SUMMARY
An important feature of glucose homeostasis is the effective release of glucagon from the pancreatic α cell. The molecular mechanisms regulating glucagon secretion are still poorly understood. We now demonstrate that human α cells express ionotropic glutamate receptors (iGluRs) that are essential for glucagon release. A lowering in glucose concentration results in the release of glutamate from the α cell. Glutamate then acts on iGluRs of the AMPA/kainate type, resulting in membrane depolarization, opening of voltage-gated Ca2+ channels, increase in cytoplasmic free Ca2+ concentration, and enhanced glucagon release. In vivo blockade of iGluRs reduces glucagon secretion and exacerbates insulin-induced hypoglycemia in mice. Hence, the glutamate autocrine feedback loop endows the α cell with the ability to effectively potentiate its own secretory activity. This is a prerequisite to guarantee adequate glucagon release despite relatively modest changes in blood glucose concentration under physiological conditions.
INTRODUCTION
Blood glucose homeostasis is controlled by the concerted secretion of pancreatic hormones from the various cell types in the endocrine part of the pancreas, the islets of Langerhans. The secretion of insulin and glucagon from the pancreatic β and α cells, respectively, is regulated by nutrients as well as by autocrine, paracrine, and nervous signals. Whereas the molecular mechanisms involved in insulin secretion are relatively well understood, those regulating glucagon secretion are not clear. A pivotal question is how glucagon can be so effectively released subsequent to the modest changes in blood glucose concentration prevailing under normal conditions.
Secretion of glucagon from α cells is increased when the blood glucose concentration decreases (Unger, 1985; Zhou et al., 2004), but the exact underlying molecular mechanisms that act on the α cell to induce glucagon release are not known. It has been proposed that high glucose levels suppress glucagon release by acting directly on α cells (Gopel et al., 2000; Unger, 1985). Mechanistically this has been explained by the selective expression of voltage-sensitive Na+ channels in α cells that contribute to the generation of neuronal-like action potentials that are inactivated by prolonged cell membrane depolarization (Gopel et al., 2000). According to this model, the inactivation of these Na+ channels suppresses α cell activity when glucose levels are high. Other studies, however, have shown that glucose activates α cells by mechanisms that mirror stimulus-secretion coupling in β cells—that is, glucose stimulates α cells and β cells alike (Olsen et al., 2005; Wendt et al., 2004). Therefore, inhibitory paracrine signals such as insulin, GABA, and Zn2+ being released from the β cell, rather than changes in the glucose concentration per se, have been suggested to be involved in the direct regulation of glucagon release (Franklin et al., 2005; Gromada et al., 2007; Ishihara et al., 2003; Kisanuki et al., 1995; Ravier and Rutter, 2005; Rorsman et al., 1989).
However, a relief of inhibitory paracrine signals cannot fully explain how relatively minor decreases in glucose concentration so effectively promote glucagon release. Therefore, we hypothesized that α cells require positive feedback loops to produce a full glucagon response (see also Hayashi et al., 2003b). Glutamate, a major excitatory neurotransmitter in the central nervous system, is of special interest because, unlike most other signals, it may stimulate rather than inhibit glucagon secretion in the islet (Bertrand et al., 1993; Hayashi et al., 2003c). Vesicular glutamate transporters that facilitate glutamate uptake into vesicles are expressed by α cells (Hayashi et al., 2001), and glutamate is secreted together with glucagon (Hayashi et al., 2003c).
Unfortunately, studies based in rodents have shown a large complexity in glutamate signaling in the islet (Moriyama and Hayashi, 2003). Results so far are conflicting, and the role of glutamate remains enigmatic. For instance, it has been reported that glutamate stimulates glucagon secretion via ionotropic glutamate receptors (iGluRs) (Bertrand et al., 1993), that it inhibits glucagon secretion via metabotropic glutamate receptors (mGluRs) (Uehara et al., 2004), and that it activates mGluRs and iGluRs in β cells to increase insulin secretion (Bertrand et al., 1992, 1995; Storto et al., 2006). To resolve this controversy, we have therefore systematically used several molecular and physiological techniques to define the role of glutamate signaling in human, monkey, and mouse islets of Langerhans.
RESULTS
Activation of iGluRs Induces Glucagon Secretion
By performing RT-PCR on isolated human and monkey islets, we could detect transcripts for the iGluR subunits GluR1, GluR2, GluR3, and GluR4 of the AMPA receptor type and GluR5, GluR6, GluR7, and KA2 of the kainate receptor type (data not shown). These results are in agreement with those published in the Beta Cell Biology Consortium database (http://www.betacell.org/resources/data/epcondb/). The expression of iGluR subunits in islets was confirmed by immunoblot analysis with antibodies against GluR2/3 (data not shown). GluR1 and GluR4 subunits could not be detected. The bands of the correct size disappeared from the immunoblots when the GluR2/3 antibody was preadsorbed, but the immunostaining in pancreas sections did not, indicating that the immunofluorescent signal obtained with the GluR2/3 antibodies was not specific. To study cell-specific expression of iGluRs, we thus decided to use alternative techniques (see below).
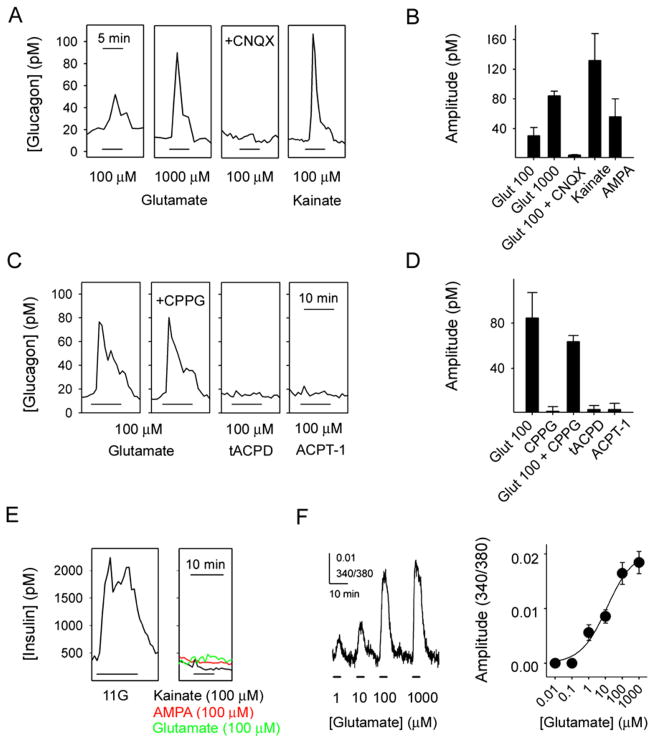
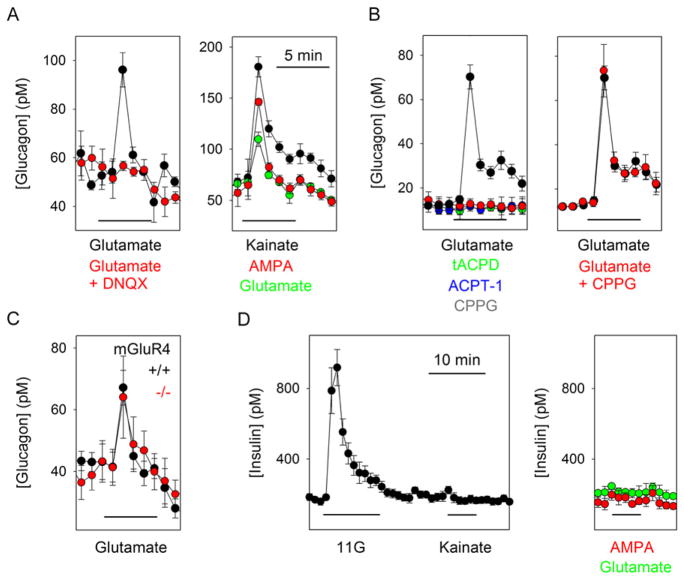
In contrast to previous results in rodent islets (Moriyama and Hayashi, 2003), we found that functional AMPA/kainate iGluRs were present exclusively in α cells of human and monkey islets (Figure 1 and Figure 2). By using the in vitro perifusion technique to detect hormone secretion, we determined that glutamate (1 μM–1 mM) stimulated large, concentration-dependent increases in glucagon release (Figures 1A and 1B). Increases in glucagon release could also be elicited by the iGluR agonists kainate (100 μM) and AMPA (100 μM; Figures 1A and 1B). CNQX (10 μM) and DNQX (10 μM; data not shown), two antagonists for iGluRs of the AMPA/kainate type, inhibited glutamate-induced glucagon release by more than 90% (Figures 1A and 1B). Metabotropic receptors have been reported to mediate negative autocrine effect on glucagon secretion in rat islets (Uehara et al., 2004). Using human islets, however, we found that the metabotropic receptor agonists trans-ACPD (100 μM) and ACPT-1 (100 μM) and the metabotropic antagonist CPPG (100 μM) did not affect basal glucagon secretion or glucagon responses to glutamate (Figures 1C and 1D).
Figure 1. Activation of Ionotropic Glutamate Receptors in Human Islets Induces Glucagon Secretion.
(A) Glutamate induced glucagon responses that could be blocked by CNQX (10 μM). Kainate and AMPA (both 100 μM) also elicited strong glucagon secretion.
(B) Quantification of results in (A) (n = 5 islet preparations).
(C) The metabotropic glutamate receptor antagonist CPPG (100 μM) did not affect the glutamate-induced glucagon response. The metabotropic glutamate receptor agonists trans-ACPD (tACPD; 100 μM) and ACPT-1 (100 μM) did not elicit changes in glucagon secretion.
(D) Quantification of results in (C) (n = 3 islet preparations).
(E) Insulin release was induced by high glucose (11 mM, 11G) but not by kainate (representative of six islet preparations).
(F) Glutamate elicited concentration-dependent [Ca2+]i responses in human islets (n = 4 islet preparations). Data were curve fitted using the Hill equation.
Results are shown as mean ± SEM.
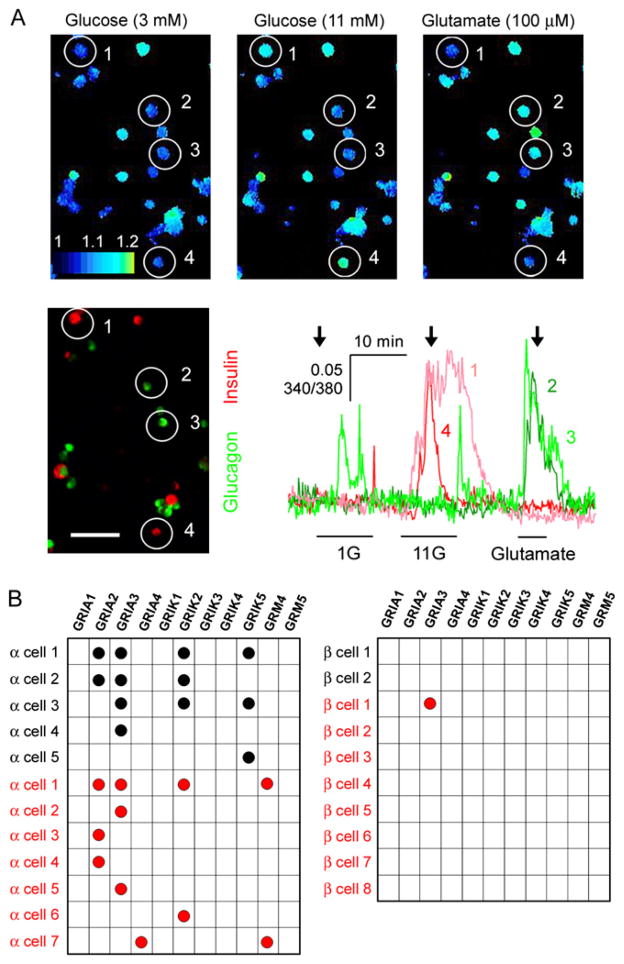
Figure 2. α Cells in Human Pancreatic Islets Express Functional Ionotropic Glutamate Receptors of the AMPA/Kainate Type.
(A) Top row: three sequential images showing [Ca2+]i responses to 3 mM glucose (3G), 11 mM glucose (11G), and 100 μM glutamate in dispersed human islet cells (pseudocolor scale). Bottom left: glucagon (green) and insulin (red) immunostaining. Glucagon-immunoreactive cells 2 and 3 responded to glutamate but not to 11G. Insulin-immunoreactive cells 1 and 4 responded to 11G but not to glutamate. Bottom right: traces of the [Ca2+]i responses of these cells. Arrows indicate the time points at which the images were taken. Bars under traces indicate stimulus application. Scale bar = 50 μm. 1G = 1 mM glucose. Results are representative of four human islet preparations.
(B) Gene expression profiling of individual α cells (left) and β cells (right) from human (red circles) and monkey (black circles) islets. Each row represents a single cell; each column represents a different glutamate receptor gene. Black or red circles denote that RT-PCR products were detected.
Neither kainate (100 μM), glutamate (100 μM), nor AMPA (100 μM) stimulated increases in insulin release in human (Figure 1E) and monkey (data not shown) islets. These effects of iGluRs agonists were similar at all glucose concentrations tested (1 mM, 3 mM, and 11 mM).
α Cells Express Functional iGluRs of the AMPA/Kainate Type
Human and monkey islets as well as dispersed single islet cells loaded with the Ca2+ indicator Fura-2 showed increases in cytoplasmic free Ca2+ concentration ([Ca2+]i) in response to glutamate (100 μM). [Ca2+]i responses to glutamate were concentration dependent (Figure 1F) and could be blocked by CNQX (10 μM) (see below). The range of glutamate concentrations that elicited [Ca2+]i responses was similar to that reported for neurons in the central nervous system (Hollmann and Heinemann, 1994; Seeburg, 1993). Cells that responded to glutamate also responded to kainate (100 μM) with large increases in [Ca2+]i (n = 12 of 12 cells). Cells that responded to glutamate in terms of increases in [Ca2+]i did not respond to high glucose concentrations (11 mM; n = 43 of 43 cells; Figure 2A), and cells that responded to high glucose did not respond to glutamate (n = 64 of 64 cells; Figure 2A). Using immunofluorescence after [Ca2+]i imaging, we found that most glutamate-responsive cells were glucagon immunoreactive (n = 34 of 37 cells; n = 4 human preparations; Figure 2A). None of the insulin-immunoreactive cells responded to kainate (n = 8 of 8), but most of the glucagon-immunoreactive cells did (n = 7 of 9). Similar results were obtained with monkey islets (data not shown).
Single-cell RT-PCR experiments showed that transcripts for the AMPA/kainate genes GRIA2, GRIA3, and GRIK2 (Figure 2B) could be detected consistently in cells identified as α cells (positive for glucagon but not insulin, somatostatin, or pancreatic polypeptide; n = 7 human cells, n = 5 monkey cells). By contrast, in identified β cells (positive for insulin only) we could not detect transcripts for AMPA/kainate genes (Figure 2B). Transcripts for the metabotropic glutamate receptor 4 gene (GRM4) were found in 2 of 7 human α cells, but not in monkey α cells or in β cells of either species (Figure 2B). None of the examined cells contained transcripts for the metabotropic glutamate receptor 5 gene (GRM5). These data indicate that human and monkey α cells, but not β cells, express functional iGluRs of the AMPA/kainate type.
Effects Induced by AMPA/Kainate Receptor Activation
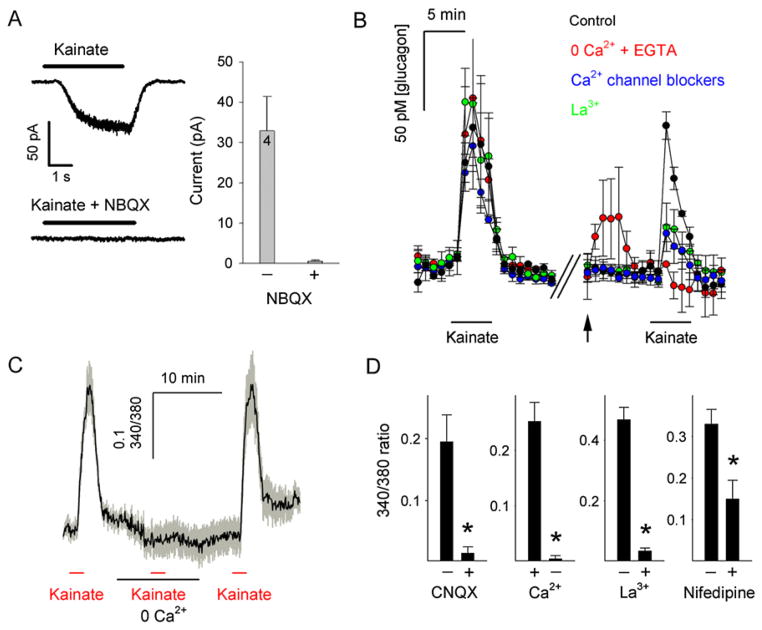
How does activation of AMPA/kainate iGluRs lead to glucagon secretion? Whole-cell patch-clamp recordings on isolated human islet cells showed that application of kainate (100 μM) elicited inward currents that could be blocked by NBQX (10 μM), an AMPA/kainate receptor antagonist (Figure 3A). [Ca2+]i responses to kainate were blocked by CNQX (10 μM) and were abolished in the absence of extracellular Ca2+ and by La3+ (30 μM), a potent blocker of voltage-gated Ca2+ channels (Figures 3C and 3D). The L-type Ca2+ channel blocker nifedipine (10 μM) reduced kainate-induced [Ca2+]i responses by ~60% (Figure 3D). We further investigated whether these mechanisms are involved in kainate-induced glucagon secretion. Using perifusion assays to detect hormone secretion, we found that kainate-stimulated glucagon secretion was abolished in the absence of extracellular Ca2+ (Figure 3B). La3+ (30 μM) and a combination of selective Ca2+ channel inhibitors greatly diminished (>90%) the glucagon response to kainate. These results indicate that kainate elicited glucagon secretion by activating inward currents through AMPA/kainate iGluRs (Hollmann and Heinemann, 1994; Mayer and Armstrong, 2004), which depolarize the α cell plasma membrane, resulting in Ca2+ influx through voltage-gated Ca2+ channels and consequently an increase in [Ca2+]i.
Figure 3. α Cell Responses to Kainate Require Ca2+ Influx through Voltage-Dependent Ca2+ Channels.
(A) Activation of ionotropic glutamate receptors (iGluRs) elicits inward currents in human islet cells. Top left: a representative whole-cell current evoked by kainate (100 μM). Bottom left: the kainate-evoked current could be blocked with the AMPA/kainate receptor antagonist NBQX (10 μM). Right: the amplitudes of these currents averaged for four cells. Holding potential = −70 mV. Bars over current traces indicate kainate application.
(B) Perifusion assays show that kainate (100 μM) stimulated large increases in glucagon secretion (left) that were abolished in the absence of extra-cellular Ca2+ (0 Ca2+ + 1 mM EGTA) and strongly diminished in the presence of the Ca2+ channel blocker La3+ (30 μM) or a combination of the specific Ca2+ channel inhibitors nimodipine (10 μM), conotoxin GVIA (1 μM), agatoxin IVA (0.1 μM), and mibefradil (1 μM). Average traces are shown (n = 3 human islet preparations). Arrow indicates switch to new solution.
(C) An averaged trace (n = 9 cells; three monkey islet preparations) showing that [Ca2+]i responses to kainate were abolished at nominal 0 Ca2+.
(D) [Ca2+]i responses to kainate were inhibited by CNQX (10 μM, n = 7 cells) and the Ca2+ channel blockers La3+ (30 μM, n = 14 cells) and nifedipine (10 μM, n = 18 cells). Shown are the means of the peak amplitudes of the [Ca2+]i responses (changes in the 340/380 fluorescence emission ratio) to kainate. Data are from three separate monkey islet preparations. *p < 0.05 by Student’s t test.
Error bars represent ±SEM.
Primate α Cells Secrete Glutamate
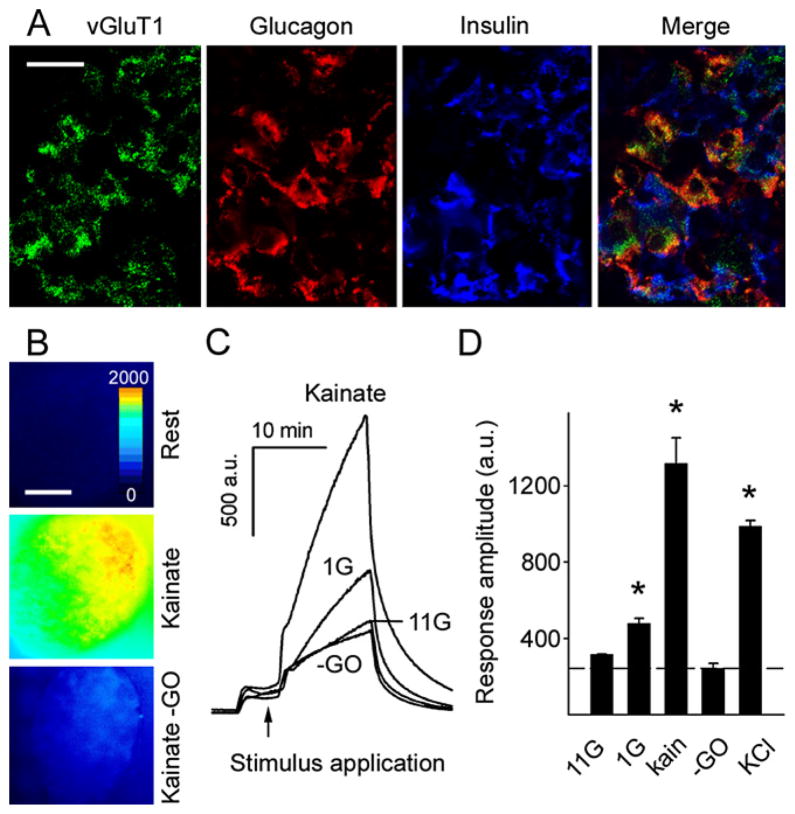
There are several putative sources of glutamate in islets, e.g., nerve terminals and endocrine cells. We therefore investigated whether glutamate is released from the glucagon-containing α cells. Cells capable of vesicular release of glutamate express vesicular glutamate transporters (vGluTs; Fremeau et al., 2004). Our RT-PCR results indicated that primate islets express vGluT1 and vGluT2 (data not shown). To investigate the localization of vGluTs in primate islets, we performed multiple immunostaining on human and monkey pancreatic sections (Figure 4A). In line with previous studies in rat (Hayashi et al., 2003a, 2003b), we found that α cells, but not insulin-containing β cells, were immunoreactive for vGluT1 (Figure 4A). These results suggest that α cells are a major source of glutamate within the primate islet.
Figure 4. Stimulated Primate α Cells Release Glutamate.
(A) Confocal images of a monkey pancreatic section containing an islet. Immunoreactivity for the vesicular glutamate transporter 1 (vGluT1) colocalized with glucagon but not insulin immunostaining. Results are representative of three human pancreata. Scale bar = 20 μm.
(B) Representative images of islets in experiments using a fluorescent enzymatic assay to detect glutamate release. In this assay, released glutamate is a substrate in an enzymatic chain reaction that generates the fluorescent product resorufin. Resorufin fluorescence is color coded; an increase from low (blue, rest) to high (yellow, kainate) indicates increased glutamate release in response to kainate. There was no fluorescence increase in the absence of the enzyme glutamate oxidase (−GO, bottom panel). Scale bar = 50 μm.
(C and D) In the absence of the enzyme glutamate oxidase (−GO), application of kainate and KCl did not increase resorufin fluorescence. Low glucose (1 mM, 1G; p = 0.005), kainate (100 μM, kain; p < 0.001), and KCl (30 mM; p < 0.001) depolarization, but not high glucose (11 mM, 11G; p = 0.289), induced significant glutamate release from islets as compared to kainate without GO (−GO; n = 3 monkey islet preparations; one-way ANOVA followed by multiple-comparisons procedure by Student-Newman-Keuls method). a.u. = arbitrary units. Results in (D) are presented as mean ± SEM.
To visualize glutamate release directly, we adapted an enzymatic assay for microfluorometric detection of extracellular glutamate (Akagi et al., 2003) and performed this assay using isolated cultured islets (Figure 4B). When the glucose concentration was lowered from 6 mM to 1 mM, fluorescence intensity increased significantly, indicating that glutamate was released (Figures 4C and 4D). By contrast, stimulation with high glucose concentrations (11 mM) did not induce glutamate secretion (Figures 4C and 4D). When stimulated with kainate (100 μM), a stimulus specific for α cells (see above), islets strongly released glutamate (Figures 4B–4D). KCl (30 mM) depolarization also elicited large increases in glutamate release (Figure 4D). Fluorescent signals were small in the absence of the glutamate-sensitive enzyme glutamate oxidase (Figures 4B–4D), indicating that the large increases in fluorescence intensity in this assay depended on the release of glutamate.
To determine whether neuronal elements could contribute to the glutamate signal, we examined isolated islets for the presence of the neuronal markers synapsin, neuron-specific enolase, neurofilament 200, and neurofilament H. We found few if any labeled fibers, boutons, or cells (data not shown), in agreement with a previous study showing that very few nerve terminals survive overnight islet culture (Karlsson et al., 1997). Because we performed our experiments using cultured islets, we conclude that intraislet glutamate is mainly derived from stimulated primate α cells.
Glutamate Signaling Provides Positive Feedback for Glucagon Secretion
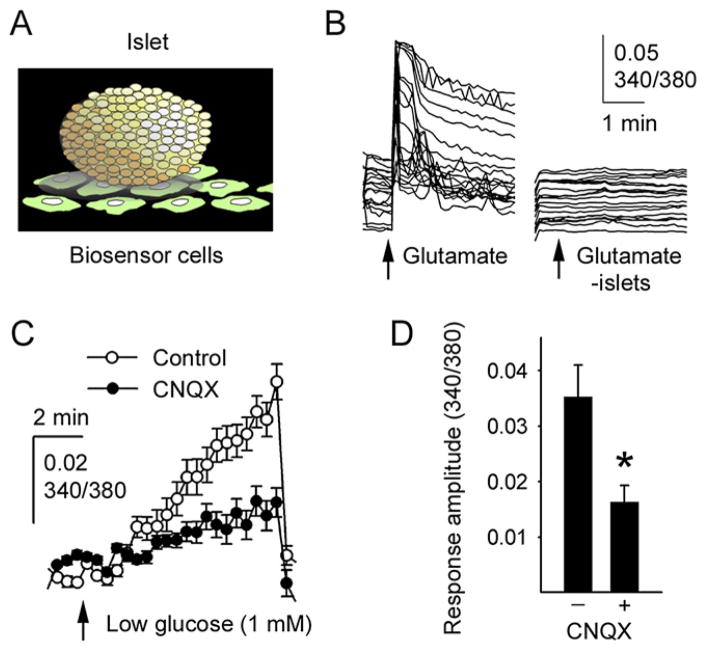
Our results indicate that α cells express iGluRs and secrete glutamate, suggesting that glutamate is an autocrine signal. We hypothesized that a decrease in glucose concentration activates a glutamate feedback loop that potentiates glucagon secretion. If so, applying an iGluR antagonist should reduce the glucagon response. Instead of testing this hypothesis with perifusion assays, which may not be sufficiently sensitive to detect responses to low concentrations of glucose (Hope et al., 2004), we decided to use a more sensitive assay, namely detection of glucagon release by biosensor cells. We placed human islets on a layer of biosensor cells expressing glucagon receptors (see Experimental Procedures) (EC50 for glucagon = 30 pM) (Figure 5A). The biosensor cells express cyclic nucleotide-gated channels, which are activated by elevated intracellular levels of cAMP subsequent to stimulation of the glucagon receptors, resulting in membrane depolarization and Ca2+ influx. Glucagon release from islets was monitored in real time by recording [Ca2+]i responses from the biosensor cells loaded with the [Ca2+]i indicator Fura-2. In the absence of islets, biosensor cells did not respond to glutamate (100 μM) (Figure 5B) or a change in glucose concentrations (data not shown). When human islets were placed on the biosensor cells, the biosensor cells showed large [Ca2+]i responses to glutamate application (Figure 5B), indicating that glucagon release was induced. Lowering the glucose concentration from 6 mM to 1 mM also induced glucagon release as measured by the [Ca2+]i responses in biosensor cells (Figure 5C). The glucagon response to decreasing the glucose concentration was inhibited by CNQX (10 μM) (54% reduction; Figure 5D). Hence, we conclude that activation of iGluRs on α cells is necessary for prompt glucagon secretion when glucose concentration decreases.
Figure 5. A Stimulatory Autocrine Glutamate Feedback Loop Is Needed for Effective Glucagon Release.
(A) Illustration of the experimental approach used to measure islet glucagon secretion in real time using glucagon-sensitive biosensor cells.
(B) Exogenous glutamate elicited glucagon secretion from human islets as measured by [Ca2+]i responses in individual glucagon biosensor cells (traces at left). No responses were seen in the glucagon biosensor cells in the absence of human islets (traces at right).
(C) Lowering the glucose concentration from 6 mM to 1 mM (arrow) induced glucagon secretion as measured by [Ca2+]i responses in biosensor cells (n = 6 regions of interest). Rinsing caused an abrupt decrease in [Ca2+]i in biosensor cells. Results in (C) and (D) are presented as mean ± SEM.
(D) Quantification of the data in (C) shows that the AMPA/kainate iGluR antagonist CNQX (10 μM) significantly inhibited the effect of glucose lowering on glucagon release by 54% (measured 8 min after reducing the glucose concentration; n = 3 islet preparations; *p = 0.042 by Student’s t test).
iGluRs on α Cells Contribute to In Vivo Glucose Homeostasis
We have found that activation of AMPA/kainate receptors is among the strongest stimuli for glucagon secretion in vitro. To determine whether activation of iGluRs on α cells contributes to in vivo glucose homeostasis, we performed experiments in mice. Mouse islets in vitro secreted glucagon, not insulin, in response to AMPA/kainate iGluR activation (Figures 6A and 6D). The metabotropic receptor agonists trans-ACPD (100 μM) and ACPT-1 (100 μM) and the metabotropic receptor antagonist CPPG (100 μM) did not affect basal or glutamate-induced glucagon secretion (Figure 6B). Moreover, glucagon responses to glutamate were indistinguishable in islets from wild-type and mGluR4 knockout mice (Figure 6C). Therefore, mouse α cells, but not β cells, responded to glutamate mainly via activation of iGluRs. These results indicate that glutamate signaling in mouse islets is similar to that in human islets.
Figure 6. Glutamate Signaling in Mouse Islets Is Similar to that in Primate Islets.
(A) Left: perifusion assays of mouse islets showed that glutamate (100 μM) stimulated glucagon release that was blocked by DNQX (10 μM) (n = 6 perifusions). Right: kainate and AMPA (both 100 μM) also stimulated glucagon secretion (n = 3 perifusions).
(B) Left: the metabotropic glutamate receptor agonists tACPD (100 μM) and ACPT-1 (100 μM) did not elicit changes in glucagon secretion. Right: the metabotropic glutamate receptor antagonist CPPG (100 μM) did not affect the glutamate-induced glucagon response (n = 3 perifusions).
(C) Glucagon responses to glutamate (100 μM) in islets from mice lacking the metabotropic glutamate receptor mGluR4 were not different from those of islets from control mice (n = 4 islet preparations per group).
(D) Insulin release was induced by high glucose (11 mM, 11G) but not by kainate (left) or by AMPA or glutamate (right) (n = 3 islet preparations).
Results are presented as mean ± SEM.
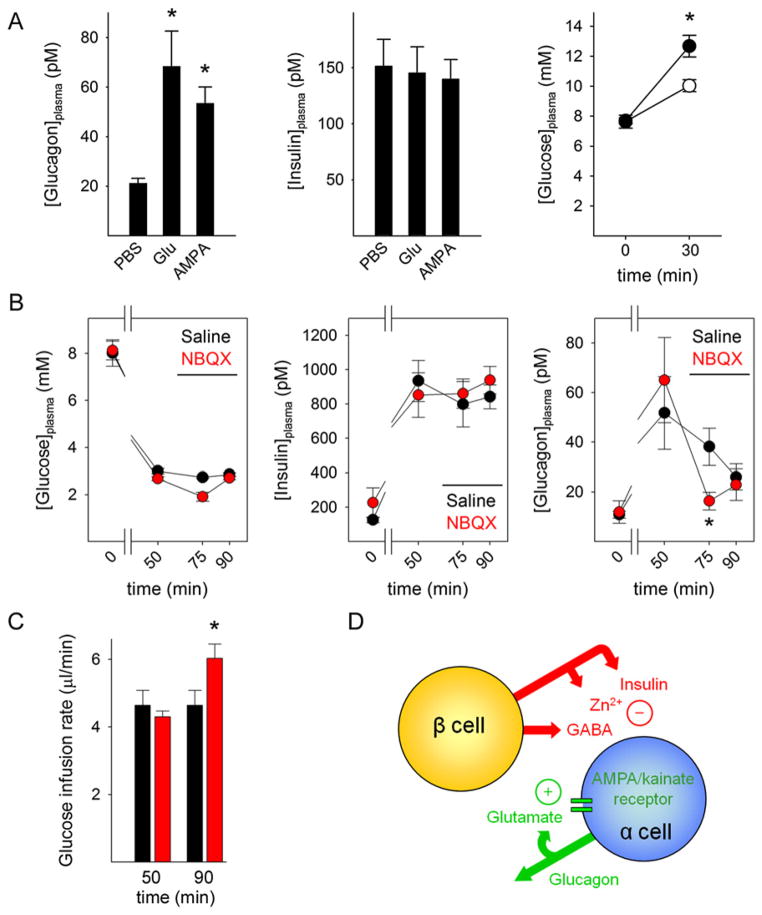
To determine whether in vivo activation of AMPA/kainate receptors affects glucagon secretion, we injected mice with glutamate and the AMPA receptor-specific agonist AMPA. Mice injected intraperitoneally (i.p.) with glutamate (30 mg/kg i.p.) and AMPA (15 mg/kg i.p.) showed increased plasma glucagon concentrations at 30 min after treatment (Figure 7A). Plasma insulin concentrations were not affected. Concomitantly, these mice had increased glucose levels (Figure 7A), which should be the result of the increased glucagon secretion. Glutamate and AMPA most likely did not have central effects because glutamate does not penetrate the blood-brain barrier (Beyreuther et al., 2007) and, at the concentrations used, AMPA did not induce convulsions indicative of central nervous system penetration (Arnt et al., 1995). Although we cannot rule out effects on peripheral neurons, the collective interpretation of our results strongly suggests that we indeed directly activated AMPA/kainate receptors on α cells and thereby stimulated glucagon secretion in mice in vivo.
Figure 7. In Vivo Activation of iGluRs Stimulates Glucagon Secretion.
(A) Left: mice treated systemically with glutamate (30 mg/kg i.p.; n = 7 mice) or AMPA (15 mg/kg i.p.; n = 8 mice) showed increased plasma glucagon concentrations (*p < 0.05 by ANOVA). Middle: plasma insulin concentrations did not change. Right: 30 min after AMPA injection, AMPA-treated mice showed increased plasma glucose concentrations (●, n = 8 mice; *p < 0.05 by Student’s t test) as compared to PBS-injected mice (○, n = 4 mice). Results in (A)–(C) are presented as mean ± SEM.
(B) Hyperinsulinemic-hypoglycemic clamp to provide a constant hypoglycemic stimulus at a blood glucose concentration of ~3 mM (left) was induced with insulin infusion (middle). Glucagon secretion in response to hypoglycemia was significantly diminished in mice after NBQX infusion (right) (10 mg/kg; red circles, n = 7) compared with saline-infused mice (black circles, n = 5; *p < 0.05 by repeated-measures ANOVA). Horizontal bar indicates drug infusion.
(C) The glucose infusion rate needed to maintain glycemia after drug infusion was significantly larger in NBQX-treated mice (red bars, n = 7) than in saline-treated mice (black bars, n = 5). *p < 0.05 by Student’s t test.
(D) Proposed model for the regulation of glucagon secretion. Activation of α cells depends on an initial stimulus as well as on positive feedback. When glucose levels fall, there is less suppression from β cell-derived GABA, Zn2+, or insulin. Positive feedback by glutamate strongly amplifies glucagon secretion. Once glucose levels increase, glucagon secretion is inhibited by insulin, Zn2+, GABA, or a combination of the three. Without glutamate feedback, α cells are not fully activated and glucagon secretion is deficient.
Increased glucagon secretion from α cells in the endocrine pancreas plays a primary role in glucose counterregulation (Banarer et al., 2002; Rizza et al., 1979). We investigated whether α cells require positive glutamate feedback loops to produce a full glucagon response to a lowering in glucose concentration. In the initial experiments, mice were injected with insulin (1.5 U/kg i.p.) to induce hypoglycemia. In these mice, systemic treatment with the AMPA/kainate iGluR antagonist NBQX (20 mg/kg i.p.) increased the insulin-induced drop in plasma glucose levels (data not shown). At this concentration, NBQX did not cause sedation, suggesting that the treatment impaired glucose counterregulation without central effects on autonomic functions (Lees, 2000).
To examine whether blocking AMPA/kainate receptors diminishes the counterregulatory response to systemic hypoglycemia, we used the hyperinsulinemic-hypoglycemic clamp technique (Figure 7B). This technique provides a standardized hypoglycemic stimulus whereby plasma glucose concentrations can be held at a desired level of glycemia (~3 mM in our study). Mice treated systemically with the AMPA/kainate iGluR antagonist NBQX (10 mg/kg intravenous; n = 7) needed larger infusion rates of glucose to maintain the desired level of glycemia, suggesting that, compared to control, saline-injected mice (n = 5), their counterregulatory response was diminished (Figure 7C). Indeed, we found that plasma glucagon levels were lower in NBQX-treated mice, indicating that activation of iGluRs is needed for efficient glucagon secretion in response to hypoglycemia (Figure 7B).
DISCUSSION
Our results firmly establish glutamate as a bona fide autocrine signaling molecule in α cells providing positive feedback for glucagon secretion in human islets. We found that glutamate is secreted by α cells and that α cells, not β cells, express iGluRs of the AMPA/kainate type. Activation of these receptors by α cell-derived glutamate generates positive feedback for α cell function and amplifies glucagon secretion. This autocrine signaling pathway helps explain how a modest decrease in blood glucose concentration effectively induces glucagon release from the pancreatic α cell.
Our results demonstrating that glutamate provides a positive feedback for glucagon release are in agreement with studies showing that glutamate stimulates glucagon secretion (Bertrand et al., 1993) but contrast with reports suggesting that the effect of glutamate is inhibitory and mediated by mGluR4 receptors (Moriyama and Hayashi, 2003). Glutamate was excitatory in all of our physiological experiments (e.g., [Ca2+]i imaging, dynamic hormone secretion assays, and in vivo plasma glucagon detection), and responses could be blocked by AMPA/kainate receptor antagonists, indicating that stimulation of glucagon release via iGluRs is the predominant effect of the glutamate autocrine feedback loop. Our findings showing that agonists and antagonists for metabotropic glutamate receptors did not affect glucagon secretion demonstrate that these receptors are not involved in glutamate signaling in human α cells. Because glucagon responses to glutamate were not altered in mice lacking mGluR4 receptors, we further conclude that this receptor likely does not contribute to α cell responses to glutamate as suggested previously (Uehara et al., 2004).
In contrast with previous results in rodents (Gonoi et al., 1994; Inagaki et al., 1995; Moriyama and Hayashi, 2003; Weaver et al., 1996), we could not find evidence for iGluRs in β cells in any of the three species examined. The discrepancies with our results may be explained by species differences and the use of different methods. It is important to note, however, that immunofluorescence studies have so far provided most of the evidence for localization of iGluRs in β cells (Muroyama et al., 2004; Weaver et al., 1996). These studies have not been verified with in situ hybridization or single-cell RT-PCR. It is therefore difficult to ascertain whether the reported immunostaining patterns represent iGluRs in β cells, particularly given that immunohistochemistry can yield spurious results (Saper and Sawchenko, 2003). In our hands, for instance, islet cell immunostaining with antibodies recognizing the AMPA receptor subunits GluR2 and GluR3 was not specific—that is, it could not be blocked by peptide preadsorption of the antibody. Because of the modest effects of glutamate receptor ligands on insulin secretion and the low incidence of glutamate-responsive β cells in previous studies, authors have hesitated to ascribe a major functional role to glutamate receptors in the regulation of insulin secretion (Molnar et al., 1995). This is in line with our single-cell RT-PCR results showing that β cells did not express iGluRs. Our findings showing that β cell activity and insulin secretion could not be stimulated with glutamate receptor agonists further strongly suggest that glutamate signaling and in particular iGluRs are not involved in the regulation of insulin secretion.
To be relevant for paracrine or autocrine signaling, glutamate must be released from islet cells in response to physiological stimulation. Studies using rodent islets have shown that α cells express vesicular glutamate transporters that facilitate glutamate uptake into secretory vesicles (Hayashi et al., 2001) and that glutamate is present in glucagon secretory granules and is coreleased with glucagon (Hayashi et al., 2003c). In the present study, we found that human and monkey α cells express the vesicular glutamate transporter vGluT1. We detected glutamate secretion in response to specific stimulation of α cells, confirming that α cells are a major source of glutamate in primate islets. Although neurons or nerve terminals could also release glutamate, our results were obtained using cultured isolated islets that did not contain neuronal elements (see also Karlsson et al., 1997). Because we found that α cells secrete glutamate and because iGluRs were exclusively expressed in α cells, the most likely scenario is that glutamate is an autocrine signaling molecule. Our results showing that blockade of iGluRs diminishes the glucagon response to a lowering in glucose concentration (see Figure 5) indicate that glutamate is endogenously released and corroborate that glutamate signaling provides a positive autocrine feedback loop.
The notion of an autocrine loop with positive feedback helps explain how α cells respond appropriately to a lowering in plasma glucose concentration. By using the hypoglycemic clamp in mice, we stimulated glucagon secretion in vivo and found that glucagon response was significantly diminished by pharmacological blockade of iGluRs. In these mice, hypoglycemia was exacerbated, indicating that iGluRs need to be activated for a full glucagon response in the context of glucose counterregulation. While we cannot rule out any additional effects that glutamate may have on central and peripheral neurons that could indirectly affect α cells, our in vitro data demonstrate that the α cell is a major direct target for glutamate and that intraislet glutamate signaling may function independently of nervous input. Therefore, the most straightforward interpretation of our results is that the glutamate autocrine feedback loop is activated in α cells to potentiate glucagon secretion.
We now put forward a model that at least in part explains how glucagon release can be effectively regulated in human pancreatic islets (Figure 7D). When there is a lowering in blood glucose concentration, the negative paracrine influence of β cells on α cells decreases (the “switch-off” hypothesis; Cryer et al., 2003; Hope et al., 2004; Zhou et al., 2004). At this stage, the glucose concentration is too low to inhibit (MacDonald et al., 2007; MacDonald and Rorsman, 2007) but still high enough to drive (Olsen et al., 2005) partial glucagon release and in parallel glutamate release, creating a positive feedback loop through iGluRs that potentiates α cell secretory activity. This is in agreement with our results showing that pharmacological blockade of iGluRs exacerbates insulin-induced hypoglycemia and reduces glucagon secretion in vivo. An interesting possibility is that in the absence of functional β cells, there is chronic release of glutamate, desensitizing α cell iGluRs. This may explain the lack of effective glucose counterregulation in individuals with diabetes.
EXPERIMENTAL PROCEDURES
Islet Isolation and Culture
Human (n = 12, age = 48 ± 7 years), monkey (Macaca fascicularis; n = 15; age > 4 years), and young adult mouse (C57BL/6; n = 15) islets were isolated and cultured as described elsewhere (Cabrera et al., 2006). Mutant mice lacking the metabotropic glutamate receptor mGluR4 (C57BL/6 background) were purchased from The Jackson Laboratory. All experimental protocols using monkeys and mice were approved by the University of Miami Animal Care and Use Committee.
Determination of Cytoplasmic Free Ca2+
Imaging of [Ca2+]i was performed as described elsewhere (Cabrera et al., 2006). Islets or dispersed islet cells were immersed in HEPES-buffered solution (in mM: 125 NaCl, 5.9 KCl, 2.56 CaCl2, 1 MgCl2, 25 HEPES; 0.1% BSA [pH 7.4]). Glucose was added to yield a final concentration of 3 mM. Islets or dispersed islet cells were incubated in Fura-2 AM (2 μM) for 1 hr and placed in a closed small-volume imaging chamber (Warner Instruments). Stimuli were applied with the bath solution. Islets loaded with Fura-2 were excited alternately at 340 and 380 nm with a Cairn Research Optoscan Monochromator light source. Images were acquired with a Hamamatsu camera attached to a Zeiss Axiovert 200 microscope. Changes in the 340/380 fluorescence emission ratio over time were analyzed in individual islets and dispersed cells using Kinetic Imaging AQM Advance software. Peak changes in the fluorescence ratio constituted the response amplitude.
Dynamic Measurements of Glucagon and Insulin Secretion
A high-capacity, automated perifusion system was developed to dynamically measure hormone secretion from pancreatic islets. A low-pulsatility peristaltic pump pushed HEPES-buffered solution (in mM: 125 NaCl, 5.9 KCl, 2.56 CaCl2, 1 MgCl2, 25 HEPES; 0.1% BSA [pH 7.4]) at a perifusion rate of 100 μl/min through a column containing 100 pancreatic islets immobilized in Bio-Gel P-4 Gel (Bio-Rad). Except where stated otherwise, glucose concentration was adjusted to 3 mM for all experiments. Stimuli were applied with the perifusion buffer. The perifusate was collected in an automatic fraction collector designed for a 96-well plate format. The columns containing the islets and the perifusion solutions were kept at 37°C, and the perifusate in the collecting plate was kept at <4°C. Perifusates were collected every minute. Hormone release in the perifusate was determined with the human or mouse Endocrine LINCOplex Kit (Linco Research) following the manufacturer’s instructions.
Real-Time Determination of Glucagon Release with Biosensor Cells
HEK293H-CNG cells stably expressing human glucagon receptor (glucagon biosensor cells) (BD Biosciences) were used to measure glucagon release in real time. The BD ACTOne technology (BD Biosciences) uses a modified cyclic nucleotide-gated (CNG) ion channel as a biosensor of cAMP activity in live cells, allowing the fluorescent detection of Gs-coupled receptor activities. The CNG channel colocalizes with adenylate cyclases on the plasma membrane and opens when the cAMP level near the plasma membrane increases subsequent to activation of the human glucagon receptor. The influx of Ca2+ through the CNG channel is quantified using fluorescent Ca2+ indicators. Glucagon elicited concentration-dependent [Ca2+]i increases in glucagon biosensor cells that were detectable at ~10 pM and saturated at ~300 pM.
Glucagon biosensor cells were grown in DMEM supplemented with FBS (10%; Invitrogen), puromycin (10 mg/ml; BD Biosciences), and G418 sulfate (500 mg/ml; Mediatech). Glucagon biosensor cells were loaded with Fura-2 AM (2 μM) for 30 min at 37°C. After washing off excess Fura-2 AM, pancreatic islets were placed on top of the glucagon biosensor cells and mounted in a closed small-volume imaging chamber (Warner Instruments) to measure [Ca2+]i as described above. Perifusion of the islets was stopped after addition of the stimulus to allow the islet secretory products to accumulate and then resumed after 10 min. The glucagon response profiles of the islets obtained with this technique resembled those obtained in perifusion studies (compare Figure 1 and Figure 6), but the biosensor cell assay was more sensitive and showed less variability.
Microfluorometric Determination of Endogenous Glutamate Release
Endogenous glutamate release from pancreatic islets was measured with the Amplex Red Glutamic Acid Assay Kit (Molecular Probes) adapted for microscopic microfluorometric detection (Akagi et al., 2003). In this assay, released glutamate is a substrate in an enzymatic chain reaction that generates the highly fluorescent product resorufin. L-glutamate is oxidized by glutamate oxidase to produce α-ketoglutarate, NH4, and H2O2. Hydrogen peroxide reacts with the Amplex Red reagent in a reaction catalyzed by horseradish peroxidase to generate resorufin. Pancreatic islets were allowed to attach to a glass coverslip previously coated with poly-D-lysine. The coverslip was mounted in a closed small-volume imaging chamber (Warner Instruments) and perifused with the HEPES-buffered solution containing the stimuli. Resorufin fluorescence was excited at 510 nm, and the emission was recorded at 590 nm using the same imaging system used to measure [Ca2+]i (see above).
Patch-Clamp Electrophysiology
Membrane currents were measured using whole-cell patch-clamp recordings of dispersed human islet cells. All recordings were amplified using an EPC10 patch-clamp amplifier and digitized and analyzed using Patchmaster software (HEKA Elektronik). Pipettes were pulled from borosilicate glass capillaries on a horizontal programmable puller (DMZ Universal Puller, Zeitz-Instrumente). Pipettes with a resistance of 5–7 MΩ were filled with a pipette solution containing (in mM) 150 N-methyl-D-glucamine (NMG), 10 EGTA, 1 MgCl2, 2 CaCl2, 5 HEPES, and 3 MgATP (pH 7.15). The bath solution contained (in mM) 138 NaCl, 5.6 KCl, 1.2 MgCl2, 2.6 CaCl2, 5 HEPES, and 10 tetraethylammonium (pH 7.4). Cells were voltage clamped at −70 mV. Ligands (e.g., kainate) were applied using the SF-77B Perfusion Fast-Step system (Warner Instruments), which allows rapid change of solutions bathing a single cell or membrane patch attached to a patch electrode. We did not attempt to identify cell type based on electrophysiological characterization because >75% of the kainate-responsive cells in our [Ca2+]i imaging experiments were glucagon-immunoreactive cells. Thus, it is most likely that the cells responding to kainate in our electrophysiological experiments were α cells.
RT-PCR
Total RNA from >90% pure human and monkey islets was isolated using the QIAGEN RNeasy Kit. cDNA was synthesized from 500 ng of total RNA using the Invitrogen SuperScript First-Strand Synthesis System. cDNA (2 μl) was used without further purification for PCR reaction (35 cycles) with a LightCycler and Roche amplification kit. Primers for human AMPA/kainate (GluR1–7, KA1, and KA2) and vesicular glutamate transporters (vGluT1–3) were purchased from QIAGEN and used at the concentrations suggested by the vendor.
Single-Cell RT-PCR
Human or monkey islets were dispersed into individual cells. Single cells were harvested using glass micropipettes (~20 μm) and placed in centrifuge tubes. mRNA was reverse transcribed using the Invitrogen SuperScript First-Strand Synthesis System. Real-time PCR was performed using TaqMan Fast Universal PCR Master Mix and the 7500 or 7900HT Fast Real-Time PCR System (Applied Biosystems). The TaqMan assays were chosen to span an exon junction and therefore did not detect genomic DNA. Each cDNA was first amplified with TaqMan probes specific for insulin, glucagon, somatostatin, and pancreatic polypeptide. Cells exclusively expressing one of the pancreatic hormones were used for further PCR to detect the glutamate receptor units GRIA1–4 (AMPA receptor subunits) and GRIK1–5 (kainate receptor subunits) and the metabotropic glutamate receptors 4 and 5 (GRM4 and GRM5).
Immunofluorescence
Immunofluorescence procedures were performed as described elsewhere (Cabrera et al., 2006). Blocks of human, monkey, or mouse pancreas (0.5 cm3) or isolated islets were fixed in 4% paraformaldehyde for 4–6 hr and frozen. Sections (14 μm) were cut on a cryostat. After a rinse with OptiMax Wash Buffer (Biogenex), sections were incubated in Universal Blocker Reagent (Biogenex) for 5–10 min, rinsed again in OptiMax Wash Buffer, and incubated in Protein Block (Biogenex) for 20 min. Thereafter, sections were incubated overnight with anti-insulin (1:500; Accurate Chemical & Scientific Corp.), anti-glucagon (1:2,000; Sigma), anti-somatostatin (1:500; Serotec), and anti-pancreatic polypeptide (1:100; Serotec) antibodies. To visualize vesicular glutamate transporters, sections were incubated in anti-vGluT1 (1:20,000; Chemicon), anti-vGluT2 (1:10,000; Chemicon), or anti-vGluT3 (1: 20,000; Chemicon) together with antisera against insulin and glucagon. To visualize neuronal elements, isolated human islets were immunostained by applying rabbit anti-synapsin 1/2 (1:500; Synaptic Systems), mouse anti-neuron-specific enolase (1:500; Chemicon), rabbit anti-neurofilament 200 (1:100; Sigma), or chicken anti-neurofilament H (1:200; Neuromix). Immunostaining was visualized using Alexa-conjugated secondary antibodies (1:400; Molecular Probes). Cell nuclei were stained with DAPI (Molecular Probes). Slides were mounted with ProLong Antifade (Molecular Probes) and coverslipped. As a negative control, we substituted the primary antibody with the serum of the animal used to raise that antibody. No staining was observed under these conditions. Pancreatic sections containing islets were examined for expression of the different endocrine markers and vGluTs using a Zeiss LSM 510 scanning confocal microscope. We chose an optical section of 1 μm for all image acquisitions. All images were digitally acquired and were not further processed. Sections were viewed at 20× and 40× magnification. Digital images were compiled using Adobe Photoshop 7.0. Only brightness and contrast were adjusted.
In Vivo Studies
We examined in vivo responses to iGluR agonists by injecting C57BL/6 mice i.p. with PBS (n = 4), monosodium glutamate (30 mg/kg; n = 7), or AMPA (15 mg/kg; n = 8). To determine whether blocking iGluRs exacerbates insulin-induced hypoglycemia, we injected the AMPA/kainate iGluR antagonist NBQX (Tocris) i.p. at 20 mg/kg 30 min before mice were treated with insulin to induce hypoglycemia.
Hyperinsulinemic-Hypoglycemic Clamp in Conscious Mice
To provide a standardized hypoglycemic stimulus, we further conducted studies using the hyperinsulinemic-hypoglycemic clamp. At least 4 days before the experiments, mice were anesthetized with isoflurane, and an indwelling catheter was inserted in the left jugular vein and externalized through an incision in a skin flap behind the head. Mice were fasted for 4 hr and placed in individual plastic containers for tail cut sampling. Tail blood samples (20 μl) were taken before the start of the experiment for determination of basal plasma glucagon and insulin secretion. A priming dose of insulin (100 mU/kg) was administered, followed by a constant infusion rate of 20 mU/kg/min (Actrapid, Novo Nordisk). The plasma glucose concentration was determined at 10 min intervals using a OneTouch Ultra glucometer. Glucose (30%) was infused at a variable rate to maintain the plasma glucose concentration at hypoglycemic levels (~3 mM). At 50 min, a hyperinsulinemic-hypoglycemic clamp was achieved. Blood glucose levels were kept at the steady state (3 mM), and NBQX was administered as a bolus (10 mg/kg) at 60 min followed by infusion (10 mg/kg) until the end of the experiment.A control experiment was performed using saline (0.9% NaCl) following the same protocol used for NBQX. Tail blood samples (20 μl) were taken at 0, 50, 75, and 90 min for determination of plasma glucagon and insulin secretion. Animals were euthanized by an overdose of pentobarbital.
Statistical Analyses
Statistical comparisons were performed using Student’s t test, one-way ANOVA, or repeated-measures ANOVA followed by multiple-comparison procedures with the Student-Newman-Keuls method.
Acknowledgments
This work was supported in part by NIH General Clinical Research Center grants MO1RR16587 and 1R01-DK55347-IU42RR016603, Islet Cell Resources grant 5U42RR016603, NIDDK grant 1R03DK075487, and Mouse Metabolic Phenotyping Center grant U24 DK59635; Juvenile Diabetes Research Foundation International grants 4-2004-361, 3-2006-853, and 3-2007-73 (to S.S.); the European Foundation for the Study of Diabetes; EuroDia grant LSHM-CT-2006-518153; the Diabetes Research Institute Foundation; the Swedish Research Council; the Novo Nordisk Foundation; the Swedish Diabetes Association; Berth von Kantzow’s Foundation; and the Family Erling-Persson Foundation. We thank G.L. Shulman and M. Gilbert for assistance with in vivo experiments and comments on the manuscript. We are further grateful to K. Johnson for technical assistance and to the members of the Human Cell Processing Facility, Translational Research Laboratory of the Cell Transplant Center, Clinical Islet Transplant Center, Organ Procurement Organizations, the ICR Basic Science Islet Distribution Program, and administrative offices at the University of Miami Diabetes Research Institute. M.C.J.-S. is a recipient of a Juvenile Diabetes Research Foundation Post-Doctoral Fellowship.
References
- Akagi Y, Hashigasako A, Degenaar P, Iwabuchi S, Hasan Q, Morita Y, Tamiya E. Enzyme-linked sensitive fluorometric imaging of glutamate release from cerebral neurons of chick embryos. J Biochem. 2003;134:353–358. doi: 10.1093/jb/mvg152. [DOI] [PubMed] [Google Scholar]
- Arnt J, Sanchez C, Lenz SM, Madsen U, Krogsgaard-Larsen P. Differentiation of in vivo effects of AMPA and NMDA receptor ligands using drug discrimination methods and convulsant/anticonvulsant activity. Eur J Pharmacol. 1995;285:289–297. doi: 10.1016/0014-2999(95)00422-h. [DOI] [PubMed] [Google Scholar]
- Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes. 2002;51:958–965. doi: 10.2337/diabetes.51.4.958. [DOI] [PubMed] [Google Scholar]
- Bertrand G, Gross R, Puech R, Loubatieres-Mariani MM, Bockaert J. Evidence for a glutamate receptor of the AMPA subtype which mediates insulin release from rat perfused pancreas. Br J Pharmacol. 1992;106:354–359. doi: 10.1111/j.1476-5381.1992.tb14340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand G, Gross R, Puech R, Loubatieres-Mariani MM, Bockaert J. Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. Eur J Pharmacol. 1993;237:45–50. doi: 10.1016/0014-2999(93)90091-u. [DOI] [PubMed] [Google Scholar]
- Bertrand G, Puech R, Loubatieres-Mariani MM, Bockaert J. Glutamate stimulates insulin secretion and improves glucose tolerance in rats. Am J Physiol. 1995;269:E551–E556. doi: 10.1152/ajpendo.1995.269.3.E551. [DOI] [PubMed] [Google Scholar]
- Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, Kempski O, Stehle P, Steinhart H, Walker R. Consensus meeting: monosodium glutamate - an update. Eur J Clin Nutr. 2007;61:304–313. doi: 10.1038/sj.ejcn.1602526. [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gonoi T, Mizuno N, Inagaki N, Kuromi H, Seino Y, Miyazaki JI, Seino S. Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. J Biol Chem. 1994;269:16989–16992. [PubMed] [Google Scholar]
- Gopel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. Regulation of glucagon release in mouse -cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol. 2000;528:509–520. doi: 10.1111/j.1469-7793.2000.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Otsuka M, Morimoto R, Hirota S, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y. Differentiation-associated Na+-dependent inorganic phosphate cotransporter (DNPI) is a vesicular glutamate transporter in endocrine glutamatergic systems. J Biol Chem. 2001;276:43400–43406. doi: 10.1074/jbc.M106244200. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Morimoto R, Yamamoto A, Moriyama Y. Expression and localization of vesicular glutamate transporters in pancreatic islets, upper gastrointestinal tract, and testis. J Histochem Cytochem. 2003a;51:1375–1390. doi: 10.1177/002215540305101014. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Otsuka M, Morimoto R, Muroyama A, Uehara S, Yamamoto A, Moriyama Y. Vesicular inhibitory amino acid transporter is present in glucagon-containing secretory granules in alphaTC6 cells, mouse clonal alpha-cells, and alpha-cells of islets of Langerhans. Diabetes. 2003b;52:2066–2074. doi: 10.2337/diabetes.52.8.2066. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamada H, Uehara S, Morimoto R, Muroyama A, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y. Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem. 2003c;278:1966–1974. doi: 10.1074/jbc.M206758200. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP. Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes. 2004;53:1488–1495. doi: 10.2337/diabetes.53.6.1488. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Kuromi H, Gonoi T, Okamoto Y, Ishida H, Seino Y, Kaneko T, Iwanaga T, Seino S. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J. 1995;9:686–691. [PubMed] [Google Scholar]
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Myrsen U, Nieuwenhuizen A, Sundler F, Ahren B. Presynaptic sympathetic mechanism in the insulinostatic effect of epinephrine in mouse pancreatic islets. Am J Physiol. 1997;272:R1371–R1378. doi: 10.1152/ajpregu.1997.272.5.R1371. [DOI] [PubMed] [Google Scholar]
- Kisanuki K, Kishikawa H, Araki E, Shirotani T, Uehara M, Isami S, Ura S, Jinnouchi H, Miyamura N, Shichiri M. Expression of insulin receptor on clonal pancreatic alpha cells and its possible role for insulin-stimulated negative regulation of glucagon secretion. Diabetologia. 1995;38:422–429. doi: 10.1007/BF00410279. [DOI] [PubMed] [Google Scholar]
- Lees GJ. Pharmacology of AMPA/kainate receptor ligands and their therapeutic potential in neurological and psychiatric disorders. Drugs. 2000;59:33–78. doi: 10.2165/00003495-200059010-00004. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Rorsman P. The ins and outs of secretion from pancreatic beta-cells: control of single-vesicle exo- and endocytosis. Physiology (Bethesda) 2007;22:113–121. doi: 10.1152/physiol.00047.2006. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PR, Cox R, Eliasson L, Rorsman P. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 2007;5:e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- Molnar E, Varadi A, McIlhinney RAJ, Ashcroft SJH. Identification of functional ionotropic glutamate receptor proteins in pancreatic beta-cells and in islets of Langerhans. FEBS Lett. 1995;371:253–257. doi: 10.1016/0014-5793(95)00890-l. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Hayashi M. Glutamate-mediated signaling in the islets of Langerhans: a thread entangled. Trends Pharmacol Sci. 2003;24:511–517. doi: 10.1016/j.tips.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Muroyama A, Uehara S, Yatsushiro S, Echigo N, Morimoto R, Morita M, Hayashi M, Yamamoto A, Koh DS, Moriyama Y. A novel variant of ionotropic glutamate receptor regulates somatostatin secretion from delta-cells of islets of Langerhans. Diabetes. 2004;53:1743–1753. doi: 10.2337/diabetes.53.7.1743. [DOI] [PubMed] [Google Scholar]
- Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, Gromada J. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology. 2005;146:4861–4870. doi: 10.1210/en.2005-0800. [DOI] [PubMed] [Google Scholar]
- Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes. 2005;54:1789–1797. doi: 10.2337/diabetes.54.6.1789. [DOI] [PubMed] [Google Scholar]
- Rizza RA, Cryer PE, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979;64:62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, Berggren PO, Bokvist K, Ericson H, Mohler H, Ostenson CG, Smith PA. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The TiPS/TINS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Pharmacol Sci. 1993;14:297–303. doi: 10.1016/0165-6147(93)90047-N. [DOI] [PubMed] [Google Scholar]
- Storto M, Capobianco L, Battaglia G, Molinaro G, Gradini R, Riozzi B, Di Mambro A, Mitchell KJ, Bruno V, Vairetti MP, et al. Insulin secretion is controlled by mGlu5 metabotropic glutamate receptors. Mol Pharmacol. 2006;69:1234–1241. doi: 10.1124/mol.105.018390. [DOI] [PubMed] [Google Scholar]
- Uehara S, Muroyama A, Echigo N, Morimoto R, Otsuka M, Yatsushiro S, Moriyama Y. Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by alpha-cells of islet of Langerhans. Diabetes. 2004;53:998–1006. doi: 10.2337/diabetes.53.4.998. [DOI] [PubMed] [Google Scholar]
- Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28:574–578. doi: 10.1007/BF00281991. [DOI] [PubMed] [Google Scholar]
- Weaver CD, Yao TL, Powers AC, Verdoorn TA. Differential expression of glutamate receptor subtypes in rat pancreatic islets. J Biol Chem. 1996;271:12977–12984. doi: 10.1074/jbc.271.22.12977. [DOI] [PubMed] [Google Scholar]
- Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes. 2004;53:1038–1045. doi: 10.2337/diabetes.53.4.1038. [DOI] [PubMed] [Google Scholar]
- Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP. Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes. 2004;53:1482–1487. doi: 10.2337/diabetes.53.6.1482. [DOI] [PubMed] [Google Scholar]