Although we have witnessed major improvements in genomic testing in the past 5 years, multiple challenges need to be solved before clinical genomic testing becomes routine (1). The clinical implementation of genomic testing parallels the challenges we have faced with other transformative technologies. For the past decade, the medical community has been discussing the impact on healthcare and society of direct-to-consumer marketed whole-body computed tomography (CT)8 scanning (2). The CT scan is a technology that uses X-rays to produce cross-sectional images of the human body. There are clinical scenarios in which the CT scan is invaluable: disease diagnosis, evaluation after trauma, and monitoring response to oncologic therapy. However, CT scan is also being marketed as a preventive diagnostic for healthy individuals (3). Genomics can parallel CT scanning in scenarios of (a) first line diagnostic testing, (b) last ditch diagnosis, and (c) inappropriate clinical use.
Genomic testing implies the examination of the nucleic acid sequence of multiple, if not all, known genes. Broadly, this includes microarray tests that survey changes in single nucleotide polymorphisms (SNPs) and copy-number variation across the entire genome, sequencing the exome or whole genome by next-generation sequencing, and sequencing large panels of clinically significant genes. Genomic testing is currently moving from academic research practice to commercialization and direct-to-consumer marketing. Although genomic testing is not yet routine, DNA testing targeted to single genes, viruses, or bacteria is commonplace and performed in many clinical laboratories. The use of genetic information has evolved over the past decade from targeted single-gene tests to broader genomic tests that encompass thousands of genes. Numerous ethical, economic, and technological challenges need to be addressed before widespread adoption of clinical genomic testing. For example, a patient may not want to know their future risk of disease; a patient (and society) may not be able to afford genomic testing. In addition, genomic testing may not be appropriate for all diseases.
Genomic Testing Is a First-line Diagnostic in Specific Scenarios
Whole-body CT scans are clearly indicated in scenarios such as trauma (4). Similarly, the use of microarrays is now recognized as a first-tier diagnostic test by the International Standard Cytogenomic Array Consortium to replace conventional cytogenetics in the diagnosis of individuals with developmental disabilities or congenital anomalies (5). In 2014, the FDA cleared the first chromosomal microarray test system (CytoScan® Dx, Affymetrix). CytoScan Dx is to be used in the context of clinical and other diagnostic findings for the diagnosis of chromosomal changes associated with “developmental delay, intellectual disability, congenital anomalies, or dysmorphic features” [FDA 510(k) approval date 1/17/2014; K130313].
Next-generation DNA sequencing enables testing of tens to thousands of genes at a cost that previously would have only enabled testing of several genes by traditional Sanger sequencing (6). For example, 1 study examined 141 patients with a familial or personal history of breast cancer who were negative for BRCA1/2 by genetic testing (7). These patients were retested by a next-generation sequencing panel test that included BRCA1/2 plus 40 additional genes. Sixteen patients were found to have pathogenic variants identified in 9 genes (not BRCA1/2) (7). The genetic changes identified by next-generation sequencing changed the management of 15 of the 16 patients. Indeed, for hereditary breast and ovarian cancer, the 2014 National Comprehensive Cancer Network has recommended next-generation gene sequencing panels when patients have tested negative for high-penetrance genes (8).
Genomic Testing May Be Appropriate as a Last-ditch Diagnostic
Sometimes patients may present with a metastatic cancer for which the organ of origin cannot be determined (cancer of unknown primary). In this deadly scenario, whole-body CT scan is a sensitive tool for the identification of the cancer primary (9). Similarly, for patients with ultra-rare genetic diseases or genetic diseases that defy clinical diagnosis, there has been anecdotal evidence that sequencing a patient’s exome or whole genome may detect a genetic defect that may lead to possible intervention to improve patient health. One example with exome sequencing was the identification of a pathogenic variant in XIAP in a 15-month-old patient with life-threatening inflammatory bowel disease (10). Because of the patient’s severity of disease and the known high mortality of XIAP defects, the patient underwent a successful bone marrow transplant. In fact, the National Institutes of Health Undiagnosed Diseases Program has demonstrated the potential utility of genomic testing in patients who cannot be diagnosed by conventional methods (11).
Genomic Testing Is Not a Universal Diagnostic Test
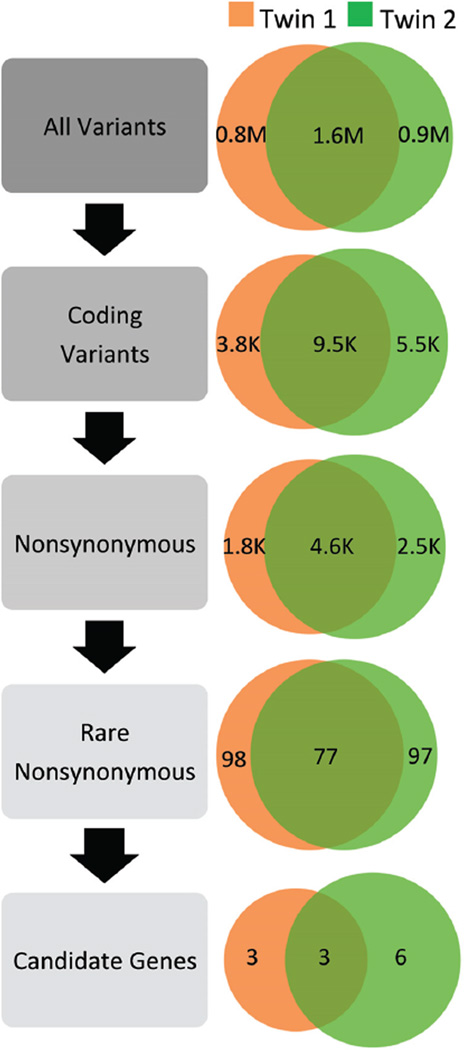
A clear controversy of whole-body CT scanning is the use of the technology for screening of healthy individuals. This type of screening may incidentally discover benign lesions that may have costly further evaluation with potential physical and mental harm (12). Similarly, the current state of genomic testing guarantees the incidental discovery of DNA variants with possible pathogenic disease association. Guidelines that have been proposed for the management of secondary findings from genomic studies have been controversial because of the potential of limiting patient autonomy (13). However, an additional complexity of genomic testing is that the numbers of genes in the human genome that are well understood remain in the minority. Of the approximately 23000 genes in the human genome, only 6137 genes are described in comprehensive databases such as the Human Gene Mutation Database (14). Thus, when we test for approximately 23000 genes in an exome study, we are guaranteed to find thousands of variants of unknown significance that are not related to the patient’s current health (15). As illustrated by a recent twin study, 9500 variants from the reference sequence could be narrowed down to 77 variants on the basis of predicted changes to amino acids and known population frequency consistent with disease (Fig. 1). However, extensive review of the literature and clinical correlation was required to select 3 likely candidate genes. Interpreting and reporting the pathogenicity of DNA variation will be a key challenge to the broader implementation of genomics in medicine (16).
Fig. 1. DNA variation present within the genomes of fraternal twins with the same genetic disease. Venn diagrams represent the number of DNA nucleotide variants that are both shared and unique to each twin at each stage of data analysis.
Compared to a standard reference genome (hg18) the twins share 1.6 million variants. Of these variants, 9500 are in protein coding regions and approximately half are predicted to result in changes at the protein level (4600; nonsynonymous). On the basis of population data on DNA variation, a subset of 77 variants are seen with a population frequency appropriate for a rare genetic disease. On the basis of extensive review of the literature and clinical correlation, 3 likely candidate genes were selected by the authors of the study. M, million; K, thousand. [Adapted from data presented by Bainbridge et al. (15).]
Conclusions
Genomics in clinical medicine is here to stay and has proven to be a useful diagnostic tool in specific clinical scenarios. However, genomic technology is not mature and is not appropriate in all clinical scenarios. Substantial research into the quality, cost-effectiveness, and societal implications is still needed before genomic testing becomes a widespread diagnostic tool. As a starting point, the CDC has a list that classifies genetic and genomic testing into tiers on the basis of the current level of evidence supporting their use (17).
Multiple issues need to be addressed as we implement genomics into everyday medicine. As a medical technology, genomics has similar challenges that have been seen before with whole-body CT scanning: diagnostic utility, incidental finding, and cost-effectiveness. In addition, the interpretation of genomic analysis is still in its infancy; we do not have a full understanding of the disease association of all genes. Finally, genetic information informs us about the potential disease status of not only the individual patient tested, but all related individuals; these data will have additional burdens in ethics and privacy that exceed CT scanning.
Footnotes
Nonstandard abbreviations: CT, computed tomography; SNP, single nucleotide polymorphism.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: No authors declared any potential conflicts of interest.
References
- 1.Chrystoja CC, Diamandis EP. Whole genome sequencing as a diagnostic test: challenges and opportunities. Clin Chem. 2014;60:724–733. doi: 10.1373/clinchem.2013.209213. [DOI] [PubMed] [Google Scholar]
- 2.Lee TH, Brennan TA. Direct-to-consumer marketing of high-technology screening tests. N Engl J Med. 2002;346:529–531. doi: 10.1056/NEJM200202143460715. [DOI] [PubMed] [Google Scholar]
- 3.Full-body CT scans - what you need to know. [Accessed July 2014]; http://www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/Medical Imaging/MedicalX-Rays/ucm115340.htm.
- 4.Huber-Wagner S, Lefering R, Qvick LM, Korner M, Kay MV, Pfeifer KJ, et al. Effect of whole-body CT during trauma resuscitation on survival: a retrospective, multicentre study. Lancet. 2009;373:1455–1461. doi: 10.1016/S0140-6736(09)60232-4. [DOI] [PubMed] [Google Scholar]
- 5.Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Gotway G, Pascual JM, Park JY. Diagnostic yield of clinical next-generation sequencing panels for epilepsy. JAMA Neurol. 2014;71:650–651. doi: 10.1001/jamaneurol.2014.405. [DOI] [PubMed] [Google Scholar]
- 7.Kurian AW, Hare EE, Mills MA, Kingham KE, McPherson L, Whittemore AS, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.53.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCCN clinical practice guidelines in oncology (NCCN guidelines) Genetic/familial high-risk assessment: breast and ovarian. Version 1.2014: National Comprehensive Cancer Network. 2014 [Google Scholar]
- 9.Hemminki K, Liu H, Heminki A, Sundquist J. Power and limits of modern cancer diagnostics: cancer of unknown primary. Ann Oncol. 2012;23:760–764. doi: 10.1093/annonc/mdr369. [DOI] [PubMed] [Google Scholar]
- 10.Worthey EA, Mayer AN, Syverson GD, Helbling D, Bonacci BB, Decker B, et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med. 2011;13:255–262. doi: 10.1097/GIM.0b013e3182088158. [DOI] [PubMed] [Google Scholar]
- 11.Gahl WA, Markello TC, Toro C, Fajardo KF, Sincan M, Gill F, et al. The National Institutes of Health undiagnosed diseases program: insights into rare diseases. Genet Med. 2012;14:51–59. doi: 10.1038/gim.0b013e318232a005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumbreras B, Donat L, Hernandez-Aguado I. Incidental findings in imaging diagnostic tests: a systematic review. Br J Radiol. 2010;83:276–289. doi: 10.1259/bjr/98067945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormick JB, Sharp RR, Farrugia G, Lindor NM, Babovic-Vuksanovic D, Borad MJ, et al. Genomic medicine and incidental findings: balancing actionability and patient autonomy. Mayo Clin Proc. 2014;89:718–721. doi: 10.1016/j.mayocp.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Human Gene Mutation Database. [Accessed July 2014]; http://www.hgmd.org. [Google Scholar]
- 15.Bainbridge MN, Wiszniewski W, Murdock DR, Friedman J, Gonzaga-Jauregui C, Newsham I, et al. Whole-genome sequencing for optimized patient management. Sci Transl Med. 2011;3:87re3. doi: 10.1126/scitranslmed.3002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, et al. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–476. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genetic testing: genomic tests and family history by levels of evidence. [Accessed July 2014]; http://www.cdc.gov/genomics/gtesting/tier.htm.