Abstract
Biomarker studies have shown that expression of the T cell co-regulatory ligand PD-L1 on tumor cells correlates with clinical responsiveness to the PD-1 antibody nivolumab. Here we report the findings of a preclinical cancer vaccine study demonstrating a similiar correlate where PD-L1 is upregulated in the tumor microenvironment. We formulated an IFNγ-inducing cancer vaccine called TEGVAX that combined GM-CSF and multiple toll-like receptor agonists to increase the number of activated dendritic cells. Treatment of established tumors with TEGVAX retarded tumor growth in a manner associated with enhanced systemic anti-tumor immunity. Unexpectedly, TEGVAX also upregulated PD-L1 expression in the tumor microenvironment, possibly explaining why tumors were not eliminated completely. In support of this likelihood, PD-L1 upregulation in this setting relied upon IFNγ-expressing tumor-infiltrating CD4+ and CD8+ T cells and administration of a PD-1 blocking antibody with TEGVAX elicited complete regression of established tumors. Taken together, our findings provide a mechanistic rationale to combine IFNγ inducing cancer vaccines with immune checkpoint blockade.
Keywords: anti-PD-1, adaptive immune resistance, tumor microenvironment, TLR agonists, cancer vaccine
Introduction
A major advance in clinical immunotherapy has been the blockade of inhibitory immune receptors and their ligands, collectively termed immune checkpoints. Specifically, blockade of CTLA-4 with ipilimumab and PD-1 with nivolumab both demonstrated clinical efficacy in a number of different advanced malignancies (1, 2). Co-localization of PD-L1 and tumor infiltrating lymphocytes (TIL) that expressed IFN-γ in the clinical specimens together with the induction of PD-L1 on tumor cell lines in response to IFN-γ have led to the idea that expression of this inhibitory ligand represents an adaptive response to “threat” by tumor-specific T cells in the microenvironment (3). This adaptive immune resistance mechanism has been hypothesized as an important means of the developing cancer cells to evade the cytotoxic anti-tumor TIL. Thus, PD-L1 expression, which correlated with clinical response to nivolumab (4), may signify the presence of anti-tumor immune responses while low expression may signify an absence of anti-tumor immunity. In accord with this notion, poorly immunogenic tumors, such as B16, have minimal PD-L1 expression (5).
These findings point towards combinatorial therapy that brings together checkpoint blockade with a driver of tumor-specific immunity in cancer patients who have an ineffective anti-tumor immune response. The most straightforward approach to accomplish this would be to combine cancer vaccines that can increase the tumor specific TH1 IFNγ secreting lymphocytes with anti-PD-1 blockade (6).
With an extensive history of safety as well as expression of diverse, unbiased tumor antigens, lethally irradiated tumor cell vaccines engineered to secrete GM-CSF have the potential to be combined with immune checkpoint blockade antibodies in patients (7, 8). Local GM-CSF can mobilize and recruit myeloid precursors into dendritic cells (DC), but this cytokine does not intrinsically induce DC activation (9). Thus, one major limitation of GM-CSF secreting tumor vaccine (GM-vaccine) has been its limited capacity to activate antigen-presenting cells (APC) necessary for optimal tumor antigen presentation in the afferent arm of the immune system (10). Towards improving the efficacy of antigen based cancer vaccines by stimulating plasmacytoid DC (pDC), Ali, et. al. formulated TLR9 agonist with tumor lysates and GM-CSF to induce improved in vivo efficacy (11). To further optimize this combinatorial method, we developed a strategy that incorporates GM-CSF, cell-based vaccine with unbiased tumor antigens, and multiple TLR agonists (11–17) that can activate both the conventional/classical (cDC) and the pDC in the innate immune system. We formulated glucopyranosyl lipid A (GLA- a TLR4 agonist) and resiquimod (R848- a TLR7/8 agonist) - two agents found to be safe in patients - with a tumor cell based vaccine to create TLR agonists enhanced GM-vaccine (TEGVAX) and studied its anti-tumor effects in an established, palpable B16 treatment model, which is resistant to most previously tested strategies of active immunotherapy (18–20).
We first demonstrated that TEGVAX significantly enhanced DC activation, tumor-specific CTL activity, and in vivo anti-tumor responses in the systemic treatment of palpable B16 melanoma. However, no mice were cured, and we observed that this vaccination/treatment induced up-regulation of PD-L1 in tumors in an IFNγ dependent manner. Addition of PD-1 blockade to this vaccine resulted in regression of a significant proportion of tumor-bearing animals.
Materials and Methods
Mice, cells, and reagents
6–8 weeks old female C57BL/6, Balb/c, and C3H/HeOUJ mice (Jackson Lab) were housed according to the Johns Hopkins Hospital (JHH) Animal Care Committee. C57BL/6 MyD88−/−TRIF−/−, and C57BL/6 (Cg) Rag2tml (Rag2−/−) mice were obtained from Drs. Franck Housseau (JHH). B16 and B16 GM-vaccine cells were cultured in RPMI1640 media containing 10% ΔFCS, penicillin (100U/ml) and streptomycin (100U/ml). In PD-L1 experiments, B16 were cultured with serum free media. CD11c+ cells were isolated by anti-mouse CD11c microBeads (MACS, Miltenyi Biotec). CD4 depleting GK1.5 antibody and CD8 depleting 2.43 (Bio X Cell) at 200μg/dose were injected intraperitoneally (i.p.) every 2 days. Hybridoma expressing blocking anti-PD-1 antibody (clone G4) was obtained from Dr. Charles Drake (JHH).
Vaccine preparation
GLA at 1.0 mg/ml and R848 at 0.2 mg/ml were prepared in 10% (w/v) squalene oil-in-water emulsion vehicle (Immune Design, Seattle, WA). GLA/R848 dissolved in emulsion was incubated with lethally irradiated (150Gy) GM-vaccine cells at 4 deg C for 0.5–2 hours and washed 4x with PBS. This GM-vaccine formulated with GLA and R848 was labeled as TEGVAX. Control GM-vaccines were treated with emulsions and washed without adjuvants. In some cases, GLA and R848 were absorbed into GM-vaccine cells with Lipofectamine and washed 4x to remove non-absorbed TLR agonists and transfectants.
Tumor treatment assay
C57BL/6 mice were injected with 1–5×104 B16 in the footpads. Once palpable tumor developed (5–10 days), 100 μl of 106 B16 GM-vaccine or B16 TEGVAX was injected subcutaneously (s.c.) into the contralateral limb. For all these experiments, 5–10 mice were used per group. All the experiments were repeated at least 5 times. Daily tumor measurements were initiated once all 3 dimensions reached anywhere from 0.5 to 4 mm and the relative tumor volume was calculated by the formula: Length(mm) × Width(mm) × Height(mm) × 0.5326 × 0.01 (41). C3H/HeOUJ mice and Balb/c mice were used with SCCFVII/SF cells and CT26 cells, respectively with comparable methods (32). In brief, CT26 TEGVAX consists of irradiated (150Gy) 1×106 CT26 and allogeneic 1×106 B78H1 GM-CSF with absorbed GLA at 1mg/ml and R848 at 0.2mg/ml as described above. SCCFVII TEGVAX was prepared from GM-CSF secreting SCCFVII cells with GLA/R848 absorbed as above (44). For all the vaccines, GM-CSF expression level ranged from 50–500ng/106 cells/24 hours. For the PD-1 blockade experiments, 100 μg/mice/injection of anti-PD-1 (G4) was injected i.p twice a week once tumor was palpable in conjunction with vaccine treatments. In some experiments, 100 μg/mice/injection of IFNγ neutralizing antibody (XMG1.2 – Bio X Cell) was injected i.p. twice/week.
DC activation assay
Draining lymph nodes (DLN) from tumor-bearing or naive mice were harvested 3–7 days post-vaccine treatments and digested in media containing DNAse I and Liberase Blendzyme 2 (20,000 Mandl U/ml) (Roche). DC-enriched populations were obtained by depleting CD3+ and CD19+, and gated for CD11c+ and B220+. These were evaluated by a multicolored FACS analysis using CD80, CD86, CD40, and MHCII antibodies (BD).
In vivo cytotoxic T-lymphocyte (CTL) assay
Splenocytes were labeled with 0.5μM and 5μM carboxyfluorescein succinimidyl ester (CFSE-Molecular Probes). The 5μM CFSE labeled cells were pulsed with 10μg/ml P15E (KSPWFTTL) peptide. The 0.5μM CFSE labeled cells pulsed with β-gal (TPHPARIGL) peptide. Mice were injected intravenously (i.v.) with a 1:1 mixture of these cells, and the splenocytes were isolated after 24 hours and analyzed by flow cytometry. Antigen-specific killing was calculated using the following formula: (1−% of CFSEP15E/% of CFSE β-gal) × 100.
Histological analysis and Immunohistochemistry (IHC)
Necrosis foci quantitation was performed on Mayer’s hematoxylin & eosin stained slides (Sigma). Necrosis foci in 10 separate regions under 20x magnification were quantitated in a blinded manner under the guidance from Dr. Janice Taube (JHH), a board certified dermatopathologist. For IHC, 10μm thick frozen sections were fixed with acetone, and blocked with 1% BSA for 30 minutes at RT. For paraffin embedded tissue, the sections were fixed in 4% paraformaldehyde prior to 1% BSA blocking. αCD4, αCD8, αCD86 FITC conjugates and αCD45 and αPD-L1 primary antibodies were incubated 1 hour and 4°C. Cy3 conjugate antibody was used as secondary antibody in some cases. DAPI was used as the nuclear counterstain. Positive cells in 10 randomly selected fields at 40x magnification were quantitated in a blinded manner. The microscope was Nikon, Eclipse E800. The camera was Nikon, DS-Qi1Mc. The software was NIS-Element AR 3.0.
Cytokines analysis
CD3 depleted DC was cultured with Golgistop™ (BD) protein transport monensin and LPS 0.1μg/ml 5hrs, and stained for anti-mouse CD11c, CD86, CD80, and MHCII. After gating for pDC and cDC populations (see Figure Legends), intracellular cytokine analysis (IL-12, IFNα and TNFα) were performed after membrane permeabilization with Cytokit (BD). For the lymphocyte analysis, harvested splenocytes or DLN cells were stimulated with 1mM/ml PMA and 1mg/ml ionomycin and Golgistop. Suspension cells were stained for anti-mouse CD3, CD4, CD8, IFNγ with isotype controls.
MRI imaging
Vaccine cells were labeled with 50 μg/ml PEG-phospholipid encapsulated ferromagnetic iron oxide (WFION) with an edge length of 22nm(42). 106 labeled vaccine cells in 40 μl were injected into the footpad and imaged on 11.7 Tesla Bruker MRI using birdcage volume resonator using T2 weighted multi-gradient echo sequence. Image parameters were: FOV=2.2×2.2 cm, Matrix size=256×192, TR=500 ms, TE=3.1 ms, slice thickness=0.5mm and 12 slices. For quantification, regions of interest (ROI) were drawn on the lymph nodes and adjacent muscle tissue as internal controls. For each ROI for every slice, pixel intensity histogram was plotted with the lowest pixel intensity in the control muscle tissue as the threshold, and quantitated as described previously (10).
Statistical Analysis
We used paired t-test to calculate two-tailed p value to estimate statistical significance of differences between two treatment groups using Excel software.
Results
TEGVAX increased DC activation as well as IFNγ secreting T-cells, but still failed to induce regression of established tumor
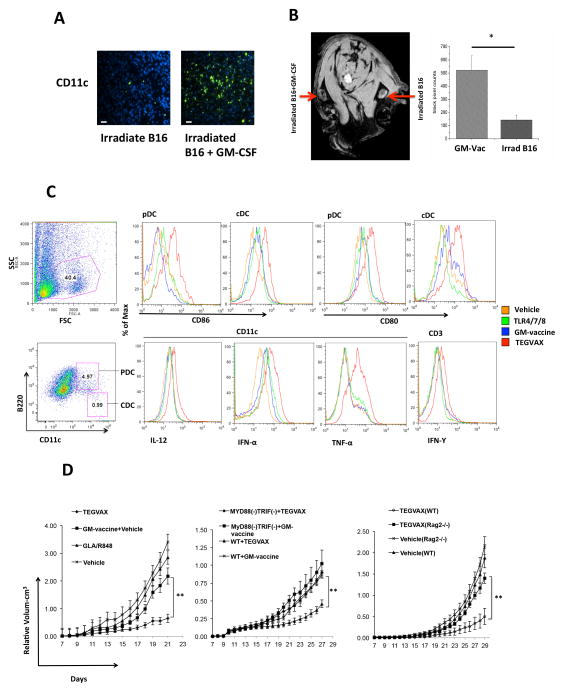
We first formulated TEGVAX that can secrete DC recruiting GM-CSF and activate these DC via multiple TLR agonists to prime tumor specific T-cells. GM-vaccine indeed increased the abundance of CD11c+ DC at the site of vaccine injection (Figure 1A). Using the magnetovaccination MRI method to quantitate the number of endogenous APC with endocytosed surrogate tumor antigen (ferromagnetic label) from the vaccine cells, we found that GM-vaccine treated mice had significantly increased APC trafficking to the DLN (Figure 1B) (21). We then tested whether GLA and R848 formulated into GM-vaccine cells could increase the number of activated DC in the DLN of non-tumor bearing mice. Compared to GM-vaccine, TEGVAX was able to significantly enhance the activation phenotype of pDC and cDC in vivo (Figure 1C). The cytokine profiles from these CD11c+ cells from the CD3 depleted DLN also demonstrated increased amounts of IL-12, IFNα, and TNFα that can skew the T-cell repertoire towards TH1 responses (Figure 1C). When we gated for CD3+cells, there were more activated IFNγ producing T-cells in mice treated with TEGVAX (Figure 1C).
Figure 1.
TEGVAX that combined GM-CSF and multiple TLR agonists (GLA and R848) increased DC trafficking and activation, and this vaccine induced an anti-tumor response. All the experiments were replicated at least 3 times. A. Immunohistochemistry of endogenous CD11c+ DC at the site of vaccine injection comparing GM-vaccine vs. irradiated B16. Bar = 200μm. B. GM-CSF increased the trafficking of endogenous DC to the DLN. Mice were simultaneously injected with paramagnetic iron oxide labeled GM-vaccine and vaccine in the footpad. The mice were imaged with microMRI at the draining popliteal lymph node site 3 days after injection, and the endogenously cell-to-cell labeled APC were quantitated in the primary DLN (10). Pixel counts on DLN were quantitated in each mice (N=3) to compare the GM-vaccine and vaccine treatment (*P<0.05). C. TEGVAX increased CD86 and CD80 activation molecules in vivo on both pDC and cDC from the draining lymph nodes in comparison to GM-vaccine 3 days after vaccine injection. pDC was gated as CD11chighMHCIIhighB220high and cDC was gated as CD11chighMHCIIhighB220low. TEGVAX treated mice also had significantly increased IL-12, TNF-α and IFN-α positive CD11c+ cells in comparison to GM-vaccine or the control treated groups. TEGVAX treated group also had increased IFN-γ secreting CD3+ T-cells in the DLN. DLN were depleted with CD3 prior to intracellular staining for CD11c+ cells in some cases. FSC is forward scatter and SSC is side scatter gating. D. TEGVAX induced significant in vivo anti-tumor response against palpable B16 tumor in comparison to GM-vaccine. The presence of emulsion vehicles did not affect these individual anti-tumor responses. All these in vivo treatment experiments are representative of at least 3–5 separate experiments, and each group in all experiments had 10 mice/group. The anti-tumor response of TEGVAX was abrogated in MyD88−/−TRIF−/− double knockout mice as well as Rag2−/− null mice (**P<0.01).
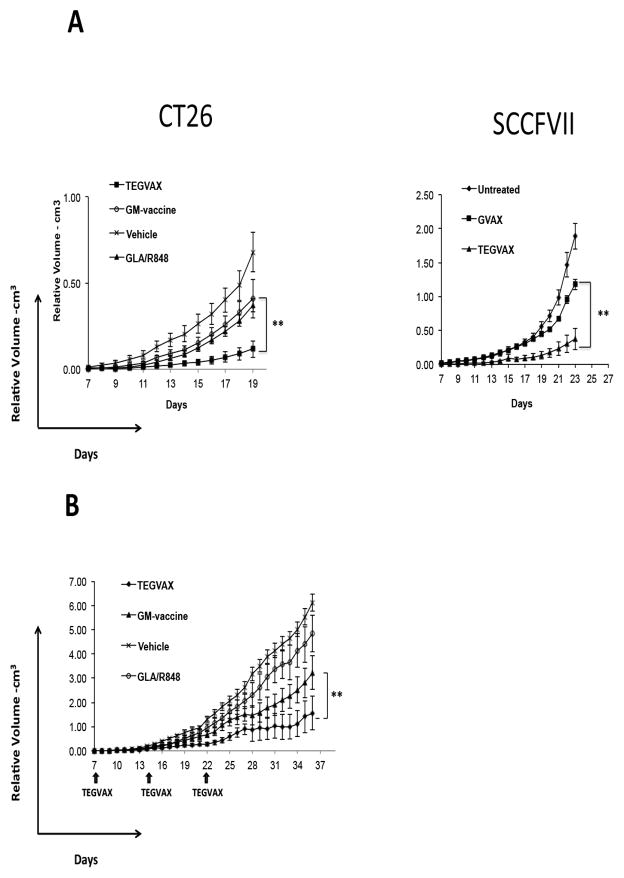
We tested TEGVAX with established B16 tumors in a therapeutic model of palpable disease. As shown in Figure 1D, mice that received TEGVAX displayed lower tumor growth rate. Both GM-vaccine alone and TLR agonists alone had some modest slowing of the tumor growth, but the combination treatment produced the best in vivo anti-tumor response. In order to assess whether T-cells were important for TEGVAX’s anti-tumor response, we performed vaccine treatment assays with Rag2 −/− mice and this anti-tumor response was abrogated in these mice (Figure 1D). We also determined that the anti-tumor effect of TEGVAX was completely abrogated in MyD88/TRIF double knockout mice, ensuring that TLR signaling was critical for its efficacy (Figure 1D). To assess whether this anti-tumor response can apply to different tumor types and murine strains, we also tested TEGVAX in the SCCFVII squamous cell carcinoma model in C3H mice as well as CT26 colon carcinoma model in Balb/c mice with comparable results, (Figure 2A). We also performed this treatment assays with multiple TEGVAX treatments in the B16 model to improve this in vivo response, but no tumor regression was noted despite the decreased growth rate of the tumor (Figure 2B).
Figure 2.
Anti-tumor responses from TEGVAX were found in multiple tumor models. A. TEGVAX induced anti-tumor response in palpable CT26 colon carcinoma and palpable SCCFVII tongue carcinoma models compared to controls (**P<0.01). CT26-GM and SCCFVII-GM vaccine was prepared as described previously (43, 44). C3H/HeOUJ mice treated with TEGVAX also demonstrated anti-tumor response (** P<0.01). B. Palpable B16 bearing mice were treated with multiple injections of vaccines and TLR agonists on day7, day14 and day21 (arrow) (** P<0.01).
TEGVAX increased lymphocytic infiltration into the tumor microenvironment as well as tumor specific CTL responses
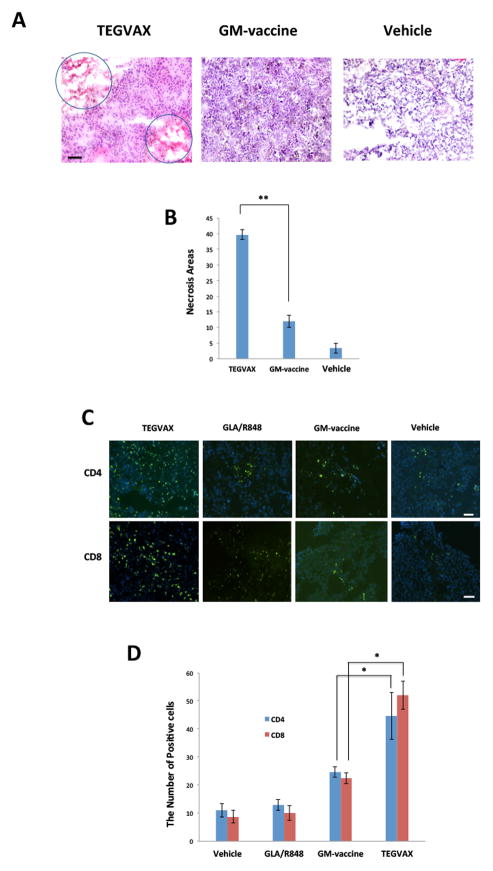
When we examined the tumor microenvironment of these treated mice, increased regions of focal necrosis were found with TEGVAX treatment in comparison to those treated with GM-vaccine or controls (Figure 3A,B). Immunohistochemistry with CD4 and CD8 antibodies showed that TEGVAX treated tumor had significantly increased infiltrating CD4 and CD8 cells within the tumor tissue in comparison to the vaccine controls (Figure 3C,D).
Figure 3.
TEGVAX treatment increased tumor necrosis and tumor infiltrating lymphocytes in the tumor microenvironment. A. H & E staining of treated B16 tumor tissue showed that TEGVAX treated tumor showed increased number of necrotic foci (circled areas) (magnification - 20X). B. Necrotic foci were quantified in 10 separate regions under 20x magnification in tumor tissue treated with TEGVAX and GM-vaccine (**P<0.01). C. TEGVAX treated B16 melanoma had increased infiltration of CD4 and CD8 T-cells in the tumor microenvironment. D. The tumor infiltrating CD4 and CD8 T-cells were counted in 10 randomly chosen fields with immunofluorescent microscope. Bar = 200 μm. TEGVAX treated B16 tumors had significantly increased infiltrating lymphocytes in comparison to GM-vaccine and GLA/R848 treated tumors (* P<0.05). All the experiments were replicated at least 3 times.
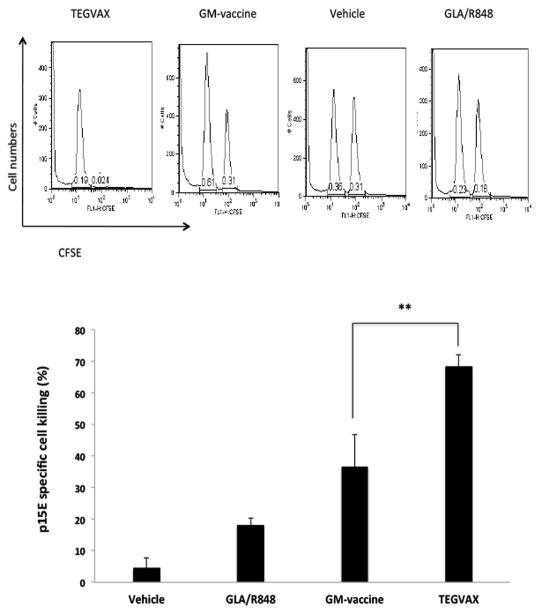
Based on the expression of the immunodominant p15E antigen in B16 tumors, we performed in vivo CTL assays to quantitate the level of p15E specific IFNγ secreting CTL. At days 21–31, when there was a clear separation in the growth rate of TEGVAX from other control groups, there were increased number of p15E specific CTL in the spleen of the mice treated with TEGVAX in comparison to GM-vaccine or other control mice (Figure 4). But despite these robust afferent immune responses, viable tumor was present in all the treated mice.
Figure 4.
TEGVAX treated mice had increased number of tumor specific CTL cells. In vivo CTL assays with P15E peptides were performed in B16 tumor bearing mice treated with vaccines. All the experiments were replicated at least 3 times. Assays were performed on day 25 after vaccine treatment from each of the treated groups (**P<0.01).
TEGVAX treatment increased IFNγ+ dependent PD-L1 up-regulation by tumor cells
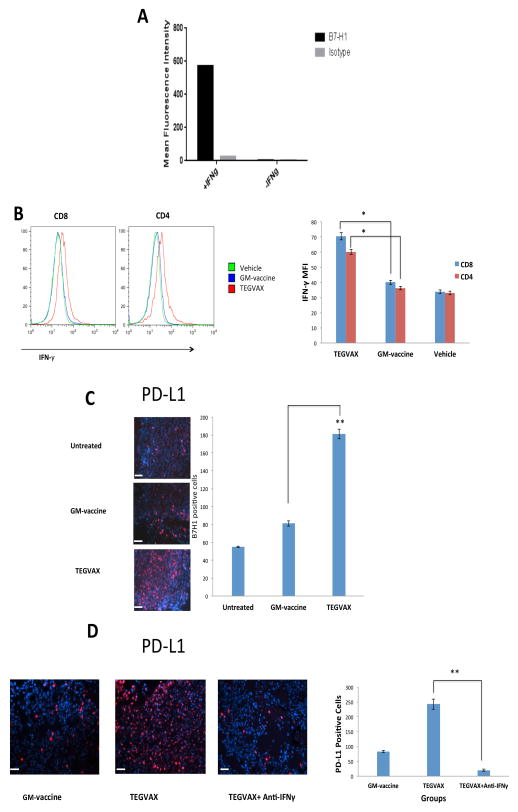
Based on the adaptive resistance hypothesis (3), we sought to determine whether the anti-tumor activity of TEGVAX could be dampened by the induction of PD-L1 in the tumor cells from the IFNγ secreting tumor specific TILs in the tumor microenvironment. As described by others, we found that B16 cells treated in vitro with IFNγ can induce the expression of PD-L1 (Figure 5A). When we examined the TIL from TEGVAX treated mice, we noted an increased level of IFNγ secreting CD4 and CD8 in the tumor microenvironment (Figure 5B). We also noted co-localization of PD-L1 and CD8 T-cells in the tumor microenvironment (Supplemental Figure 1). To test whether these increased TH1 and CTL responses in vivo after TEGVAX administration is associated with the upregulation of PD-L1 on the tumor cells, we stained the tumor tissue and found that TEGVAX significantly increased the expression of PD-L1 in comparison to GM-vaccine or vehicle treated mice (Figure 5C). This increased PD-L1 expression in the tumor microenvironment was significantly diminished when TEGVAX-treated animals were treated with IFNγ neutralizing antibody in vivo. (Figure 5D).
Figure 5.
TEGVAX induced T-cell driven IFNγ dependent upregulation of PD-L1 expression in the tumor microenvironment. A. B16 melanoma cells were cultured in serum free media with or without IFNγ. Cells were stained with anti-murine PD-L1 conjugates, and its mean fluorescent intensity (MFI) was measured using flow cytometry. B. Tumor infiltrating T-cells were harvested from the treated mice and intracellular staining of IFNγ from both CD4 and CD8 populations were performed. MFI of CD4+ IFNγ+ and CD8+ IFNγ+ were quantitated in each of the treated groups (*P<0.05). C. Treated tumor tissue were harvested and stained with rat anti-mouse PD-L1 antibodies and anti-rat Cy3 conjugates to assess PD-L1 positive cells. Bar = 200 μm. Blinded quantitation was performed in 10 different fields at 40x magnification (*P<0.01). D. Tumor tissue from mice treated with GM-vaccine, TEGVAX, or TEGVAX administered with neutralizing IFNγ antibody were harvested and stained for PD-L1 expression. Bar = 200 μm. All the experiments were replicated at least 3 times (*P<0.01).
Anti-PD-1 antibody with TEGVAX can induce regression of established B16 tumors to reverse the adaptive immune resistance mechanism
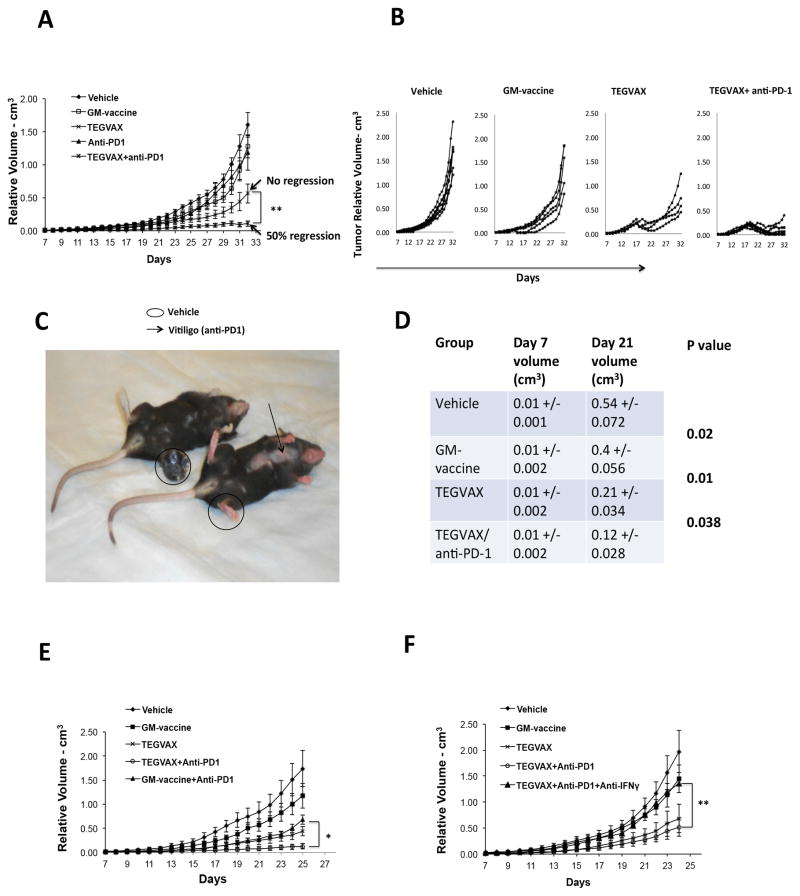
Based on these results, we hypothesized that adding PD-1 pathway blockade to TEGVAX treatment might unleash the potent TEGVAX dependent T-cell responses to mediate a stronger anti-tumor effect, a finding with important clinical relevance. When the blocking anti-PD-1 antibody treatment was combined with TEGVAX, established B16 tumors were noted to regress in over 50% of the mice treated, while TEGVAX treated mice could not induce tumor regression (Figure 6A,B). In these mice that responded with the combined treatment, vitiligo was noted as shown in Figure 6C. Mice treated with anti-PD-1 alone had essentially minimal anti-tumor response in comparison to the untreated group. The poor response to anti-PD-1 alone is likely due to the fact that B16 growth does not induce enough of an endogenous anti-tumor response to be significantly enhanced by PD-1 pathway blockade. Mice treated with combined anti-PD-1/TEGVAX that showed regressions of tumor were followed for another month (day 70) and no tumor developed. When these mice were challenged with B16 tumor at another site, no tumor developed (data not shown). These experiments were replicated 5 times with over 50 mice per group with comparable results. Figure 6D summarizes the cumulative data of the tumor volume of the TEGVAX/anti-PD-1 group that is statistically significant smaller than the other groups.
Figure 6.
TEGVAX with anti-PD-1 treatment induced regression of established B16 tumors. All the experiments were replicated at least 5 times. A. Left panel shows tumor growth rate of established B16 tumor treated with various combinations of vaccines and blocking anti-PD-1 antibody. Vaccines and anti-PD-1 treatments were performed weekly. B. Right panel shows the tumor measurements of individual mice in each of the treated groups. C. Treated mice with regressed tumor demonstrated autoimmune phenotype. The right mice is one of the mice treated with TEGVAX/anti-PD-1, left mice is one of the mice treated with GM-vaccine. Arrow points to site of vitiligo. D. Cumulative volume measurements of 5 experiments comparing TEGVAX/anti-PD-1 treatment to controls demonstrate significantly consistent anti-tumor responses. A total of 50 mice per group were included in this analysis (N=50/group). The mean volumes with standard deviations from 5 different tumor treatment assays were combined. The P values were generated using paired Student t-test comparing volume measurements with standard deviation with the adjacent rows. E. TEGVAX + anti-PD-1 treatment has greater anti-tumor response in comparison to GM-vaccine + anti-PD-1 treatment in vivo. F. Neutralization of IFNγ abrogates the improved anti-tumor response of combinatorial TEGVAX and anti-PD-1 treatment. All the experiments above were replicated at least 3 times. Each treatment group had 10 mice/group.
When PD-1 blockade was combined with GM-vaccine alone, there was a modest anti-tumor response that approximated the growth rate seen with TEGVAX group, but none of these mice demonstrated regression as seen with TEGVAX/anti-PD-1 treated groups (Figure 6E). Control experiments with neutralizing IFNγ antibody abrogated the anti-tumor response of TEGVAX/anti-PD-1, which is consistent with adaptive immune resistance mechanism (Figure 6F).
Discussion
Cancer immunotherapy strategies have been limited due to multiple resistance mechanisms, but two clinical studies demonstrated that immune checkpoint inhibitors can improve clinical responses in multiple tumor types (1, 2). Our findings provide in vivo mechanistic demonstration that vaccines should be coupled with anti-PD-1 blockade in future clinical trials. In our B16 model, PD-L1 induction by IFNγ producing CTL was critical in the efficacy of the anti-PD-1 blockade (22–24). PD-1 blockade by itself did not have significant anti-tumor response (Figure 6), and only when combined with PD-L1 inducing TEGVAX did the tumor regress. This apparent discrepancy with the success of anti-PD-1 clinical trial as a monotherapy underscores the critical role of adaptive immune resistance mechanism. Clinical responses were tightly correlated with the colocalized expression of PD-L1 on the tumor and TIL, suggestive of endogenous anti-tumor immunity held in check. Our B16 tumors had minimal baseline expression of PD-L1 (Figure 5C) and the lack of in vivo response with anti-PD-1 alone implicates a limited endogenous anti-tumor immune response that cannot be reversed. Only the induction of sufficient tumor specific CTL with immune checkpoint molecule blockade did we observe regression in vivo. In support of our mechanistic findings, Spranger et. al. recently showed tight correlation between CD8+ TIL associated with PD-L1 in melanoma patients, and CD8+ dependent PD-L1 induction in preclinical models (25).
The objective responses in advanced cancer patients with anti-PD-1 monotherapy ranged from 18–28%, which implies there is much room for clinical improvements with the addition of IFNγ-producing vaccines. Towards this clinical goal, we formulated a GM-CSF secreting tumor cell vaccine with GLA (TLR4) and R848 (TLR7/8) agonists and demonstrated significant augmentation of in vivo anti-tumor response in comparison to GVAX alone or TLR agonists alone in 3 different murine models. We showed that these adjuvants increased the number of activated plasmacytoid DC as well as classical/conventional DC and their anti-tumor effects were MyD88/TRIF dependent. These in vivo anti-tumor responses were T-cell dependent and associated with an elevation in p15E specific T-cells in the B16. Despite this anti-tumor response, the lack of tumor regression was associated with PD-L1 induction in the tumor microenvironment. To directly test the adaptive immune resistance mechanism (3, 5), TEGVAX was combined with anti-PD-1 blockade, and we observed regression of established B16 tumors as shown in Figure 6. These treatment assays with neutralizing IFNγ antibody suppressed the TEGVAX dependent upregulation of PD-L1 in the tumor microenvironment (Figure 5D), and the regression of tumor seen with TEGVAX/anti-PD-1 blockade was abrogated (Figure 6E). It should be noted that there is no published clinical data that have combined vaccines with immune checkpoint blockade.
Previous reports showed that the combination of anti-PD-1, anti-CTLA-4, and vaccine may promote anti-tumor responses, but their in vivo assay involved initiating treatment on non-palpable, non-established B16 tumor (26, 27). Both groups showed that anti-PD-1 and cellular vaccines can be combined to induce an anti-tumor response in vivo, but these reports utilized a non-palpable tumor inoculation method, which may not model clinical scenarios with established tumor, and they did not examine the upregulation of PD-L1 expression on the tumor cells. Our therapeutic assay was much more stringent in that we initiated treatment 7–10 days after B16 inoculation, at which point an organized immune inhibitory tumor microenvironment can be established, which cannot be treated with known formulations of vaccines. We saw no outgrowth of initially palpable B16 tumors that regressed even 2 months after the initiation of treatments, and a secondary challenge did not show any tumor growth (Figure 6A,B). Moreover, we did not need to combine anti-PD-1 with other immune checkpoint blockade antibodies to demonstrate regression of established tumor. Addition of other immune checkpoint blockades to further improve the cure rates in our system remains an open question. Duraiswamy et. al. showed that the addition of anti-CTLA-4 with anti-PD-1 and vaccine can also induce partial regression of established colon and ovarian carcinoma model (28). Our report, however, demonstrate the mechanistic link between IFNγ producing TIL that can upregulate PD-L1 in the tumor microenvironment to render this poorly immunogenic B16 tumor amenable to anti-PD-1 treatment in a stringent model of adaptive immune resistance. Optimizing the various combinations of translatable blocking checkpoint molecules (anti-CTLA-4, anti-LAG3, anti-TIM3) can be performed with a bulky B16 tumor in the future, but our data clearly demonstrates that an important starting point for a combinatorial regimen is IFNγ producing vaccine with anti-PD-1.
It should be reiterated that all the components of TEGVAX/anti-PD-1 have been tested in patients to be relatively safe. With its demonstration as a potent anti-tumor agent by itself, TEGVAX in combination with anti-PD-1 antibody has high translational potential for clinical trials. GLA and R848 were initially selected because these adjuvants significantly induced enhanced anti-tumor cytokine profiles compared to other TLR agonists, and both have been tested in patients to be safe (17, 29–31). We previously demonstrated an in vivo response with intratumoral injection using GM-vaccine formulated with LPS, but now we report a much more potent response with TEGVAX, a therapeutic vaccine administered distant from the site of established tumor (32). The mechanisms that underlie the intratumoral injection in comparison to the systemic injection in these treatment models are different, but cumulatively, these experiments demonstrate that TLR agonists formulated with a cellular vaccine injected intratumorally or systemically are unlikely to have a pro-carcinogenic effect (17, 33–36).
Immune evasion mechanisms of the established tumor are multifactorial, and we have not addressed the role of stromal tissue (37, 38) or myeloid cells (MDSC or macrophages) (39, 40) in their response to the combination of TEGVAX and anti-PD-1 blockade. Cumulatively, however, this report demonstrates that TEGVAX that can significantly augment the priming of tumor specific T-cells, even for established tumors, and it is an excellent candidate to be a component in a combinatorial therapy with anti-PD-1 blocking antibody or other forms of immune checkpoint blockade in cancer patients.
Supplementary Material
Acknowledgments
Grant Support: NIH K23-DE018464 and Flight Attendant Medical Research Institute Grant (YJK), Melanoma Research Alliance (YJK and DMP), and Alpha Omega Alpha Carolyn L Kuckein Student Research Fellowship (IJM). Dr. Janice Taube, a board certified dermatopathologist, analyzed the tissue necrosis on the H&E stains on the tumor.
Footnotes
Disclosure of potential conflicts of interest: Hy Levitsky is employed as Head of Cancer Immunology Experimental Medicine at Roche and as Adjunct Professor at Johns Hopkins. Young Kim has received sponsored research grant from Aduro BioTech, Inc. Drew Pardoll is an uncompensated consultant for Amplimmune, Aduro BioTech, and ImmuneXcite.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank C, Brown I, Peterson AC, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64(3):1140–5. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90(8):3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long CM, van Laarhoven HW, Bulte JW, Levitsky HI. Magnetovaccination as a novel method to assess and quantify dendritic cell tumor antigen capture and delivery to lymph nodes. Cancer Res. 2009;69(7):3180–7. doi: 10.1158/0008-5472.CAN-08-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1(8):8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garaude J, Kent A, van Rooijen N, Blander JM. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Sci Transl Med. 2012;4(120):120ra16. doi: 10.1126/scitranslmed.3002868. [DOI] [PubMed] [Google Scholar]
- 13.Zheng R, Cohen PA, Paustian CA, et al. Paired toll-like receptor agonists enhance vaccine therapy through induction of interleukin-12. Cancer Res. 2008;68(11):4045–9. doi: 10.1158/0008-5472.CAN-07-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura K, Jain A, Allen HE, et al. Selective targeting of antitumor immune responses with engineered live-attenuated listeria monocytogenes. Cancer Res. 2006;66(2):1096–104. doi: 10.1158/0008-5472.CAN-05-2307. [DOI] [PubMed] [Google Scholar]
- 15.Paustian C, Caspell R, Johnson T, et al. Effect of multiple activation stimuli on the generation of Th1-polarizing dendritic cells. Hum Immunol. 2011;72(1):24–31. doi: 10.1016/j.humimm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Dubensky TW, Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22(3):155–61. doi: 10.1016/j.smim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204(6):1441–51. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behzad H, Huckriede AL, Haynes L, et al. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis. 2012;205(3):466–73. doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu JJ, Huang DB, Tyring SK. Resiquimod: A new immune response modifier with potential as a vaccine adjuvant for Th1 immune responses. Antiviral Res. 2004;64(2):79–83. doi: 10.1016/j.antiviral.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 21.King IL, Kroenke MA, Segal BM. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in the effector cell differentiation after subcutaneous immunization. J Exp Med. 2010;207(5):953–61. doi: 10.1084/jem.20091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 23.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–8. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–53. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 25.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Simmons A, Du T, et al. Allogeneic GM-CSF-secreting tumor cell immunotherapies generate potent anti-tumor responses comparable to autologous tumor cell immunotherapies. Clin Immunol. 2009;133(2):184–97. doi: 10.1016/j.clim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T cell rejection function in tumors. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coler RN, Baldwin SL, Shaverdian N, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One. 2010;5(10):e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauder DN, Smith MH, Senta-McMillian T, Soria I, Meng TC. Randomized, single-blind, placebo-controlled study of topical application of the immune response modulator resiquimod in healthy adults. Antimicrob Agents Chemother. 2003;47(12):3846–52. doi: 10.1128/AAC.47.12.3846-3852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coler RN, Bertholet S, Moutaftsi M, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6(1):e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis MB, Vasquez-Dunddel D, Fu J, Albesiano E, Pardoll D, Kim YJ. Intratumoral administration of TLR4 agonist absorbed into a cellular vector improves antitumor responses. Clin Cancer Res. 2011;17(12):3984–92. doi: 10.1158/1078-0432.CCR-10-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swann JB, Vesely MD, Silva A, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105(2):652–6. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317(5834):121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 35.Szczepanski MJ, Czystowska M, Szajnik M, et al. Triggering of toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69(7):3105–13. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murad YM, Clay TM, Lyerly HK, Morse MA. CPG-7909 (PF-3512676, ProMune): Toll-like receptor-9 agonist in cancer therapy. Expert Opin Biol Ther. 2007;7(8):1257–66. doi: 10.1517/14712598.7.8.1257. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204(1):49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochsenbein AF, Klenerman P, Karrer U, et al. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci U S A. 1999;96(5):2233–8. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–54. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 42.Lee N, Choi Y, Lee Y, et al. Water-dispersible ferrimagnetic iron oxide nanocubes with extremely high r(2) relaxivity for highly sensitive in vivo MRI of tumors. Nano Lett. 2012;12(6):3127–31. doi: 10.1021/nl3010308. [DOI] [PubMed] [Google Scholar]
- 43.Jain A, Slansky JE, Matey LC, Allen HE, Pardoll DM, Schulick RD. Synergistic effect of a granulocyte-macrophage colony-stimulating factor-transduced tumor vaccine and systemic interleukin-2 in the treatment of murine colorectal cancer hepatic metastases. Ann Surg Oncol. 2003;10(7):810–20. doi: 10.1245/aso.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Couch M, Saunders JK, O’Malley BW, Jr, Pardoll D, Jaffee E. Genetically engineered tumor cell vaccine in a head and neck cancer model. Laryngoscope. 2003;113(3):552–6. doi: 10.1097/00005537-200303000-00029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.