585 | 0.512 | 0.672 | 0.583 | 0.658 | 0.703 |
| 25–49 | 0.181 | 0.208 | 0.261 | 0.333 | 0.125 | 0.217 | 0.038 | 0.080 |
| 50–79 | 0.060 | 0.089 | 0.180 | 0.484 | 0.104 | 0.311 | 0.115 | 0.385 |
| ≥ 80 | 0.119 | 0.271 | 0.079 | 0.167 | 0.184 | 0.467 | 0.021 | 0.072 |
For the temporalis muscle during the awake time period, the MMA of the pain-related TMD diagnoses group is higher at the <25% and ≥80% ranges although this pattern is less distinct than the one observed for masseter muscle. Similar trends are also observed during the sleep time period (table 3).
Discussion
The purpose of this study was to evaluate the association between MMA recorded in individual’s natural environments during awake and sleep time periods among individuals with pain-related TMD diagnoses. Multi-step analyses of the interactions within subject and between subject variables indicated statistically no significant interactions among muscle, time period, MMA range and diagnostic group. However, among the main effects, it was observed that there was statistically significant differences in MMA at <25% magnitude. There was a generalized trend for masseter and temporalis MMA, where subjects with pain-related TMD diagnoses had higher activities than during awake and sleep time periods.
Masseter MMA during the awake time period was higher among all threshold ranges, while during the sleep more variability was observed. Studies evaluating the association between low-magnitude masticatory muscle activities during awake and sleep time periods and TMD have consistently reported similar results (17, 18). However, studies evaluating the association between high-magnitude masticatory muscle activities, such as during a defined parafunctional behavior like bruxism, and TMD have reported inconsistent findings (11, 19–21). A reason for such inconsistency can be attributed to poor methodological quality (11). Most of the studies reporting association between high-magnitude muscle activities and TMD have relied on nonstandardized self–report questionnaires, or only on oral examination for tooth wear; both with poor reliability and validity in characterizing parafunction (11, 19).
The temporalis muscle activity trend suggest that at either low–magnitude muscle activities (<25%) or high–magnitude muscle activities (≥80%), MMA was higher in the pain-related TMD diagnoses group. However, at other magnitude levels variability was observed. One possible explanation for such trends may be because differing muscle activities are associated with specific oral behaviors (22). Additionally, it has also been postulated that similar oral behaviors in participants with and without TMD may result in different levels of muscle activation (22, 23).
In general, our findings are consistent with those previously reported (18), in which investigators have shown a statistically significant association between background nocturnal low-magnitude masticatory muscle activities and myofascial TMD. Our study, overcame several methodological limitations presented in the literature (25). First, there was no frequency spectral analysis of EMG recordings to decrease spillover of activity at one frequency into neighboring frequencies. This was overcome in our study design by calculating ranges activity magnitudes relative to those for a known bite force (20 N) to ensure mutually exclusive data (figure 3). Secondly, background masticatory muscle activity magnitudes were only recorded during the sleep time period whereas, in our study masticatory muscle activity was recorded during both awake and sleep time periods. Additionally, masseter and temporalis muscle activity data were not differentiated during the analyses, although it has been shown previously that masseter and temporalis muscles are activated dissimilarly across different oral tasks (22, 23). In this study however, masseter and temporalis muscle MMA were analyzed separately.
The observed variability between the diagnostic groups could be explained by the influence of Axis II variables on masticatory muscle activity. It has been proposed that anxiety, depression, and coping ability have an effect on the masticatory muscle activity (22, 24–26). Anxiety disorders in particular have been associated with parafunctional activities such as sleep – related bruxism, day – time clenching and nail biting. It has been proposed that a high level of stress is a risk factor for hyperactivity of masticatory muscles (9) (8, 26). However, clinical evidence in literature regarding this association has been poor and inconsistent (11, 25, 26).
There were several limitations in our investigation. First, we utilized non-invasive surface electrodes for ambulatory EMG recording. It has been shown that non-invasive electrodes are less accurate than intramuscular fine-wire electrodes for EMG recording (27). Nevertheless, surface electrodes may have an advantage over fine-wire electrodes when characterizing “whole muscle” behavior rather than single motor units. Another reason for using surface electrodes was the extent and the nature of recording period (infield recording for total of 6 time periods, each lasting for at least 6 hours). It would have not been practical to train the participants and send them into the field with fine-wire electrodes. Secondly, placement of electrodes for EMG recording was not standardized. All of the participants were instructed to place electrodes on themselves during the in-field recording period. This may have introduced an error in measurement; however, investigators trained all of the participants so as to minimize errors associated with electrode position. Third, the smallest duration threshold in current study was set at ≥1 second. This means any rhythmic functional and parafunctional activity at rate of less than 1 Hertz would have not been recorded. A final limitation is the use of conservative post-hoc tests, which may increase the possibility of type II error. Therefore, further investigations with larger sample size are needed to elucidate the potential presence of interaction among the diagnostic group.
Conclusion
Although, the current study does not identify an explanatory mechanism through which pain-related TMD diagnoses group elicits relatively higher activity in masseter and temporalis muscle across various thresholds, it suggests that masticatory muscle activity is a risk indicator.
Clinical Relevance.
Pain-related TMD have a poorly understood multifactorial etiology. There is contrasting evidence available for association of pain-related TMD and masticatory muscle activity. This is primarily due to presences of multiple potential biases and confounders, particularly at the diagnostic levels. In this publication, we assess if any associations exist between activity levels of masseter and temporalis muscles during awake and sleep time periods and groups of subjects with pain-related TMD diagnoses using a standardized and validated TMD examination to categorize the participants and a state-of-the-art monitoring system to measure masticatory muscle activities.
Acknowledgments
The authors wish to thank study participants and contributions of Dr. Hongzeng Liu for customizing software programs used in data processing and analyses. This study was supported by NIH grant# 5R01DE016417 – 07).
Footnotes
The authors declare no potential conflicts of interest with respect to the authorship and/ or publication of this article.
References
- 1.de Leeuw R, Klasser GD. In: Orofacial pain: guidelines for assessment, diagnosis, and management. 5. de Leeuw R, Klasser GD, editors. Chicago, IL: Quintessence Publishing Co, Inc; 2008. [Google Scholar]
- 2.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohrbach R, Burgess J. Temporomandibular Disorders and Orofacial Pain. In: Bope, Rakel, Kellerman, editors. Conn’s Current Therapy. Philadelphia: Saunders; 2010. pp. 992–997. [Google Scholar]
- 4.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- 5.Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain. 2011;12:T12–26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, et al. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: implications and future directions. J Pain. 2013;14:T116–124. doi: 10.1016/j.jpain.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene CS. Diagnosis and treatment of temporomandibular disorders: emergence of a new care guidelines statement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:137–139. doi: 10.1016/j.tripleo.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Flor H, Turk DC. Psychophysiology of chronic pain: do chronic pain patients exhibit symptom-specific psychophysiological responses? Psychol Bull. 1989;105:215–259. doi: 10.1037/0033-2909.105.2.215. [DOI] [PubMed] [Google Scholar]
- 9.Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–694. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 10.Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain. 2009;23:153–166. [PubMed] [Google Scholar]
- 11.Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e26–50. doi: 10.1016/j.tripleo.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Bair E, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Diatchenko L, et al. Multivariable modeling of phenotypic risk factors for first-onset TMD: the OPPERA prospective cohort study. J Pain. 2013;14:T102–115. doi: 10.1016/j.jpain.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12:T27–45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickel JC, Gonzalez YM, McCall WD, Ohrbach R, Marx DB, Liu H, et al. Muscle organization in individuals with and without pain and joint dysfunction. Journal of Dental Research. 2012;91:568–573. doi: 10.1177/0022034512445909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nickel JC, Iwasaki LR, Walker RD, McLachlan KR, McCall WD., Jr Human masticatory muscle forces during static biting. J Dent Res. 2003;82:212–217. doi: 10.1177/154405910308200312. [DOI] [PubMed] [Google Scholar]
- 16.Nickel JC, Yao P, Spalding PM, Iwasaki LR. Validated numerical modeling of the effects of combined orthodontic and orthognathic surgical treatment on TMJ loads and muscle forces. Am J Orthod Dentofacial Orthop. 2002;121:73–83. doi: 10.1067/mod.2002.120138. [DOI] [PubMed] [Google Scholar]
- 17.Glaros AG, Williams K, Lausten L. The role of parafunctions, emotions and stress in predicting facial pain. J Am Dent Assoc. 2005;136:451–458. doi: 10.14219/jada.archive.2005.0200. [DOI] [PubMed] [Google Scholar]
- 18.Raphael KG, Janal MN, Sirois DA, Dubrovsky B, Wigren PE, Klausner JJ, et al. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J Oral Rehabil. 2013;40:883–891. doi: 10.1111/joor.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raphael KG, Sirois DA, Janal MN, Wigren PE, Dubrovsky B, Nemelivsky LV, et al. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012;143:1223–1231. doi: 10.14219/jada.archive.2012.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaros AG, Burton E. Parafunctional clenching, pain, and effort in temporomandibular disorders. J Behav Med. 2004;27:91–100. doi: 10.1023/b:jobm.0000013646.04624.8f. [DOI] [PubMed] [Google Scholar]
- 21.Manfredini D, Winocur E, Guarda-Nardini L, Lobbezoo F. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain. 2013;27:99–110. doi: 10.11607/jop.921. [DOI] [PubMed] [Google Scholar]
- 22.Ohrbach R, Markiewicz MR, McCall WD., Jr Waking-state oral parafunctional behaviors: specificity and validity as assessed by electromyography. Eur J Oral Sci. 2008;116:438–444. doi: 10.1111/j.1600-0722.2008.00560.x. [DOI] [PubMed] [Google Scholar]
- 23.Farella M, Palla S, Erni S, Michelotti A, Gallo LM. Masticatory muscle activity during deliberately performed oral tasks. Physiol Meas. 2008;29:1397–1410. doi: 10.1088/0967-3334/29/12/004. [DOI] [PubMed] [Google Scholar]
- 24.Ohrbach R, McCall W, editors. Pain Forum. Elsevier; 1996. The stress-hyperactivity-pain theory of myogenic pain: proposal for a revised theory. [Google Scholar]
- 25.Manfredini D, Landi N, Fantoni F, Segu M, Bosco M. Anxiety symptoms in clinically diagnosed bruxers. J Oral Rehabil. 2005;32:584–588. doi: 10.1111/j.1365-2842.2005.01462.x. [DOI] [PubMed] [Google Scholar]
- 26.Manfredini D, Fabbri A, Peretta R, Guarda-Nardini L, Lobbezoo F. Influence of psychological symptoms on home-recorded sleep-time masticatory muscle activity in healthy subjects. J Oral Rehabil. 2011;38:902–911. doi: 10.1111/j.1365-2842.2011.02226.x. [DOI] [PubMed] [Google Scholar]
- 27.Chapman AR, Vicenzino B, Blanch P, Knox JJ, Hodges PW. Intramuscular fine-wire electromyography during cycling: repeatability, normalisation and a comparison to surface electromyography. J Electromyogr Kinesiol. 2010;20:108–117. doi: 10.1016/j.jelekin.2008.11.013. [DOI] [PubMed] [Google Scholar]