Supplemental Digital Content is available in the text.
Key Words: preventable hospitalization, multilevel modelling, primary care
Abstract
Background:
Geographic rates of preventable hospitalization are used internationally as an indicator of accessibility and quality of primary care. Much research has correlated the indicator with the supply of primary care services, yet multiple other factors may influence these admissions.
Objective:
To quantify the relative contributions of the supply of general practitioners (GPs) and personal sociodemographic and health characteristics, to geographic variation in preventable hospitalization.
Methods:
Self-reported questionnaire data for 267,091 participants in the 45 and Up Study, Australia, were linked with administrative hospital data to identify preventable hospitalizations. Multilevel Poisson models, with participants clustered in their geographic area of residence, were used to explore factors that explain geographic variation in hospitalization.
Results:
GP supply, measured as full-time workload equivalents, was not a significant predictor of preventable hospitalization, and explained only a small amount (2.9%) of the geographic variation in hospitalization rates. Conversely, more than one-third (36.9%) of variation was driven by the sociodemographic composition, health, and behaviors of the population. These personal characteristics explained a greater amount of the variation for chronic conditions (37.5%) than acute (15.5%) or vaccine-preventable conditions (2.4%).
Conclusions:
Personal sociodemographic and health characteristics, rather than GP supply, are major drivers of preventable hospitalization. Their contribution varies according to condition, and if used for performance comparison purposes, geographic rates of preventable hospitalization should be reported according to individual condition or potential pathways for intervention.
Preventable hospitalizations (also known as hospitalizations for “ambulatory care sensitive conditions,” “potentially avoidable hospitalizations,” and “potentially preventable hospitalizations”) are those considered to be preventable through timely access to quality primary and preventive care.1–3 Rates of preventable hospitalization are reported internationally as an indicator of health system performance and, in Australia, are used to guide the allocation of health service resources.4,5 Typically, this reporting involves comparing rates of preventable hospitalizations between geographic or health administrative areas,5,6 with the underlying rationale that variation in admission rates is related to the accessibility or quality of primary care, based on measures such as the density of the general practitioner (GP) workforce,7–9 perceived availability of health services,10,11 the presence of community health centers,12 or having a regular source of care.13,14
Health system performance indicators should reflect factors that can be influenced by, and are responsive to, health policy change.15,16 Policy interventions to reduce preventable hospitalizations usually address health care systems, such as incentives to increase equity in the distribution of GPs.17,18 However, multiple factors influence variation in preventable hospitalization, and interventions can also target clinical and self-management of conditions (such as chronic disease management and telemedicine programs) and primary prevention at population level (such as health promotion campaigns). Accordingly, the valid use and interpretation of preventable hospitalization as a measure of health system performance requires an understanding of the relative contributions of personal and health care factors,15 particularly because more proximal interventions would be expected to drive change more quickly than those operating through primary prevention.
Most attempts to explore the multiple factors that drive preventable hospitalizations have used an ecological approach, analyzing area-based measures such as disease prevalence, average income, racial composition of the population, or area-level deprivation.8,10,19–21 Interpretation of such analyses can be limited because they are subject to “ecological fallacy” by inferring risk factors for individuals based on population-level information, while it is not known which members of the population were actually hospitalized.22,23 Few studies of preventable hospitalization have collected detailed sociodemographic or health data for individuals, and these have used these data only to construct aggregate area-level variables,10,11 or else did not explore the role of personal characteristics in driving geographic variation in admission.23–25
Multilevel modelling, a statistical technique that structures data into hierarchies, such as individuals nested within their geographic area of residence, can estimate the relative contributions of factors at each of these levels to the total variation in an outcome.26 Although multilevel modelling has increasingly been used to explore personal and contextual drivers of preventable hospitalizations, analyses to date have been limited by the use of administrative hospital27–29 or US Medicare claims7 data, which did not include detailed information about individual patients.
This study used multilevel modelling and detailed person-level data from a large-scale cohort study linked to routinely collected health data to investigate the relative contributions of the supply of GP services, relative to the contribution of personal sociodemographic, health and behavioral characteristics, to geographic variation in preventable hospitalizations.
METHODS
Study Population
This observational cohort study used data from the Assessing Preventable Hospitalisation InDicators (APHID) study, details of which have been published elsewhere.30 Briefly, APHID includes participants from the Sax Institute’s 45 and Up Study,31 a prospective cohort of over 267,000 men and women aged over 45 in New South Wales (NSW), Australia. Study participants were recruited from 2006 to 2009 through Medicare Australia (Australia’s national universal health insurer), and joined the study by completing a self-administered questionnaire, including information on demographic characteristics, indicators of socioeconomic status, self-reported health, number, and type of comorbidities and behavioral risk factors. Participants also provided consent for long-term follow-up, including linkage to administrative health data sets.
Self-reported survey data for 45 and Up Study participants were linked with hospital admissions data from the NSW Admitted Patient Data Collection, a census of all hospital separations (discharges, transfers, and deaths) from all NSW public and private sector hospitals and day-procedure centers, and mortality data from the NSW Registry of Births Death and Marriages mortality data file, which contains fact-of-death information on death registrations within Australia. Probabilistic data linkage was performed by the NSW Centre for Health Record Linkage (http://www.cherel.org.au/) using ChoiceMaker software. A manual clerical review on a sample of linkage records found a false-positive linkage rate of 0.3%.
Ethics approval for the 45 and Up Study was granted by the University of New South Wales Human Research Ethics Committee, and approval for the APHID study was granted by the NSW Population and Health Services Research, Aboriginal Health & Medical Research Council, and University of Western Sydney Research Ethics Committees.
Preventable Hospitalizations
Preventable hospitalizations were identified using the linked Admitted Patient Data Collection hospital admissions data and defined according to the preventable hospitalization indicator in the Australian 2012 National Healthcare Agreement.32 This indicator is composed of admissions for 21 conditions, broadly categorized as “chronic,” “acute,” and “vaccine-preventable” (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/MLR/A897). To assess whether hospitalizations for these conditions differed from other hospitalizations, an additional category of “nonpreventable” hospitalizations was defined as all emergency hospitalizations not included in the preventable hospitalization indicator.
Personal-level Variables
Self-reported information from the 45 and Up Study baseline survey was used to identify characteristics of the study participants (Table 1). Sociodemographic characteristics included age, sex, Aboriginal or Torres Strait Islander status, annual household income, highest level of education, speaking a language other than English at home, marital status, health insurance status, and number of people outside their home they can depend on. Health and behavioral characteristics included body mass index (using self-reported height and weight), self-reported health status, level of functional limitation (using the Medical Outcomes Study physical functioning scale), level of psychological distress (using the K10 Scale), number of comorbidities (heart disease, high blood pressure, stroke, diabetes, blood clot, asthma, Parkinson’s disease, and any cancer except skin cancer), and a positive health behavior score33 calculated as the total number of the following reported behaviors: nonsmoking status, safe level of alcohol consumption (<14 drinks/wk), at least 2.5 hours of intensity-weighted physical activity per week, and meeting daily dietary guidelines for fruit (2 serves) and vegetable (5 serves) consumption.
TABLE 1.
Person-level and Area-level Covariates Used in Models Predicting Rates of Preventable Hospitalization
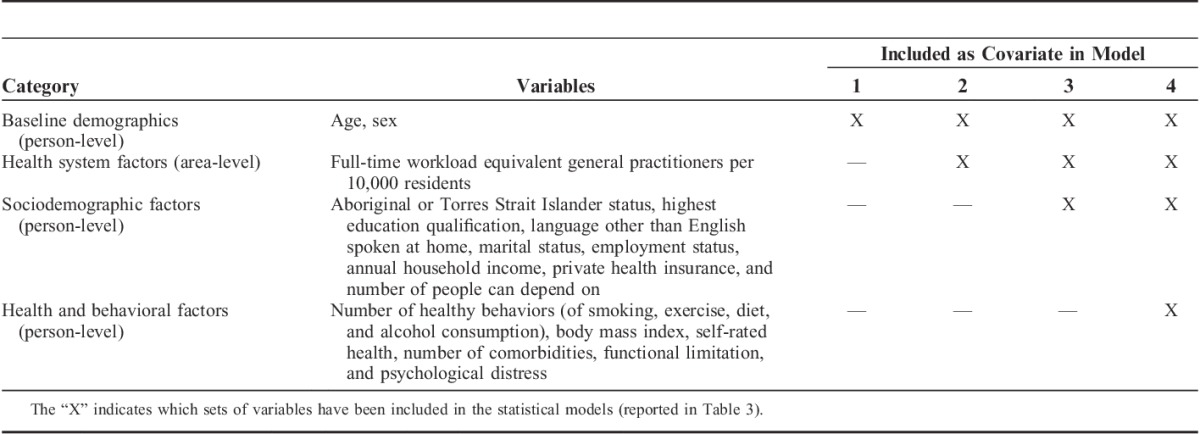
Geographic-level Variables
Geographic areas of residence were identified from the 45 and Up Study using Statistical Local Areas (SLAs), one of the smallest geographic units available in the Australian Standard Geographical Classification.34 SLAs were defined using boundaries from the 2006 Australian Census. The 199 SLAs differ in size and population across the state due to variation in remoteness from urban centers (Supplementary Figure 1, Supplemental Digital Content 1, http://links.lww.com/MLR/A897), with mean population 33,883 (range, 357–138,322).35
The number of full-time workload equivalent (FWE) GPs within each SLA, measured the effective supply of primary care services.18,36 It was estimated using aggregate state-level data from the Department of Health and Ageing37 and aggregate SLA-level data from the 2011 Social Health Atlas of Australia.38 FWE GPs were calculated as the number of Medicare claims for GP services for residents of each SLA, divided by the average number of claims per FWE GP in NSW. Population estimates were used to calculate the density of FWE GPs per 10,000 residents of each SLA, and divided into quintiles. A sensitivity analysis treated FWE GPs as population-weighted quintiles, and produced similar results (data not shown).
Statistical Methods
Multilevel Poisson models were used to analyze rates of preventable hospitalization, with individuals as the unit of analysis. Counts of the number of preventable hospitalizations for each individual were taken between the date of study entry and the end of follow-up through the linked hospital data (December 30, 2010), or death, whichever came first. The log of the follow-up time was used as an offset. Individuals were clustered in their geographic area of residence (SLA) using a random intercept parameter, which allowed the baseline risk of admission to vary between these geographic areas. Separate analyses were run for the 3 major categories of preventable admission, and where numbers allowed, the individual conditions.
Geographic variation in risk of preventable hospitalization was first quantified using multilevel models adjusted for age and sex (Model 1). The variance (σ2) of the random intercept parameter for the SLAs was used to quantify the amount of variation in the risk of admission between geographic areas.39
Quintiles of the density of FWE GPs were added to the model as an area-level covariate (Model 2). Subsequent models (Table 1) sequentially added person-level confounders, starting with sociodemographic variables considered to be nonmodifiable and largely outside the scope of health policy action (Model 3), followed by health and behavioral characteristics considered potentially amenable to health interventions targeting populations or individuals (Model 4). Incidence rate ratios and 95% confidence intervals (CIs) were calculated for each of the variables by exponentiating the regression parameters. The amount of geographic variation in admission (from Model 1) which was explained by the variables in each subsequent model (Model n), was calculated as the proportional change in variance (PCV),39 where PCV=(σ2(Model 1)−σ2(Model n))/σ2(Model 1).
Missing values were treated as additional categories; incidence rate ratios for these “missing” categories are reported in Supplementary Figures 2–6, Supplemental Digital Content 1, http://links.lww.com/MLR/A897. A sensitivity analysis excluding (n=90,678) persons with missing data on any variable found no notable changes in the patterns of individual-level predictors of admission or changes in area-level variation between models (data not shown). All models used a second-order penalized quasi-likelihood estimation procedure, and all analyses were performed in SAS 9.3 and MLwiN 2.25.
RESULTS
Of the N=267,091 45 and Up Study participants, 1.6% (n=4336) were excluded because their age or geographic area of residence was unknown, they resided outside of NSW, or had incompatible dates for records in the linked data (eg, death before study entry), leaving n=262,755 for analysis (Table 2) over an average of 2.8 years of follow-up between 2006 and 2010. At the area-level, the rate of FWE GPs ranged from 2.6 to 13.3 per 10,000 residents (Supplementary Figure 1, Supplemental Digital Content 1, http://links.lww.com/MLR/A897).
TABLE 2.
Cohort Characteristics and Average Rate of Preventable Hospitalizations Per 100 Person-years of Follow-up
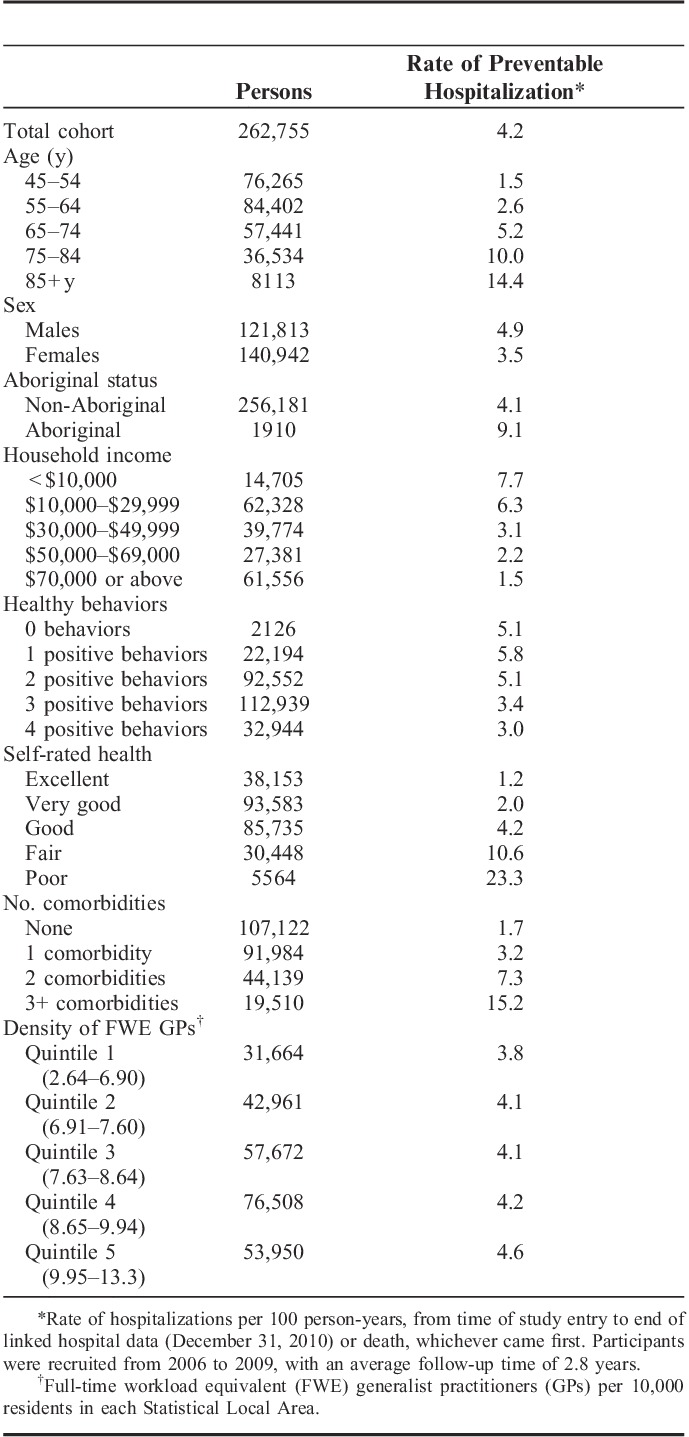
Of the study participants, n=20,009 (7.6%) participants had a preventable hospitalization, with n=14,525 having 1, n=3425 having 2, and n=2059 having 3 or more admissions, giving a total of 30,553 hospitalizations. More participants had preventable hospitalizations for chronic conditions than for acute or vaccine-preventable conditions (Table 3), and the mean number of admissions per admitted person was greater for the chronic than for the acute or vaccine-preventable conditions (mean of 1.6, 1.2, and 1.1 admissions/y, respectively).
TABLE 3.
Area-level Variance, σ2, Across 199 Statistical Local Areas in Rate of Preventable Hospitalizations, and the Proportional Change in Area-level Variance (PCV)* Between an Age and Sex-adjusted Multilevel Poisson Model (Model 1) With Additional Models Sequentially Adjusted for General Practitioner Workforce Supply (Model 2), Sociodemographic Factors (Model 3), and Health and Behavioral Factors (Model 4)
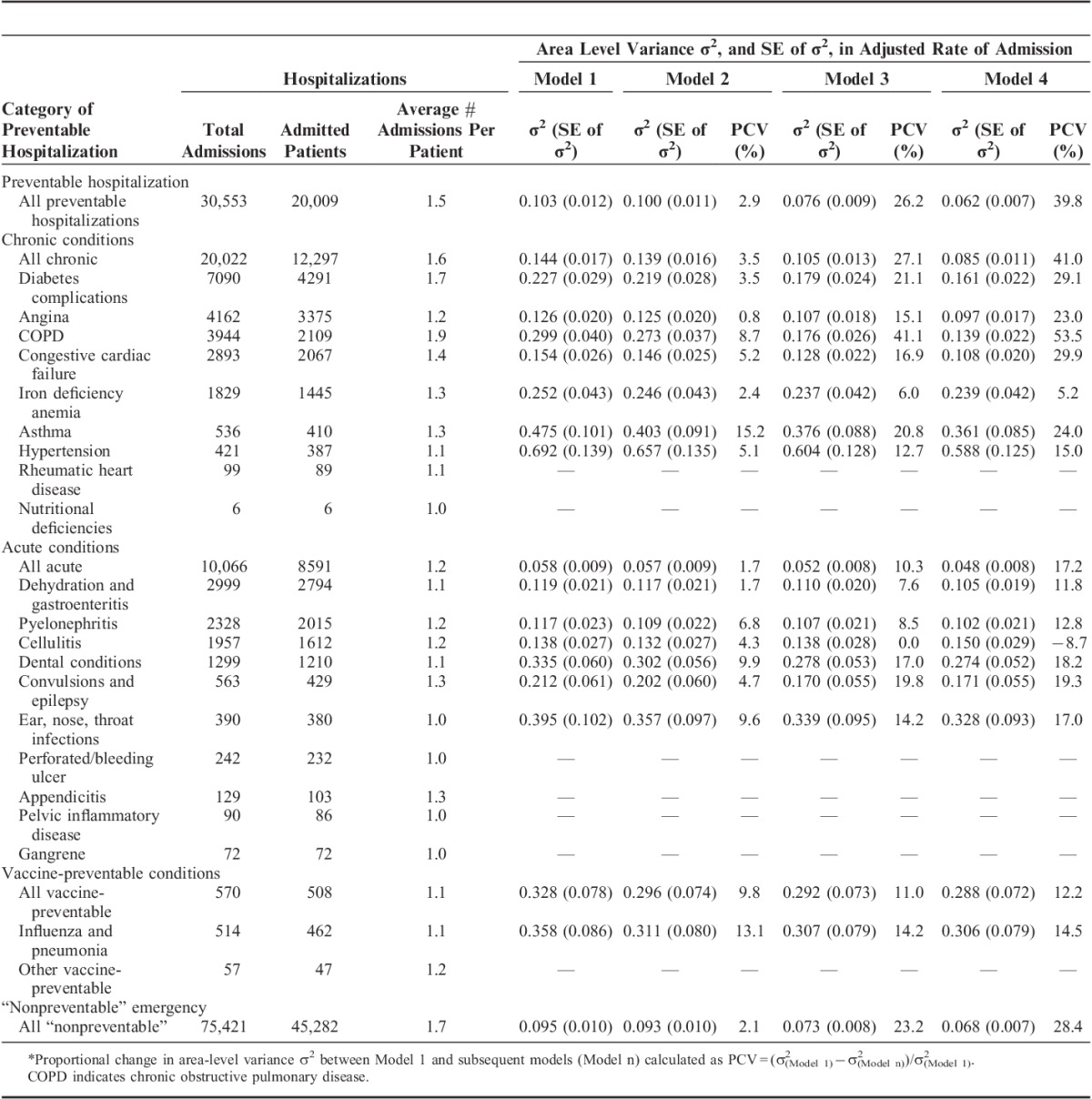
There was significant variation between areas in the age-adjusted and sex-adjusted rate of preventable hospitalization (σ2=0.103, P<0.001). The amount of variation differed across major categories of conditions (Table 3), and was greater for admissions for vaccine-preventable (σ2=0.328, P=0.003), than for chronic (σ2=0.144, P<0.001) or acute (σ2=0.058, P<0.001) conditions, although vaccine-preventable conditions had a larger standard error due to the low number of events.
The inclusion of area-level FWE GPs in the model (Table 3) explained little of the area-level variation in preventable hospitalization (PCV=2.9%), and the rate of preventable hospitalization was not significantly related to area-level quintiles of FWE GPs in either an age-sex adjusted model, or models further adjusted for personal sociodemographic or health characteristics (Fig. 1). Similarly, no clear trend was evident across major categories of preventable hospitalization (Fig. 1) and most individual conditions (Supplementary Figure 7, Supplemental Digital Content 1, http://links.lww.com/MLR/A897). There was an inverse association between quintiles of FWE GPs and the rate of hospitalizations for vaccine-preventable conditions (primarily influenza and pneumonia), and a higher rate of hospitalization in the upper quintiles for dental conditions, although CIs for these estimates were wide.
FIGURE 1.
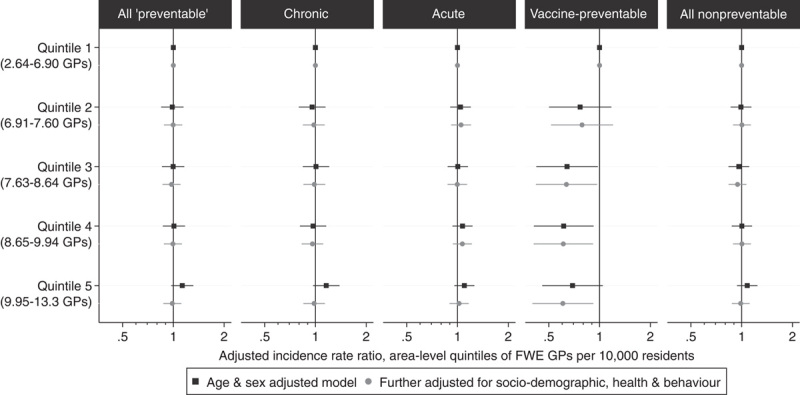
Association between quintiles of the density of full-time workload equivalent (FWE) general practitioners (GPs) per capita within Statistical Local Areas, with the rate of preventable and “nonpreventable” hospitalizations, from multilevel Poisson models adjusted for age and sex, and further adjusted for personal sociodemographic, health, and behavioral characteristics.
The addition of person-level sociodemographic characteristics to the model (Table 3) explained an additional 23.3% of area-level variation in preventable hospitalizations (PCV=26.2%), whereas a further 13.6% was explained by the addition of person-level health and behavioral characteristics (PCV=39.8%). Combined, these person-level characteristics explained 36.9% of the area-level variation in preventable hospitalizations, with this proportion being greater for admissions for chronic (37.5%) than for acute (15.5%) or vaccine-preventable (2.4%) conditions. Among individual causes, person-level characteristics explained the greatest area-level variation for chronic obstructive pulmonary disease (COPD) (44.8%), diabetes (25.6%), congestive cardiac failure (24.7%), and angina (22.2%), the 4 most common chronic causes of preventable admissions. However, small numbers of admissions for the less common causes limited the extent to which cause-specific comparisons could be drawn.
Most person-level variables in the fully adjusted model were found to be significant predictors of preventable hospitalization (Fig. 2). Overall, admission rates were highest for participants who were older, had poorer self-reported health, greater functional limitation, greater number of comorbidities, or were Aboriginal or Torres Strait Islander. Admission rates were lower for females, participants who were employed, had higher levels of income, or reported greater numbers of positive health behaviors. Predictors of admission differed slightly between the major categories of preventable hospitalization, with the higher rate of admissions associated with older age and poorer health being most pronounced for chronic conditions, and a slightly different pattern of association for acute admissions among females and participants who speak a language other than English at home.
FIGURE 2.
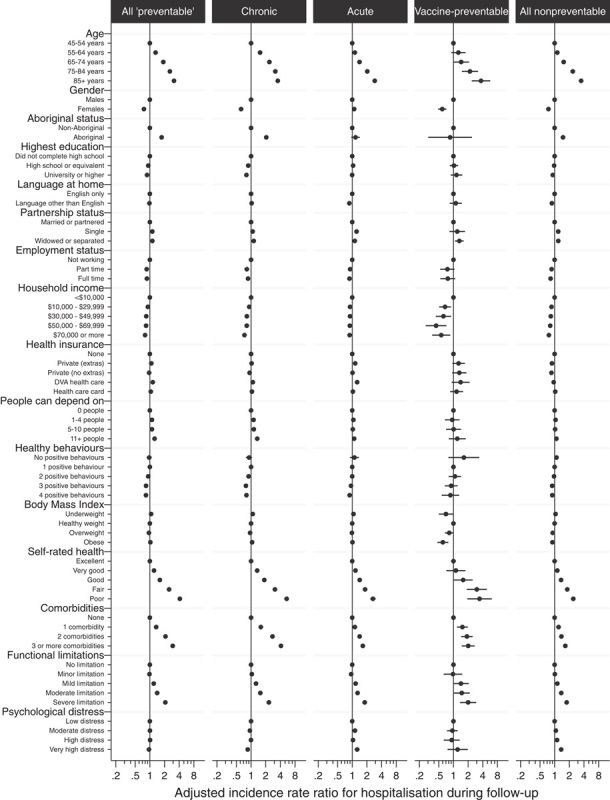
Incidence rate ratios for person-level predictors of preventable hospitalization, in multilevel Poisson models simultaneously adjusted for all person-level variables and area-level quintiles of full-time workload equivalent general practitioners.
Study participants had 75,421 “nonpreventable” emergency hospitalizations during the corresponding period. There was a significant area-level variation in the rates of “nonpreventable” hospitalization (σ2=0.095, P<0.001), of which 2.1% was explained by the inclusion of FWE GPs in the model, and a further 26.3% by the sociodemographic, health, and behavioral characteristics of the population (Table 3). As for preventable hospitalizations, there was no significant association between the rates of “nonpreventable” hospitalization and the area-level quintile of FWE GPs (Fig. 1).
DISCUSSION
This study was the first to use detailed person-level data to assess how both the supply of GP services and the composition of the population influences geographic variation in preventable hospitalizations—a key consideration in the valid use of preventable hospitalizations as a health system performance indicator. We found that supply of GP services explained only a small amount (2.9%) of the geographic variation in rates of preventable hospitalization, and that these rates did not vary significantly according to quintiles of GP supply, but that more than one-third (36.9%) of geographic variation in preventable hospitalizations was driven by personal sociodemographic and health characteristics.
The lack of a significant association between the supply of GP services and preventable hospitalizations was unexpected, because much of the literature has demonstrated inverse associations.7–9,40 However, results have been inconsistent,19–21,28 and much of the research has used practitioner headcount measures or self-rated access to care rather than more objective measures of effective supply. Most of the existing research was performed in the United States with few studies in Australia.11 Australia has a higher number of annual physician visits per capita (6.5) than the United States (3.9), United Kingdom (5.0), and Canada (5.5), with a “safety net” scheme to improve access to health care services for low-income groups, and targeted interventions to reduce health disparities for more vulnerable populations.41 It may be that current strategies to improve access to GPs have been effective, with fewer barriers to accessing care than in countries such as the United States, and the use of primary care services in Australia may be more reflective of the underlying health need of the population. Although previous ecological-level research in Australia found an inverse association between full-time equivalent GPs and preventable hospitalizations,11 this association disappeared after adjusting for sociodemographic and health characteristics of areas.
This study instead indicated that preventable hospitalizations may be more representative of gradients in health than in health care.20 Prior research has found that up to half the variation in preventable hospitalization between areas was attributed to factors other than accessibility of primary care, such as sociodemographic, health, or hospital service factors,11,15 although interpretation of these findings was limited by the use of aggregate area-level measures of risk exposure, or a small sample size for geographic areas. Many studies have adjusted for sociodemographic or health characteristics, and such adjustment is recommended for the standard reporting of the indicator.42 This study shows that care should be taken to unpack, not just adjust for, the contribution of these factors, as good performance measures should be both attributable and responsive to policy change,16 and such adjustment may actually mask the most important drivers of admission.
Few prior studies have detailed person-level data with which to investigate person-level predictors of hospitalization,23,24 with much of the evidence coming from aggregate ecological analyses or analyses on specific conditions.2,42,43 Our findings with regard to the demographic characteristics of the population are consistent with the literature, with higher rates of preventable hospitalization among men, older persons, and Aboriginal or Torres Strait Islander people.23,43,44 Similarly, the inverse associations between markers of socioeconomic status—such as income, education, and employment—are consistent with strong associations reported in the literature, as are the higher rates among participants with poorer self-rated health, greater number of comorbidities, and higher levels of functional limitation.2,23,43,45 Fewer studies have investigated the role of social support, health behaviors, and mental health, and the findings have been less consistent.33,43,45
Although it is well understood that chronic, acute, and vaccine-preventable conditions in the indicator relate to primary care in different ways,2 only some reporting systems stratify their results accordingly.3,6 It is argued there may be insufficient events to analyze conditions separately,42 and that the use of condition-specific indicators can lead to “tunnel vision” with a concentration of performance efforts around those conditions being monitored.27 This study found the contribution of various factors to geographic variation in preventable hospitalization varied markedly according to condition, and vaccine-preventable conditions alone appeared to have an inverse association with GP supply. Conversely, the high-volume chronic conditions—diabetes complications, COPD, congestive cardiac failure, and angina—were most strongly driven by the sociodemographic and health characteristics of the population. Our finding that area-level supply of primary care services and person-level sociodemographic factors made similar contributions to geographic variation in “preventable” and “nonpreventable” hospitalizations casts further doubt on the value of the aggregate indicator. Where possible we suggest that it is desirable to separate the indicator according to conditions that present different pathways for intervention.
Our findings do not downplay the potential role of primary care, and the broader health system, in reducing rates of unnecessary hospitalization for chronic conditions. However, they point to the need for further work to identify effective interventions and appropriate performance measures for these. Although social determinants of health may be targeted through long-term primary prevention, the responsiveness of these strategies may be low and influenced by factors outside of the health system. Admissions for chronic conditions may be more amenable to disease management and strategies to improve the quality of care, because multimorbid patients require complex case management, patient adherence to guidelines is often poor,42 and medication-related hospitalizations for people with chronic disease are common.46 Quality of care may also be improved by focussing on the primary care system more broadly, not just GP care, such as support of pharmacist and physician assistants for check-ups, diagnoses, and repeat prescriptions.18
The core strengths of this study include the availability of detailed person-level information with linked hospital admissions data, and the use of multilevel modelling to examine how population composition influences geographic variation in admission. Reliable area-level data that is representative of the population, such as disease prevalence, can be difficult to obtain,47 and while a number of studies have had either detailed person-level data,23,24 or used multilevel modelling to incorporate individual factors into small-area analyses of preventable admission,7,27,28 this is the first study to our knowledge to incorporate both. This study is also one of the few to present results stratified by both major categories and individual conditions6,11,15,20,42 that are included in the indicator. This is especially useful because a number of versions of the indicator have been used over time and in different jurisdictions,6,48 hindering direct comparisons between these different aggregate indicators.
A limitation of this study is that participants in the 45 and Up Study are older and potentially healthier than the general population,31 and given the low participation rate (18%) there may be concerns about generalizability. However, persons aged 45 years and above have the highest rate of preventable admissions per capita, and contribute two-thirds of preventable hospitalizations in Australia.6 As it is a healthier cohort, participants may be more likely to access primary care services. However, internal relative risk estimates from the 45 and Up Study have found to be comparable with those from population health surveys,49 and the large sample size provides substantial heterogeneity to allow for valid within-cohort comparisons.50 Another potential limitation of the study was its reliance on the FWE GP measure as a sole measure of GP supply. However, the use of FWE GPs accounted for multiple worksites and differing caseloads of GPs in regional and rural areas, and is theoretically preferable to headcounts as a measure of realized access to primary care services.18 The study was also unable to account for all potential drivers of admission, such as variations in hospital characteristics, which would require assigning potential pools of patients to their likely hospital(s) of admission.51 Residual overdispersion in the model may have also resulted in less accurate variance estimates and CIs.
This study has confirmed that personal characteristics are major drivers of preventable hospitalization, and importantly, the contribution of these factors varies according to condition. In the Australian setting at least, variations in GP supply explain little of the geographic variation in rates of preventable hospitalization. Our findings suggest the need for caution in the international adoption of health system performance indicators that have largely been developed and tested within the US health care system. International comparative work using similar individual-level data and multilevel modelling methods will potentially shed light on how the use and interpretation of this performance indicator may vary across countries and according to health system characteristics.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the many thousands of people participating in the 45 and Up Study. The authors also thank the Sax Institute, the NSW Ministry of Health, and the NSW Register of Births, Deaths, and Marriages for allowing access to the data, and the Centre for Health Record Linkage for conducting the probabilistic linkage of records.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.lww-medicalcare.com.
The APHID study is funded by a National Health and Medical Research Council Partnership Project Grant (#1036858) and by partner agencies the Australian Commission on Safety and Quality in Health Care, the Agency for Clinical Innovation and the NSW Bureau of Health Information. The 45 and Up Study is funded by the Sax Institute with support from major partner Cancer Council NSW and other partners which, at the time of writing, include: Heart Foundation (NSW Division), NSW Ministry of Health, beyondblue: the national depression initiative, Ageing, Disability and Home Care, NSW Family and Community Services and Australian Red Cross Blood Service. A.H.L.’s work at the Social and Public Health Sciences Unit, University of Glasgow, is core funded by the UK Medical Research Council (MC_UU_12017/5) and the Chief Scientist Office of the Scottish Government Health Directorates (SPHSU2).
The APHID Study investigator team comprises Louisa Jorm, Alastair Leyland, Fiona Blyth, Robert Elliot, Kirsty Douglas, Sally Redman, Michael Falster, Bich Tran, Sanja Lujic, Deborah Randall, Marjon van der Pol, Damilola Olajide, Danielle Butler, Neville Board, Douglas Lincoln, Kim Sutherland, Chris Shipway, and Nigel Lyons. This research was completed using data collected through the 45 and Up Study (http://www.saxinstitute.org.au). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW; and partners: the National Heart Foundation of Australia (NSW Division); NSW Ministry of Health; beyondblue; Ageing, Disability and Home Care, Department of Family and Community Services; and the Australian Red Cross Blood Service.
The authors declare no conflict of interest.
REFERENCES
- 1.Billings J, Zeitel L, Lukomnik J, et al. Impact of socioeconomic status on hospital use in New York City. Health Aff (Milwood). 1993;12:162–173. [DOI] [PubMed] [Google Scholar]
- 2.Ansari Z. The concept and usefulness of ambulatory care sensitive conditions as indicators of quality and access to primary health care. Aust J Prim Health. 2007;13:91–110. [Google Scholar]
- 3.Kruzikas DT, Jiang HJ, Remus D, et al. Preventable Hospitalisations: A Window into Primary and Preventive Care, 2000 HCUP Fact Book No 5; AHRQ Publication No 04-0056. 2004Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- 4.Council of Australian Governments. Intergovernmental Agreement (IGA) on Federal Financial Relations: Schedule F National Healthcare Agreement. 2012Council of Australian Governments; Available at: http://www.federalfinancialrelations.gov.au/content/national_agreements.aspx. Accessed March 20, 2013. [Google Scholar]
- 5.National Health Performance Authority. Healthy Communities: Selected Potentially Avoidable Hospitalisations in 2011–12. 2013Sydney: National Health Performance Authority. [Google Scholar]
- 6.Page A, Ambrise S, Glover J, et al. Atlas of Avoidable Hospitalisations in Australia: Ambulatory Care-Sensitive Conditions. 2007Adelaide, South Australia: Public Health Information Development Unit, The University of Adelaide. [Google Scholar]
- 7.Chang CH, Stukel TA, Flood AB, et al. Primary care physician workforce and Medicare beneficiaries’ health outcomes. JAMA. 2011;305:2096–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laditka JN, Laditka SB, Probst JC. More may be better: evidence of a negative relationship between physician supply and hospitalization for ambulatory care sensitive conditions. Health Serv Res. 2005;40:1148–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu J, Friedman B, Burstin H. Primary care, HMO enrollment, and hospitalization for ambulatory care sensitive conditions: a new approach. Med Care. 2002;40:1260–1269. [DOI] [PubMed] [Google Scholar]
- 10.Bindman AB, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA. 1995;274:305–311. [PubMed] [Google Scholar]
- 11.Ansari Z, Laditka JN, Laditka SB. Access to health care and hospitalization for ambulatory care sensitive conditions. Med Care Res Rev. 2006;63:719–741. [DOI] [PubMed] [Google Scholar]
- 12.Probst JC, Laditka JN, Laditka SB. Association between community health center and rural health clinic presence and county-level hospitalization rates for ambulatory care sensitive conditions: an analysis across eight US states. BMC Health Serv Res. 2009;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill JM, Mainous AG., III The role of provider continuity in preventing hospitalizations. Arch Fam Med. 1998;7:352–357. [DOI] [PubMed] [Google Scholar]
- 14.Menec VH, Sirski M, Attawar D, et al. Does continuity of care with a family physician reduce hospitalizations among older adults? J Health Serv Res Policy. 2006;11:196–201. [DOI] [PubMed] [Google Scholar]
- 15.Giuffrida A, Gravelle H, Roland M. Measuring quality of care with routine data: avoiding confusion between performance indicators and health outcomes. Br Med J. 1999;319:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adair CE, Simpson E, Casebeer AL, et al. Performance measurement in healthcare: part II—state of the science findings by stage of the performance measurement process. Healthc Policy. 2006;2:56–78. [PMC free article] [PubMed] [Google Scholar]
- 17.Scott A, Witt J, Humphreys J, et al. Getting doctors into the bush: general practitioners’ preferences for rural location. Soc Sci Med. 2013;96:33–44. [DOI] [PubMed] [Google Scholar]
- 18.Duckett S, Breadon P, Ginnivan L. Access All Areas: New Solutions for GP Shortages in Rural Australia. 2013Melbourne: Grattan Institute. [Google Scholar]
- 19.Ricketts TC, Randolph R, Howard HA, et al. Hospitalization rates as indicators of access to primary care. Health Place. 2001;7:27–38. [DOI] [PubMed] [Google Scholar]
- 20.Roos LL, Walld R, Uhanova J, et al. Physician visits, hospitalizations, and socioeconomic status: ambulatory care sensitive conditions in a canadian setting. Health Serv Res. 2005;40:1167–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krakauer H, Jacoby I, Millman M, et al. Physician impact on hospital admission and on mortality rates in the Medicare population. Health Serv Res. 1996;31:191–211. [PMC free article] [PubMed] [Google Scholar]
- 22.Diez-Roux AV. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am J Public Health. 1998;88:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Culler SD, Parchman ML, Przybylski M. Factors related to potentially preventable hospitalizations among the elderly. Med Care. 1998;36:804–817. [DOI] [PubMed] [Google Scholar]
- 24.Laditka JN. Physician supply, physician diversity, and outcomes of primary health care for older persons in the United States. Health Place. 2004;10:231–244. [DOI] [PubMed] [Google Scholar]
- 25.Chew RB, Bryson CL, Au DH, et al. Are smoking and alcohol misuse associated with subsequent hospitalizations for ambulatory care sensitive conditions? J Behav Health Serv R. 2011;38:3–15. [DOI] [PubMed] [Google Scholar]
- 26.Leyland AH, Groenewegen PP. Multilevel modelling and public health policy. Scand J Public Health. 2003;31:267–274. [DOI] [PubMed] [Google Scholar]
- 27.Fiorentini G, Iezzi E, Lippi Bruni M, et al. Incentives in primary care and their impact on potentially avoidable hospital admissions. Eur J Health Econ. 2011;12:297–309. [DOI] [PubMed] [Google Scholar]
- 28.Deraas TS, Berntsen GR, Jones AP, et al. Associations between primary healthcare and unplanned medical admissions in Norway: a multilevel analysis of the entire elderly population. BMJ Open. 2014;4:e004293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berlin C, Busato A, Rosemann T, et al. Avoidable hospitalizations in Switzerland: a small area analysis on regional variation, density of physicians, hospital supply and rurality. BMC Health Serv Res. 2014;14:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorm LR, Leyland AH, Blyth FM, et al. Assessing Preventable Hospitalisation InDicators (APHID): protocol for a data-linkage study using cohort study and administrative data. BMJ Open. 2012;2:e002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banks E, Redman S, Jorm L, et al. Cohort profile: the 45 and up study. Int J Epidemiol. 2008;37:941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Australian Institute of Health and Welfare. PI 22-selected potentially preventable hospitalisations, 2012. Available at: http://meteor.aihw.gov.au/content/index.phtml/itemId/443687. Accessed May 18, 2012.
- 33.Tran B, Falster MO, Douglas K, et al. Health behaviours and potentially preventable hospitalisation: a prospective study of older Australian adults. PloS one. 2014;9:e93111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trewin D. Statistical Geography Volume 1—Australian Standard Geographical Classification (ASGC), ABS Catalogue No 12160. 2006Canberra: Australian Bureau of Statistics. [Google Scholar]
- 35.Australian Bureau of Statistics. Regional Population Growth, Australia, 2012, ABS Catalogue No 32180. 2013Canberra: Australian Bureau of Statistics. [Google Scholar]
- 36.Mazumdar S, Konings P, Butler D, et al. General practitioner (family physician) workforce in Australia: comparing geographic data from surveys, a mailing list and medicare. BMC Health Serv Res. 2013;13:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Department of Health. General practice workforce statistics—1984-85 to 2011-12. 2013. Available at: http://www.health.gov.au/internet/main/publishing.nsf/Content/General+Practice+Statistics-1. Accessed October 21, 2013.
- 38.Public Health Information Development Unit, The Universit of Adelaide. Social health atlas of Australia. 2011. Available at: http://www.publichealth.gov.au/data/a-social-health-atlas-of-australia_-2011.html. Accessed April 10, 2014.
- 39.Merlo J, Yang M, Chaix B, et al. A brief conceptual tutorial on multilevel analysis in social epidemiology: investigating contextual phenomena in different groups of people. J Epidemiol Community Health. 2005;59:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parchman ML, Culler SD. Preventable hospitalizations in primary care shortage areas. An analysis of vulnerable Medicare beneficiaries. Arch Fam Med. 1999;8:487–491. [DOI] [PubMed] [Google Scholar]
- 41.Thomson S, Osborn R, Squires D, et al. International Profiles of Health Care Systems. 2011New York, NY: The Commonwealth Fund. [Google Scholar]
- 42.Agency for Healthcare Research and Quality (AHRQ). Refinement of the HCUP Quality Indicators. 2001Rockdale: Agency for Healthcare Research and Quality (AHRQ). [Google Scholar]
- 43.Katteri R, Anikeeva O, Butler C, et al. Potentially Avoidable Hospitalisations in Australia: Causes for Hospitalisaitons and Primary Health Care Interventions PHC RIS Policy Issue Review. 2012Adelaide: Primary Health Care Research & Information Service. [Google Scholar]
- 44.Harrold TC, Randall DA, Falster MO, et al. The contribution of geography to disparities in preventable hospitalisations between indigenous and nonindigenous Australians. PloS one. 2014;9:e97892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muenchberger H, Kendall E. Predictors of preventable hospitalization in chronic disease: priorities for change. J Public Health Policy. 2010;31:150–163. [DOI] [PubMed] [Google Scholar]
- 46.Caughey GE, Ellett LMK, Wong TY. Development of evidence-based Australian medication-related indicators of potentially preventable hospitalisations: a modified RAND appropriateness method. BMJ Open. 2014;4:e004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shwartz M, Pekoz EA, Ash AS, et al. Do variations in disease prevalence limit the usefulness of population-based hospitalization rates for studying variations in hospital admissions? Med Care. 2005;43:4–11. [PubMed] [Google Scholar]
- 48.Purdy S, Griffin T, Salisbury C, et al. Ambulatory care sensitive conditions: terminology and disease coding need to be more specific to aid policy makers and clinicians. Public Health. 2009;123:169–173. [DOI] [PubMed] [Google Scholar]
- 49.Mealing NM, Banks E, Jorm LR, et al. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol. 2010;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ponsonby AL, Dwyer T, Couper D. Is this finding relevant? Generalisation and epidemiology. Aust N Z J Public Health. 1996;20:54–56. [DOI] [PubMed] [Google Scholar]
- 51.Shwartz M, Pekoz EA, Labonte A, et al. Bringing responsibility for small area variations in hospitalization rates back to the hospital: the propensity to hospitalize index and a test of the Roemer’s Law. Med Care. 2011;49:1062–1067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


