Supplemental Digital Content is Available in the Text.
Analysis of a propensity-matched cohort determined that 82% to 84% of patients with spinal cord stimulation (SCS) will need at least 1 magnetic resonance imaging within 5 years of implant, with 59% to 74% requiring nonspine magnetic resonance imaging within 10 years. Magnetic resonance imaging–conditional SCS devices are needed that grant access of patients with SCS to this imaging modality.
Keywords: spinal cord simulation, SCS, neuromodulation, magnetic resonance imaging (MRI), chronic pain, back pain, chronic back and leg pain, diagnostic imaging, radiology, spine
Abstract
Study Design.
Analysis of use of magnetic resonance imaging (MRI) in the chronic back and leg pain spinal cord stimulation (SCS)–implanted population was conducted using a propensity-matched cohort population.
Objective.
To project the percentage of patients with SCS expected to need at least 1 MRI within 5 years of implant.
Summary of Background Data.
Patients experiencing pain, including those who underwent implantation with SCS systems, are likely to have comorbidities and ongoing pain issues that may require diagnostic imaging. MRI is the most common diagnostic imaging modality for evaluating patients with new or worsening low back pain. However, patients with SCS are typically excluded from receiving MRI because of the safety risks related to the interactions of MRI fields and implantable devices.
Methods.
To provide an accurate estimate of the need for MRI in the SCS-implanted population, Truven Health MarketScan Commercial Claims and Medicare Supplemental databases were used to perform analysis of SCS-implanted patients propensity score matched to a nonimplanted population–based cohort. Four years of paid and adjudicated claims data were used to determine the magnetic resonance (MR) images received, which was exponentially projected to estimate MRI within 5 and 10 years of implant.
Results.
Approximately 82% to 84% of SCS-implanted patients are expected to need at least 1 MRI within 5 years of implant. Furthermore, 59% to 74% of patients will require nonspine MRI within 10 years.
Conclusion.
There is a high need for MRI in this chronic back and leg pain SCS population, with a significant portion being completed on locations outside of the spine. This analysis highlights a need for MRI-conditional SCS devices that grant access of patients with SCS to this imaging modality.
Level of Evidence: 3
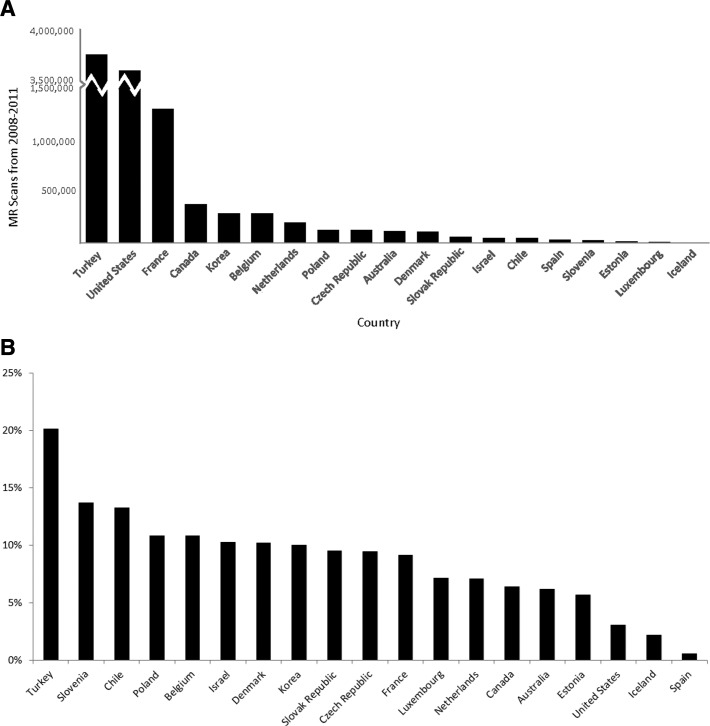
Over the past quarter century, the use of diagnostic imaging has substantially increased globally with the fastest growth being outside the United States and in emerging markets (Figure 1A, B).1 Sixty million computed tomographic (CT) and 20 million nuclear medicine examinations were completed in the United States in 2005, compared with 3 million CT and 7 million nuclear medicine examinations in 1980.2 Concerns regarding the risks of ionizing radiation have accompanied this rise. Protracted exposure to ionizing radiation, which world and United States agencies classify as a carcinogen, increases the risk for developing cancer.2–5 This issue may be mitigated by employing the imaging modality with the lowest radiation dose but optimal examination for the disease state.6,7
Figure 1.
A, Magnetic resonance imaging growth (2008–2011). B, Magnetic resonance imaging average annual growth rate (2008–2011). MR indicates magnetic resonance.
Magnetic resonance imaging (MRI) is now the preferred mode of diagnostic imaging for many disease states, both in terms of guidelines and clinical practice.7–9 Use of MRI in the diagnosis of acute stroke is expanding, partially replacing CT in the acute phase to stratify stroke patients.10–12 For breast cancer, there is no optimal alternative to MRI in some situations. As an example of evolving techniques, breast MRI utilization has increased between 300% and 1600% from 2000 to 2011 in the United States.13,14
For purposes of diagnostics and to gauge progression, imaging is often used in patients with chronic pain. Chronic pain impacts approximately 100 million Americans, greater than the number impacted by cancer, heart disease, and diabetes combined.15 Globally, chronic pain is a significant public health issue, with high prevalence in both developed and developing countries and increasing prevalence with age.16 The Institute of Medicine estimates that the annual global cost of chronic pain is $560 to $635 billion.15
Patients with chronic spinal pain have an amplified need for MRI as they often experience comorbid conditions. A recent study estimated that 87.1% of individuals with chronic spinal pain have at least 1 other comorbid condition.17 Disease states such as arthritis, migraine, stroke, epilepsy, cancer, and vision problems are all significantly associated with chronic spinal pain, and many of these conditions (migraine, stroke, epilepsy) are optimally evaluated with MRI.7,17 Consequently, access to magnetic resonance (MR) technology is particularly relevant for patients experiencing chronic pain for these reasons as well as the ongoing progression of their underlying chronic pain pathology.
Chronic pain of various etiologies including post–spine surgery syndrome, complex regional pain syndrome (CRPS), and lumbar radiculopathy is often treated by spinal cord stimulation (SCS), a clinically and cost-effective treatment for chronic back and leg pain (CBLP).18 SCS is a therapy for chronic pain whereby electrical stimulation is typically delivered to the dorsal columns of the spinal cord via epidural placement of leads containing an array of percutaneous or paddle-type electrodes. Although the mechanisms of SCS are not completely elucidated, trunk and extremity pain is replaced with painless paresthesia. The efficacy of SCS has been demonstrated via randomized studies in disease states such as failed back surgery syndrome, chronic lumbar radiculopathy, and CRPS.19–21
Historically, neurostimulation systems were contraindicated for MRI. The next step in the evolution of these devices was conditionally safe labeling by the Food and Drug Administration under specific controlled situations for head imaging. These restrictions stem from concerns of potential adverse interactions between implanted devices and MRI, along with the increased likelihood that a patient will be recommended for MRI.22 Several risks exist for MRI of neurostimulator patients, including patients' injury or discomfort, technical complications, and device damage. MRI exposure can affect the operation of the neurostimulator, including changes to the parameter settings, telemetry failure, and power on resets.23–25 Furthermore, induced currents (from the magnetic field of the MRI) have the potential to cause tissue heating, tissue stimulation, or device malfunction/failure.24–28
Although head-only MR image labeling provides important options for device patients requiring MRI of the brain, only about a quarter of all MR images obtained in the United States are of the head and the neck.29 There are centers that perform MRI of carefully selected patients when the benefits outweigh the risks. However, these are typically performed with strict protocols, extensive precautions, and by those with significant expertise in MRI; few, if any, authors advocate routine standardized scanning of patients with active implantable medical devices.25,28,30–32
To provide an accurate estimate of MRI utilization in the SCS-implanted population, insurance claims databases were used to perform analysis of propensity-matched population cohort to the SCS-implanted patients. Using 4 years of paid and adjudicated claims, exponential projection estimates were used to approximate the number of patients with SCS who received at least 1 MRI within 5 and 10 years of implant.
MATERIALS AND METHODS
Data Source
Data from Truven Health MarketScan Research Databases, which contain individual-level, de-identified, health care claims information from employers, health plans, hospitals, Medicare Supplemental and Medicaid programs, were used.33 Often used for research, these databases are Health Insurance Portability and Accountability Act compliant. A protocol describing the study objectives, criteria for patient selection, data elements of interest, and statistical methods was submitted to the New England Institutional Review Board and deemed exempt from review (exemption #14-234).
Patient Selection and Identification
Patient-level data were extracted from the MarketScan Commercial and Medicare databases for the years 2008–2011 (Figure 2). Patients with a record of an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for the following diseases were included: CBLP, including failed back surgery syndrome, radiculopathy, or CRPS (see Supplemental Digital Content Table A, available at http://links.lww.com/BRS/A968). Patients with claims for a cardiac implant that would be contraindicated for MRI were excluded from this analysis. Patients were then divided into 2 groups: those with and without SCS implant (see Supplemental Digital Content Table A, available at http://links.lww.com/BRS/A968). Continuous enrollment was required for 12 months before and after the date of the SCS implant. For the SCS-indicated cohort, additional inclusion criteria consisted of 4 years of continuous health plan coverage (2008–2011).
Figure 2.
Patient flow (inclusion/exclusion). CBLP indicates chronic back and leg pain; SCS, spinal cord stimulation.
Statistical Analysis
All data were imported and maintained in SAS data files. Tabulation of summary statistics, graphical presentations, and data analyses were performed using SAS Software, Version 9.2 (SAS Institute Inc., Cary, NC).
Propensity Matching
The SCS-indicated patients were matched 1:1 using a combination of direct (age and sex) and propensity score matching (comorbid conditions) to SCS-implanted patients. The goal of propensity matching is to remove biases in cohort estimates that would otherwise be present due to differences between the cohorts. Adjusting for a patient's propensity score, or conditional probability of assignment to a particular treatment given a set of observed characteristics, has been shown to remove bias because of these characteristics. In this study, all covariates were obtained from the MarketScan databases and were clinically relevant to the outcomes of interest. A SAS macro from the Mayo Clinic (gmatch) was used to generate the match. Gmatch employs a greedy algorithm to choose a fixed number of controls for each patient in a treatment cohort (e.g., 1:1 or 2:1), whereby each patient is matched to his or her nearest neighbor with respect to all factors simultaneously, using various distance measures and calipers.34
Propensity scores were calculated from a logistic regression model as the probability that a patient had an SCS implant given the patient's characteristics. These characteristics were the covariates in the logistic regression model and included age (within 3 yr), sex (direct match), and probability score on the basis of comorbidities. Assessment of bias reduction was conducted by evaluating the differences in the distributions of patient characteristics among the cohorts before versus after matching.
Predicting MRI Utilization
After matching, the survival probability to first MR image was estimated in the SCS-indicated cohort during a 4-year period (2008–2011). Using these data, a linear best-fit model was used to project the data out to 5 years. Then, the data were fitted with exponential functions to project a range of best-fit scenarios, as measured by the coefficient of determination, out to 10 years.
RESULTS
There were 5,751,174 patients with a diagnosis code from an inpatient or outpatient claim in 2008–2011 designating CBLP, including failed back surgery syndrome, radiculopathy, or CRPS. Of those patients, 117,366 were excluded because they had a claim for a cardiac implant and, therefore, were contraindicated (Figure 2). There were 13,995 patients with an inpatient or outpatient claim with a procedure code for SCS (SCS-implanted), and there were 5,619,813 patients who did not (SCS-indicated). Significant differences between the 2 cohorts were resolved upon matching. After matching, there were a total of 3325 SCS-indicated patients tracked for more than 4 years: 60% of patients were female, the majority were 50 years of age or older, and 71% had commercial insurance (Table 1). Frequently occurring comorbid conditions included lumbar disc disease (66%), osteoarthritis (62%), hypertension (52%), depressive disorders (39%), hyperlipidemia (38%), and diabetes (20%) (see Supplemental Digital Content Table B, available at http://links.lww.com/BRS/A969).
TABLE 1. Patient Demographics.
| Category | Total | SCS-Implimented | SCS-Indicated | |||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | N | Percent | |
| Total patients | 6650 | 100 | 3325 | 100 | 3325 | 100 |
| Age group, yr | ||||||
| < 18 | 16 | 0 | 8 | 0 | 8 | 0 |
| 18–29 | 106 | 2 | 53 | 2 | 53 | 2 |
| 30–39 | 578 | 9 | 290 | 9 | 288 | 9 |
| 40–49 | 1355 | 20 | 677 | 20 | 678 | 20 |
| 50–59 | 1905 | 29 | 952 | 29 | 953 | 29 |
| 60–69 | 1335 | 20 | 669 | 20 | 666 | 20 |
| 70–79 | 959 | 14 | 478 | 14 | 481 | 14 |
| 80 + | 396 | 6 | 198 | 6 | 198 | 6 |
| Sex | ||||||
| Female | 3994 | 60 | 1997 | 60 | 1997 | 60 |
| Male | 2656 | 40 | 1328 | 40 | 1328 | 40 |
| Insurance coverage | ||||||
| Commercial | 4724 | 71 | 2350 | 71 | 2374 | 71 |
| Medicare | 1926 | 29 | 975 | 29 | 951 | 29 |
| Insurance product type | ||||||
| Preferred Provider Organization | 3657 | 55 | 1832 | 55 | 1825 | 55 |
| Comprehensive | 1112 | 17 | 574 | 17 | 538 | 16 |
| Health Maintenance Organization | 952 | 14 | 428 | 13 | 524 | 16 |
| Point of Service | 559 | 8 | 256 | 8 | 303 | 9 |
| Missing | 169 | 3 | 93 | 3 | 76 | 2 |
| Consumer-Driven Health Plans | 121 | 2 | 81 | 2 | 40 | 1 |
| Exclusive Provider Organization | 40 | 1 | 34 | 1 | 6 | 0 |
| Point of Service with Capitation | 15 | 0 | 7 | 0 | 8 | 0 |
| High Deductible Health Plan | 25 | 0 | 20 | 1 | 5 | 0 |
| Region | ||||||
| South | 2997 | 45 | 1683 | 51 | 1314 | 40 |
| North Central | 1818 | 27 | 875 | 26 | 943 | 28 |
| West | 1060 | 16 | 520 | 16 | 540 | 16 |
| Northeast | 734 | 11 | 238 | 7 | 496 | 15 |
| Unknown | 41 | 1 | 9 | 0 | 32 | 1 |
| SCS indicates spinal cord stimulation. | ||||||
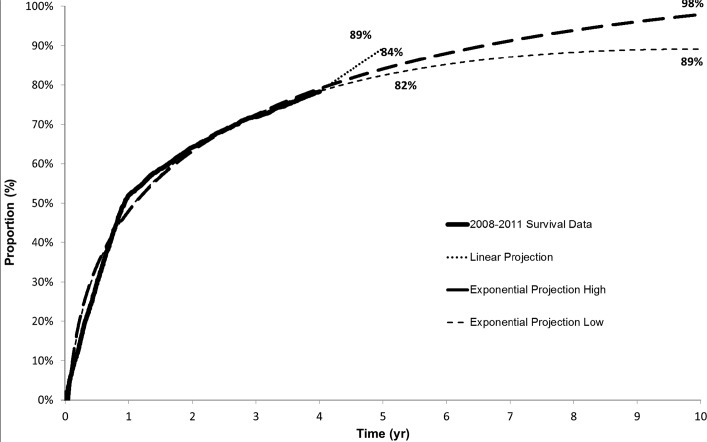
For the SCS-indicated cohort, the rate of patients receiving MRI was identified after 1 year (52%), 2 years (64%), 3 years (72%), and 4 years (78%). Using a linear projection, the estimated need for MRI within 5 years is 89%. Using a more robust exponential projection, MRI within 5 years is 82% to 84%. Approximately 89% to 98% of SCS-implanted patients are expected to need at least 1 MRI within 10 years of implant (Figure 3). When limited to nonspine MR images, approximately 59% to 74% of SCS-implanted patients are expected to need at least 1 nonspine MRI within 10 years of implant (Figure 4).
Figure 3.
Proportion of patients having any magnetic resonance imaging over 5- and 10-year time horizon.
Figure 4.
Proportion of patients having nonspine magnetic resonance imaging over 5- and 10-year time horizon.
For SCS-implanted patients, a total of 2021 (61%) patients receiving an MRI within 12 months prior to implant were identified (Table 2). These 2021 patients obtained a total of 3719 MR images in the 12 months prior to implant with an average of 1.84 per patient, with 76% being MR images of the spine (Table 2).
TABLE 2. Twelve Months Pre–Spinal Cord Stimulation Implant Frequency of Type of Magnetic Resonance Images.
| Count | % | |
|---|---|---|
| Total patients | 3325 | |
| Total patients with MR images | 2021 | 61 |
| Total MR images | 3718 | |
| Spine | 2812 | 76 |
| Nonspine | 907 | 24 |
| Average MR images for patients with MR images | 1.84 | |
| MR indicates magnetic resonance. |
DISCUSSION
MRI is often the diagnostic imaging modality of choice in a myriad of medical conditions. This is particularly true for spine and pain-related pathologies, where utilization is high. It is quite clear that the need for MRI is high within the CBLP population, with 82% to 84% of patients projected to need MRI within 5 years of SCS implant. This underscores the persistence and evolution of this disease state.
Pain physicians and spine surgeons may need to look beyond their focus on the spine and consider the impact of any clinical decision upon the patient's future access to medical treatments, often at the hands of other physician specialties. Increasingly, the use of MRI is not limited to disease of the spine. This study makes it clear that there is a need for nonspine MRI in this patient subset. Approximately 59% to 74% of patients with CBLP will need at least one nonspine MRI within 10 years of implant. If one was to assume that with the use of implantable therapies, CBLP was effectively treated, this would not eliminate the ongoing workup and treatment of other disease states. These data correspond with general imaging trends: an industry analysis of MR images obtained in the United States in 2013 showed that 76% of all MR procedures are outside of the lumbar and thoracic spine.29 MRI of the brain and extremities accounted for 20% and 22%, respectively, of all MRIs performed in the United States.29 Recent trends and the growing sophistication of this imaging modality underscore the broadening indication for MRI, with an increasing use in areas such as the breast and stroke.10–14 Also of note is the use of MRI immediately prior to SCS implant. Clinically, this might correspond to the workup of patients in preparation for SCS implant but is also often associated with a baseline acquisition of imaging data in preparation for patients' transition to a state where therapy will preclude access to MRI. Interestingly, this group averaged 1.84 in the year prior to implant, with the majority of images being those of the spine.
Although MRI is not the appropriate diagnostic modality for all patients or disease states, it will continue to be integral and sometimes irreplaceable to patients' care. CT, CT enhanced with myelography, plain radiographs, and ultrasonography are available options and might be preferable in certain conditions. However, MRI is unquestionably the optimal option in a growing array of disease states, including spinal disorders, disease states of the large peripheral joints (shoulders, knees), stroke, multiple sclerosis, malignant breast cancer, and malignancy.7 One must consider the experience of a patient with an implantable device now facing new medical issues or an evolution of existing diagnoses. One option for these patients is to undergo explantation of their devices, thereby facing additional surgical risks and costs to the health care system. It has been suggested that explantation rates might more closely estimate the need for MRI, this tantamount to suggesting that cancer rates are better estimated by cancer surgery rates. Clinically speaking, when faced with the need for further diagnostic imaging for patients, each provider likely evaluates his or her patient to determine his or her pathway, reserving explantation as a last resort. Alternatively, and more likely, patients undergo suboptimal imaging modalities for their condition. CT is a useful tool in certain disease states, but it is not ideal in complicated low back and leg pain, where MRI is the preferred imaging modality.35 Thus, in some cases, not only is the alternative, such as CT, providing less diagnostic information, but it is also exposing patients to a large radiation dose. CT doses of radiation are much higher than conventional radiography, with the effective dose for CT 100 to 1000× higher than for a corresponding radiography.36 Additional examples include the reliance on arthroscopy to diagnose intra-articular joint pathology after trauma to the knee rather than on MRI. Finally, many patients are either not offered implantable therapies because of continued concerns regarding the ongoing need for diagnostic MRI or patients who underwent implantation are receiving MRIs contrary to labeling.
When relating the importance of MRI to the comorbid conditions reported for the patients with SCS in this analysis, it is of note that for several of these key conditions such as lumbar disc disease, osteoarthritis, and a variety of neurological disorders, the American College of Radiology appropriateness criteria rate MRI as most appropriate.7 In some cases, there is no equivalent imaging test with a similar rating.7
Despite the potential for adverse outcomes and Food and Drug Administration warnings, a few centers have performed MRI of SCS-implanted patients to prospectively assess safety or retrospectively report on individual cases.23–25,27,28,37 Six reports in the peer-reviewed literature report on 54 patients who underwent implantation with SCS systems and received MRI, with 19 adverse events.23–25,27,28,37 There were 10 reports of device function compromise (18.5%), 3 of which required explant and/or replacement. There were 6 reports of device heating (11.1%) as assessed by patients' report or cutaneous temp measurement over implantable pulse generator pocket. Three patients reported painful dysesthesias during the scan (5.6%). Only 2 studies conducted follow-up assessment after the MRI, 1 with 3-month duration and the other with 12-month duration. These heating and/or pain events suggest the possibility of initial asymptomatic tissue damage that might make one more vulnerable to future injury. These off-label MR images of patients with SCS describe considerable safety risks, yet the potential for much more damaging thermal injury from MRI with an active implantable device is possible (J. Welter et al, unpublished data, 2014).38
Given the safety hazards of off-label MR images, the growing utilization of MRI, and the evolving pain and comorbidities in the chronic pain patient population, there is a clear need for MR-compatible devices to treat chronic pain conditions. This analysis shows that 82% to 84% of patients with SCS are expected to need an MRI within 5 years of implant.
Important strengths of this analysis include specific utilization information that is both recent and based on a large group of patients from all regions of the United States. In addition, the analysis uses 4 years of paid and adjudicated claims data to determine actual MR images completed and has, therefore, met payer medical necessity criteria. Limitations of this research are that data were sourced from large claims databases designed for billing purposes and not research. For example, this SCS population is more concentrated in the south region, which may be a product of the MarketScan database being a convenience sample. In addition, claims are only reflective of what was coded, with the assumption that the coding is accurate and complete. Finally, these findings are generalizable only to other United States commercially insured and Medicare populations.
Key Points
Eighty-two percent to eighty-four percent of SCS-implanted patients will need at least 1 MRI within 5 years of implant. This is based on exponential projection estimate using SCS-implanted patients and a propensity-matched cohort.
Fifty-nine percent to seventy-four percent of patients will need nonspine MRI within 10 years.
This analysis highlights a need for MRI-conditional SCS devices that grant access of patients with SCS to this imaging modality.
Acknowledgments
Supplemental digital content is available for this article. Direct URL citations appearing in the printed text are provided in the HTML and PDF version of this article on the journal's Web site (www.spinejournal.com).
Footnotes
Acknowledgment date: June 11, 2014
The device(s)/drug(s) is/are FDA approved or approved by corresponding national agency for this indication.
This study was funded by Medtronic, plc.
Relevant financial activities outside the submitted work: consultancy, payment for lectures, employees, stocks.
References
- 1.Organisation for Economic Co-operation and Development. Health care utilization. Available at: http://stats.oecd.org/Index.aspx?DataSetCode=HEALTH_PROC. Accessed February 21, 2014.
- 2.Amis ES, Jr, Butler PF, Applegate KE, et al. American College of Radiology. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol 2007;4:272–84. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 75 Available at: http://monographs.iarc.fr/ENG/Monographs/vol75/mono75.pdf. Accessed January 17, 2014. [Google Scholar]
- 4.Agency for Toxic Substances and Disease Registry. Toxicological profile for ionizing radiation. Available at: http://www.atsdr.cdc.gov/toxprofiles/tp149.pdf. Accessed January 17, 2014. [PubMed]
- 5.US Department of Health and Human Services, Public Health Service, National Toxicology Program. Report on Carcinogens. 12th ed. Available at: http://ntp.niehs.nih.gov/ntp/roc/twelfth/roc12.pdf. Accessed January 17, 2014.
- 6.European Commission. Radiation protection 118: referral guidelines for imaging. Available at: http://ec.europa.eu/energy/nuclear/radioprotection/publication/doc/118_en.pdf. Accessed April 8, 2014.
- 7.American College of Radiology. November 2013 ACR appropriateness criteria. Available at: http://www.acr.org/Quality-Safety/Appropriateness-Criteria. Accessed February 9, 2014.
- 8.National Institute for Health and Care Excellence. Low back pain: early management of persistent non-specific low back pain. Available at: http://publications.nice.org.uk/low-back-pain-cg88. Accessed February 9, 2014.
- 9.Airaksinen O, Brox JI, Cedraschi C, et al. ; COST B13 Working Group on Guidelines for Chronic Low Back Pain. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J 2006;15(suppl 2):S192–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schellinger PD, Bryan RN, Caplan LR, et al. ; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010;75:177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latchaw RE, Alberts MJ, Lev MH, et al. American Heart Association Council on Cardiovascular Radiology and Intervention, Stroke Council, and the Interdisciplinary Council on Peripheral Vascular Disease. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke 2009;40:3646–78. [DOI] [PubMed] [Google Scholar]
- 12.Giles MF, Albers GW, Amarenco P, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology 2011;77:1222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wernli KJ, DeMartini WB, Ichikawa L, et al. ; Breast Cancer Surveillance Consortium. Patterns of breast magnetic resonance imaging use in community practice. JAMA Intern Med 2014;174:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stout NK, Nekhlyudov L, Li L, et al. Rapid increase in breast magnetic resonance imaging use: trends from 2000 to 2011. JAMA Intern Med 2014;174:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Relieving pain in America. A blueprint for transforming prevention, care, education, and research. Available at: http://www.iom.edu/∼/media/Files/Report%20Files/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research/Pain%20Research%202011%20Report%20Brief.pdf. Accessed March 26, 2014.
- 16.Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008;9:883–91. [DOI] [PubMed] [Google Scholar]
- 17.Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical-mental comorbidity in the united states: results from the national comorbidity survey replication. Pain 2005;113:331–9. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RS, Desai MJ, Rigoard P, et al. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract 2014;14:489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar K, North R, Taylor R, et al. Spinal cord stimulation vs. conventional medical management: a prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (PROCESS study). Neuromodulation 2005;8:213–8. [DOI] [PubMed] [Google Scholar]
- 20.North RB, Kidd DH, Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery 2005;56:98–106; discussion 106–7. [DOI] [PubMed] [Google Scholar]
- 21.Kemler MA, Barendse GAM, Van Kleef M, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med 2000;343:618–24. [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration. A Primer on medical device interactions with magnetic resonance imaging systems [FDA Web site]. Available at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm107721.htm Published February 7, 1997. Updated June 18, 2009. Accessed January 23, 2014.
- 23.Liem LA, van Dongen VC. Magnetic resonance imaging and spinal cord stimulation systems. Pain 1997;70:95–7. [DOI] [PubMed] [Google Scholar]
- 24.Tronnier VM, Staubert A, Hahnel S, et al. Magnetic resonance imaging with implanted neurostimulators: an in vitro and in vivo study. Neurosurgery 1999;44:118–25; discussion 125–6. [DOI] [PubMed] [Google Scholar]
- 25.De Andres J, Valía JC, Cerda-Olmedo G, et al. Magnetic resonance imaging in patients with spinal neurostimulation systems. Anesthesiology 2007;106:779–86. [DOI] [PubMed] [Google Scholar]
- 26.Henderson JM, Tkach J, Phillips M, et al. Permanent neurological deficit related to magnetic resonance imaging in a patient with implanted deep brain stimulation electrodes for Parkinson's disease: case report. Neurosurgery 2005;57:E1063. [DOI] [PubMed] [Google Scholar]
- 27.Simopoulos TT, Gill JS. Magnetic resonance imaging of the lumbar spine in a patient with a spinal cord stimulator. Pain Physician 2013;16:E295–300. [PubMed] [Google Scholar]
- 28.Mutter UM, Bellut D, Porchet F, et al. Spinal magnetic resonance imaging with reduced specific absorption rate in patients harbouring a spinal cord stimulation device—a single-centre prospective study analysing safety, tolerability and image quality. Acta Neurochir (Wien) 2013;155:2327–32. [DOI] [PubMed] [Google Scholar]
- 29.IMV 2013 MR Benchmark Report.
- 30.Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 2005;28:326–8. [DOI] [PubMed] [Google Scholar]
- 31.Wilkoff BL, Bello D, Taborsky M, et al. Magnetic resonance imaging in patients with a pacemaker system designed for the magnetic resonance environment. Heart Rhythm 2011; 8:65–73. [DOI] [PubMed] [Google Scholar]
- 32.Shellock F. MRI safety and neuromodulation systems. In:Krames E, Peckham P, Rezai A, eds. Neuromodulation. London: Elsevier Academic Press; 2009:243–85. [Google Scholar]
- 33.Hansen LG, Chang S. White Paper. Health Research Data for the Real World: The MarketScan Databases. Truven Health Analytics; 2012. [Google Scholar]
- 34.Mayo Clinic. Gmatch macro developed by Erik Bergstralh and Jon Kosanke. Available at: http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros Updated 2003. Accessed August 20, 2013.
- 35.Davis PC, Wippold FJ, II, Brunberg JA, et al. ACR appropriateness criteria on low back pain. J Am Coll Radiol 2009;6:401–7. [DOI] [PubMed] [Google Scholar]
- 36.Semelka RC, Armao DM, Elias J, Jr, et al. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging 2007;25:900–9. [DOI] [PubMed] [Google Scholar]
- 37.Shah RV, Smith HK, Chung J, et al. Cervical spinal cord neoplasm in a patient with an implanted cervical spinal cord stimulator: the controversial role of magnetic resonance imaging. Pain Physician 2004;7:273–8. [PubMed] [Google Scholar]
- 38.Coffey RJ, Kalin R, Olsen JM. Magnetic resonance imaging conditionally safe neurostimulation leads: investigation of the maximum safe lead tip temperature. Neurosurgery 2014;74:215–24; discussion 224–5. [DOI] [PubMed] [Google Scholar]