Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) is amenable to early detection and specialty care. Thus, while important to patients with the condition, end-stage renal disease (ESRD) from ADPKD may also be an indicator of the overall state of nephrology care.
Study Design
Retrospective cohort study of temporal trends in renal replacement therapy (RRT)-requiring ESRD from ADPKD and pre-RRT nephrologist care, 2001-2010 (n = 23,772).
Setting & Participants
US patients who initiated maintenance RRT between 2001 and 2010 (n = 1,069,343), from United States Renal Data System data.
Predictor
RRT-requiring ESRD from ADPKD.
Outcomes
Death, wait-listing for renal transplant, renal transplant.
Measurements
US census data were used as population denominators. The Poisson distribution was used to compute incidence rates. Incidence ratios were standardized to rates in 2001-2002 for age, sex, and race/ethnicity. Patients with and without ADPKD were matched to compare clinical outcomes. Poisson regression was used to calculate incidence rates and adjusted hazards ratios for clinical events after inception of RRT.
Results
General population incidence ratios in 2009-2010 were unchanged from 2001-2002 (incidence ratio 1.02). Of patients with ADPKD, 48.1% received > 12 months of nephrology care before RRT; preemptive transplant was the initial RRT in 14.3% and fistula the initial hemodialysis access in 35.8%. Over 4.9 years of follow-up, patients with ADPKD were more likely to be listed for transplant (11.7 [95% CI 11.5-12.0] per 100 person-years vs. 8.4 [8.2-8.7]) and to undergo transplant (9.8 [9.5-10.0] vs. 4.8 [4.7-5.0]), and less likely to die (5.6 [5.4-5.7] vs. 15.5 [15.3-15.8]) than matched controls without ADPKD.
Limitations
Retrospective, nonexperimental, registry-based study of associations; cause-and-effect relationships cannot be determined.
Conclusions
While outcomes on dialysis are better for ADPKD than for non-ADPKD patients, access to predialysis nephrology care and non-declining ESRD rates may be a cause for concern.
Keywords: Dialysis, end-stage renal disease, polycystic kidney disease, renal replacement therapy, renal transplant
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary form of kidney disease and has been described in all racial and ethnic groups. In the United States, for example, ADPKD is thought to be responsible for one in every twenty cases of end-stage renal disease (ESRD) requiring renal replacement therapy (RRT).1 Enumerating the clinical epidemiology of ESRD from ADPKD could be useful for several reasons. While much research is ongoing in genetics and in diagnostic and therapeutic domains, up-to-date disease-specific information on risk factors and outcomes of ESRD from ADPKD would help long-term decision-making for at-risk individuals. In addition, nationally representative epidemiological data could help with trial design and cost-benefit analysis of innovative interventions designed specifically to slow disease progression.2-4 From a broader perspective, ADPKD differs from most other causes of ESRD because it can be detected early in life. Hence, it has the potential to illuminate issues such as non-disease-specific interventions to prevent ESRD and patterns of nephrology care in late-stage chronic kidney disease.
As information of this nature is surprisingly sparse, we set out to describe the clinical epidemiology of ESRD from ADPKD in the United States from 2001 to 2010. The principal objectives of this study were to elucidate trends in incidence ratios, standardized to rates in 2001-2002, of ESRD due to ADPKD requiring RRT in the US from 2001 to 2010. Regarding clinical outcomes after beginning RRT, we set out to compare rates of wait-listing for renal transplant, transplant, and death in matched patients with and without ADPKD. An additional objective was to calculate hazards ratios for these outcomes specific to patients with ADPKD.
Methods
Subjects
In this retrospective study, United States Renal Data System (USRDS) standard analysis files were used to evaluate US patients who initiated maintenance RRT between 2001 and 2010 (n = 1,069,343). Baseline characteristics at initiation of RRT were obtained from the Centers for Medicare & Medicaid (CMS) Medical Evidence Report (form CMS-2728), and corresponding data fields residing in the USRDS Medevid95 and Medevid05 files. By federal requirement, the Medical Evidence Report must be submitted for all new maintenance RRT patients in the US. The form underwent structural changes in 1995 and 2005. Unlike previous versions, the 2005 version collects information regarding duration of nephrologist care before RRT initiation and hemodialysis vascular access at initiation. In both the 1995 and 2005 versions, one of 82 causes is entered as the primary cause of ESRD; options are identical between forms.
Defining ADPKD
Cases of ESRD due to ADPKD were those with primary cause of ESRD listed as “Polycystic kidneys, autosomal dominant” on the Medical Evidence Report (form CMS-2728).
Covariates
The covariates used for analysis were obtained from the USRDS Medevid95 and Medevid05 files and included sex; ethnicity defined as Hispanic or Latino or not Hispanic or Latino; race defined as white, black or African American, and other defined as non-white, non-African American; and several comorbid conditions, including atherosclerotic heart disease and any history of diabetes. Information on prior ESRD therapy, including nephrology care and access type at initiation, was obtained from the Medevid05 file only, as previous versions did not include this information. Continuous variables including albumin, creatinine, and hemoglobin were converted to categorical variables for analysis. Body mass index expressed as kg/m2 was calculated based on reported height and weight in the Medevid95 and Medevid05 files. Two eras used for comparison were defined as 2001-2005 and 2006-2010.
Outcome Assessment
Dates of death and first renal transplant were obtained from the USRDS Patients file, and first listing for transplant from the USRDS Waitlist_ki and Waitlist_kp files.5 Using death as an outcome of interest, the time interval was defined as the [Death date] – [Date of dialysis initiation]. Specific outcomes of interest included dates of death, wait-listing for renal transplant, and renal transplant.
Analysis
US census data were used as population denominators for the years examined, with age in 5-year increments and race/ethnicity classified as non-Hispanic white, non-Hispanic black, Hispanic, and other.6 The Poisson distribution was used to compute incidence rates of RRT-requiring ESRD due to ADPKD. For standardized incidence ratios (with observed rates as numerator and expected rates as denominator), expected incidence rates were calculated by applying incidence rates from 2001-2002 to each individual permutation of age, sex, race, and ethnicity to the corresponding subgroup of the US population in subsequent 2-year periods. Chi-square analysis was used for unadjusted comparisons of patients with and without ESRD due to ADPKD, and logistic regression with adjustment for age, sex, race, and ethnicity was used for adjusted comparisons. Percentages of missing values were calculated and reported. To compare clinical outcome rates of patients with and without ADPKD, patients were matched according to age (in 1-year intervals), sex, race, and ethnicity. Poisson regression was used to calculate incidence rates and adjusted hazards ratios for clinical events after inception of RRT, with follow-up ending on June 30, 2011. Person-time was calculated with start time as first service date and end of follow-up time as the date of first occurrence of the outcome of interest, death, or survival to June 30, 2011. Similar calculations for follow-up time were performed for listing for renal transplant and transplant. SAS, v9.1.3 (Cary, North Carolina) was used for data analysis.
Results
In 2001-2002, 4282 patients began RRT because of ESRD due to ADPKD, a rate of 7.5 cases per million per year (Table 1); rates were higher for groups characterized by age 40-64 (17.3) and ≥ 65 years (15.3), and non-Hispanic white (8.2) and African American race/ethnicity (7.8). The mean age (standard deviation) at initiation in 2001-2002 was 55.7 (13.2) years and remained largely unchanged over time. For the overall population, standardized incidence ratios (SIRs) exceeded 1 for all biennia after 2003-2004, with significant increases in 2005-2006 (1.07) and in 2007-2008 (1.06). For patients aged 40-64 years, the ratios increased and remained significantly higher in 2003-2004 (1.11) and in 2005-2006 (1.16), 2007-2008 (1.16), and 2009-2010 (1.11). The SIR appeared to decrease for patients of Hispanic ethnicity in the most recent biennia (0.84), and to increase significantly for patients of other race/ethnicity in 2007-2008 (1.64) and 2009-2010 (1.48).
Table 1.
Rates (2001-2002) and Standardized Incidence Ratios (2003-2010) of ESRD Due to Autosomal Dominant Polycystic Kidney Disease Requiring Renal Replacement Therapy
| Incidence rate per million, 2001-2002 |
SIR 2003-2004, vs. 2001-2002 |
SIR 2005-2006, vs. 2001-2002 |
SIR 2007-2008, vs. 2001-2002 |
SIR 2009-2010, vs. 2001-2002 |
|
|---|---|---|---|---|---|
| US Population | 286,297,074 | 291,456,616 | 296,948,256 | 302,662,587 | 308,060,609 |
| ADPKD Cases | 4282 | 4423 | 4940 | 5070 | 5055 |
|
| |||||
| Non ADPKD | 334.9 (0.8) | 1 (0) | 1.01 (0) | 0.98 (0)c | 0.97 (0)c |
| ADPKD | 7.5 (0.1) | 0.99 (0.01) | 1.07 (0.02)a | 1.06 (0.01)a | 1.02 (0.01) |
| Age, yrs. | |||||
| Mean (SD) | 55.7 (13.2) | 55.3 (12.7) | 55.6 (12.8) | 55.3 (12.9) | 55.6 (12.9) |
| 0-40 | 1.2 (0.1) | 0.91 (0.05) | 1.07 (0.05) | 1.13 (0.05) | 1.13 (0.06) |
| 40-64 | 17.3 (0.3) | 1.11 (0.02)b | 1.16 (0.02)c | 1.16 (0.02)c | 1.11 (0.02)b |
| ≥ 65 | 15.3 (0.5) | 0.92 (0.03) | 1.02 (0.03) | 0.98 (0.03) | 0.96 (0.03) |
| Sex | |||||
| Men | 8.2 (0.2) | 0.97 (0.02) | 1.08 (0.02)a | 1.06 (0.02) | 1.03 (0.02) |
| Women | 6.8 (0.2) | 1.02 (0.02) | 1.06 (0.02) | 1.06 (0.02) | 1.02 (0.02) |
| Race/ethnicity | |||||
| White | 8.2 (0.1) | 1.01 (0.02) | 1.08 (0.02)a | 1.05 (0.02) | 1.03 (0.02) |
| African American | 7.8 (0.3) | 0.98 (0.04) | 1.1 (0.04) | 1.06 (0.04) | 1.02 (0.04) |
| Hispanic | 5.1 (0.3) | 0.92 (0.05) | 0.96 (0.05) | 0.91 (0.04) | 0.84 (0.04)a |
| Other | 4.3 (0.4) | 0.98 (0.08) | 1.07 (0.08) | 1.64 (0.09)c | 1.48 (0.08)b |
Note: Parameter estimates are either rates per million per year or standardized (to 2001-2002) incidence ratios with standard error (SE) in parentheses. With PE denoting “point estimate” and Obs as “observed incidence rate,” Exp “expected incidence rate from rates seen in 2001-2002,” standardized incidence ratios were calculated and reported as [PEObs/PEExp]. P values refer to comparisons of observed rates and rates expected when those seen in 2001-2002 were applied to the years under consideration. P ≥ 0.05 unless otherwise indicated.
0.01 ≤ P value < 0.05.
0.001 ≤ P value < 0.01.
P value < 0.001.
Table 2 shows comparisons of ESRD patients with and without ADPKD and of patients with ESRD due to ADPKD in two eras. Patients with ESRD from ADPKD were more likely than patients without ADPKD to be aged 40-64 years (68.1% vs. 41.2%), female (46.1% vs. 44.4%), white (72.9% vs. 52.9%), and non-Hispanic (90.7% vs. 86.5%). After adjustment for age, sex, race, and ethnicity, associations of ESRD from ADPKD at baseline included younger age, female sex, white race, non-Hispanic ethnicity, absence of ischemic heart disease, absence of diabetes, peritoneal dialysis and preemptive transplant for RRT, fistulas for hemodialysis, longer duration of nephrologist care, lower estimated glomerular filtration rate (eGFR), lower body mass index, and higher serum albumin and hemoglobin levels. Ranked by magnitude, odds ratios adjusted for age, sex, race, and ethnicity (AORs) for ADPKD were ≥ 2.0 for preemptive transplant (7.2 for living donor transplant and 4.29 for deceased donor transplant vs. hemodialysis) and peritoneal dialysis (2.54 vs. hemodialysis), and ≤ 0.5 for age ≥ 65 years (0.39 vs. < 40 years), African American race (0.26 vs. white), Hispanic ethnicity (0.39 vs. non-Hispanic), diabetes (0.07), serum albumin < 3.5 g/dL (0.19 vs. ≥ 3.5 g/dL), catheter for hemodialysis (0.27 vs. fistula), nephrology care ≤ 12 months (0.35 vs. > 12 months), and ischemic heart disease (0.35). AORs for more recent era of dialysis initiation were highest for eGFR > 15 mL/min/1.73 m2 (2.27), preemptive living donor kidney transplant (1.41), and deceased donor kidney transplant (1.55), and lowest for ischemic heart disease (0.7) and graft for dialysis initiation. No AORs ≤ 0.5 occurred between eras. AORs for preemptive transplant vs. maintenance dialysis were highest for other race (2.61), eGFR > 15 mL/min/1.73 m2 (5.08), and hemoglobin ≥ 9 g/dL (4.33), and ≤ 0.5 for age > 65 years (0.28), African American race (0.2), Hispanic ethnicity (0.3), presence of diabetes (0.26), presence of ischemic heart disease (0.43), and albumin < 3.5 g/dL (0.31).
Table 2.
Baseline Characteristics at Initiation of Renal Replacement Therapy, 2001-2010 (n = 1,069,343)
| All Patients |
Patients with ADPKD |
Preemptive Transplant, ADPKD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADPKD | No ADPKD |
P | AOR ADPKD | 2006- 2010 |
2001- 2005 |
P | AOR 2006- 2010 |
P | Yes | No | AOR, PET | P | |
| n | 23,772 | 1,045,571 | - | 12,656 | 11,116 | - | - | 3403 | 20,369 | ||||
| Dialysis initiation yr. |
|||||||||||||
| 2001-2005 | 46.8 | 47.5b | 0.02 | 1 (Reference) | - | - | - | - | 39.3 | 48 | 1 (Reference) | 0 | |
| 2006-2010 | 53.2 | 52.5 | - | 1.02 (0.99-1.04) | - | - | - | - | 60.7 | 52 | 1.41 (1.31-1.52) | 0 | |
| Age, yrs. | |||||||||||||
| < 40 | 8.4 | 9.2 | 0 | 1 (Reference) | 8.3 | 8.5 | 0.21 | 1 (Reference) | - | 8.5 | 8.3 | 1 (Reference) | 0 |
| 40-64 | 68.1 | 41.2 | - | 1.65 (1.57-1.73) | 68.5 | 67.4 | - | 1.06 (0.96-1.16) | 0.21 | 82.8 | 65.6 | 1.1 (0.96-1.26) | 0 |
| ≥ 65 | 23.6 | 49.6 | - | 0.39 (0.37-0.41) | 23.3 | 24.2 | - | 1.04 (0.94-1.15) | 0.78 | 8.6 | 26.1 | 0.28 (0.23-0.33) | 0 |
| Sex | |||||||||||||
| Men | 53.9 | 55.6 | 0 | 1 (Reference) | 54.3 | 53.4 | 0.20 | 1 (Reference) | - | 53.7 | 54 | 1 (Reference) | 0 |
| Women | 46.1 | 44.4 | - | 1.17 (1.14-1.2) | 45.7 | 46.6 | - | 0.98 (0.93-1.03) | 0.34 | 46.3 | 46 | 1.05 (0.98-1.14) | 0 |
| Race/ethnicity | |||||||||||||
| White | 72.9 | 52.9 | 0 | 1 (Reference) | 70.4 | 72.5 | 0 | 1 (Reference) | - | 81.3 | 71.5 | 1 (Reference) | 0 |
| Black | 13.1 | 28.2 | - | 0.26 (0.25-0.27) | 15.2 | 14 | - | 1.1 (1.02-1.19) | 0.08 | 3.5 | 14.7 | 0.2 (0.16-0.24) | 0 |
| Hispanic | 9.3 | 13.5 | - | 0.39 (0.38-0.41) | 10.7 | 9.7 | - | 1.06 (0.97-1.15) | 0.23 | 3.8 | 10.2 | 0.3 (0.25-0.35) | 0 |
| Other | 4.8 | 5.4 | - | 0.53 (0.5-0.57) | 3.6 | 3,7 | - | 0.98 (0.85-1.13) | 0.30 | 11.5 | 3.7 | 2.61 (2.29-2.97) | 0 |
| Ischemic heart disease |
|||||||||||||
| No | 90.6 | 76.1 | 0 | 1 (Reference) | 91.6 | 88.9 | 0 | 1 (Reference) | - | 97.7 | 89.5 | 1 (Reference) | 0 |
| Yes | 9.4 | 23.9 | - | 0.35 (0.34-0.37) | 8.4 | 11.1 | - | 0.7 (0.64-0.76) | 0 | 2.3 | 10.5 | 0.26 (0.2-0.32) | 0 |
| Diabetes | |||||||||||||
| No | 91.8 | 47.1 | 0 | 1 (Reference) | 91.1 | 93 | 0 | 1 (Reference) | - | 96.4 | 91 | 1 (Reference) | 0 |
| Yes | 8.2 | 52.9 | - | 0.07 (0.07-0.07) | 8.9 | 7 | - | 1.31 (1.19-1.44) | 0 | 3.6 | 9 | 0.43 (0.35-0.52) | 0 |
| Dialysis mode | |||||||||||||
| Hemodialysis | 70.4 | 91.9 | 0 | 1 (Reference) | 68.6 | 73.5 | 0 | 1 (Reference) | - | - | 82.2 | - | |
| Peritoneal dialysis |
15.3 | 6.4 | - | 2.54 (2.44-2.63) | 15.5 | 14.9 | - | 1.12 (1.04-1.20) | 0.04 | - | 17.8 | - | |
| Preemptive transplant |
|||||||||||||
| Living donor | 11.0 | 1.1 | - | 7.2 (6.87-7.54) | 12.4 | 9.3 | - | 1.41 (1.29-1.55) | - | 77.1 | - | - | - |
| Deceased donor | 3.3 | 0.6 | 4.29 (3.97-4.63) | 3.9 | 2.7 | - | 1.55 (1.32-1.82) | 0.001 | 22.9 | - | - | - | |
| Vascular access | |||||||||||||
| Fistula | 35.8 | 13.4 | 0 | 1 (Reference) | 35.9 | 26.3 | 0.68 | 1 (Reference) | - | - | 35.8 | - | |
| Graft | 5.2 | 3.6 | - | 0.62 (0.56-0.68) | 5.2 | 5.3 | - | 0.71 (0.54-0.94) | 0.04 | - | 5.2 | - | |
| Catheter | 59 | 83 | - | 0.27 (0.26-0.28) | 59 | 68.4 | - | 0.9 (0.79-1.04) | 0.43 | - | 59 | - | |
| Predialysis nephrol. care, mo. |
|||||||||||||
| > 12 | 48.1 | 23.7 | 0 | 1 (Reference) | 48.3 | 13.4 | 0 | 1 (Reference) | - | 62.5 | 45.3 | 1 (Reference) | 0 |
| ≤ 12 | 51.9 | 76.3 | - | 0.35 (0.34-0.36) | 51.7 | 86.6 | - | 0.78 (0.7-0.87) | 0 | 37.5 | 54.7 | 0.51 (0.46-0.5) | 0 |
| GFR, mL/min/1.73 m2 |
|||||||||||||
| ≤15 | 93.7 | 87.3 | 0 | 1 (Reference) | 92.1 | 96.4 | 0 | 1 (Reference) | - | 81.6 | 95.7 | 1 (Reference) | 0 |
| >15 | 6.3 | 12.7 | - | 0.44 (0.42-0.47) | 7.9 | 3.6 | - | 2.27 (2.02-2.55) | 0 | 18.4 | 4.3 | 5.08 (4.51-5.71) | 0 |
| BMI, kg/m2 | |||||||||||||
| < 30 | 71.2 | 66.4 | 0 | 1 (Reference) | 68.4 | 74.2 | 0 | 1 (Reference) | - | 72.1 | 71 | 1 (Reference) | 0.214 |
| ≥ 30 | 28.8 | 33.6 | - | 0.64 (0.62-0.66) | 31.6 | 25.8 | - | 1.32 (1.25-1.4) | 0 | 27.9 | 29 | 0.87 (0.8-0.94) | 0 |
| Serum albumin, g/dL |
|||||||||||||
| ≥3.5 | 73.6 | 34 | 0 | 1 (Reference) | 74.4 | 72.5 | 0.00 3 |
1 (Reference) | - | 90.2 | 70.4 | 1 (Reference) | 0 |
| < 3.5 | 26.4 | 66 | - | 0.19 (0.18-0.2) | 25.6 | 27.5 | - | 0.9 (0.85-0.97) | 0.003 | 9.8 | 29.6 | 0.31 (0.27-0.35) | 0 |
| Hemoglobin, g/dL | |||||||||||||
| < 9 | 16 | 26 | 0 | 1 (Reference) | 15.2 | 17 | 0 | 1 (Reference) | - | 4.5 | 18 | 1 (Reference) | 0 |
| ≥ 9 | 84 | 74 | - | 1.8 (1.73-1.87) | 84.8 | 83 | - | 1.15 (1.07-1.23) | 0 | 95.5 | 82 | 4.33 (3.63-5.15) | 0 |
Note: Parameter estimates are presented as column percentages or odds ratios, with 95% confidence intervals in parentheses. Reference categories for binary variables are those without the condition. As data fields for predialysis vascular access for hemodialysis and nephrology care before renal replacement therapy were not available before the 2005 version of the Medical Evidence Report, the denominators for these variables consisted of 58.9% of the study population (n = 14,002) for whom the 2005 version of the form was completed.
Missing data (%) were as follows: access, 46.6%; predialysis nephrology care, 40.9%; eGFR, 0.6%); BMI, 1.4%); Hemoglobin, 8.3%; Albumin, 24.5%.
ADPKD, autosomal dominant polycystic kidney disease; AOR, adjusted odds ratio; BMI, body mass index; eGFR, estimated glomerular filtration rate; PET, preemptive transplant; RRT, renal replacement therapy.
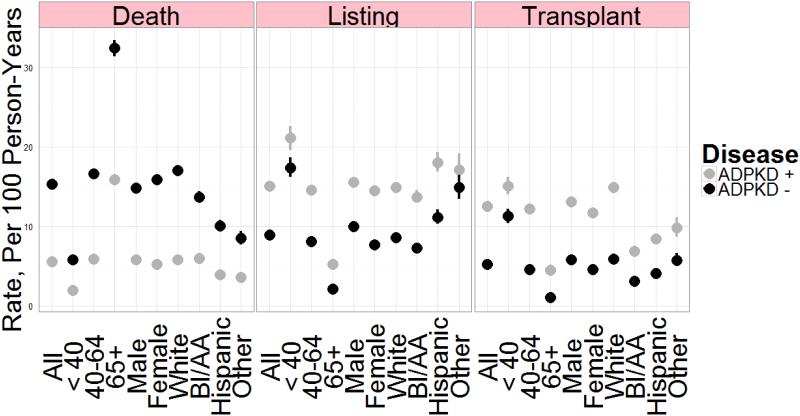
Figure 1 and Table S1 show rates of death, listing for transplant, and transplant in patients with ADPKD and in a cohort of patients without ADPKD matched for age, sex, and race. Listing and transplant rates were higher and mortality rates lower for patients with ADPKD, both overall and within all subgroups examined, except that transplant rates were similar for patients aged < 40 years.
Figure 1.
Rates of death, listing for renal transplant, and transplant in patients with ADPKD (n = 23,619, 99.4% of the ADPKD cohort) and an equal number of matched control patients without ADPKD. Controls were matched by age, sex, and race at initiation of renal replacement therapy. Parameters shown are rates per 100 person-years, with error bars showing 95% confidence intervals. A formal tabulation of numerical estimates appears in Supplemental Table S1. ADPKD, autosomal dominant polycystic kidney disease.
Table 3 shows hazards ratios for death, listing, and transplant adjusted for age, sex, race, and ethnicity in patients with ESRD due to ADPKD. Over a mean follow-up of 4.3 years, 27.9% of ADPKD patients died. Adjusted hazard ratios associated with a greater likelihood of survival were preemptive transplant (0.13 for living donor, 0.21 for deceased donor), initiation in a more recent era (0.86), female sex (0.86), and Hispanic ethnicity (0.73). Factors associated with increased likelihood of death were age 40-64 (1.76 vs. < 40 years) and ≥ 65 (7.19 vs. < 40 year) years, presence of ischemic heart disease (1.69), presence of diabetes (1.52), eGFR > 15mL/min/1.73 m2 at initiation (1.76), and serum albumin < 3.5 g/dL (1.86). Factors associated with decreased likelihood of listing for renal transplant were age 40-64 (0.88) and ≥ 65 (0.21) years, presence of ischemic heart disease (0.61), presence of diabetes (0.69), eGFR > 15 mL/min/1.73m2 (0.74), and albumin < 3.5 g/dL (0.67). Factors associated with decreased likelihood of transplant were age 40-64 (0.92) and ≥ 65 (0.26) years, African American race (0.45), other race (0.68), Hispanic ethnicity (0.54), presence of ischemic heart disease (0.58), presence of diabetes (0.53), nephrology care ≤ 12 months (0.69), eGFR > 15mL/min/1.73 m2 (0.73), body mass index > 30 kg/m2 (0.80), and serum albumin < 3.5 g/dL (0.68).
Table 3.
Adjusted hazards ratios for outcomes on dialysis therapy, patients with ADPKD (n = 23,772)
| Group | Reference | Death | Listing | Transplant |
|---|---|---|---|---|
| 27.9 %/4.3 years | 53 %/1.8 years | 35.7 %/2.9 years | ||
| Initial RRT 2006-2010 | 2001-2005 | 0.86 (0.81-0.92) | 0.99 (0.95-1.04)a | 0.86 (0.82-0.9) |
| Age 40-64 yrs. | < 40 | 1.76 (1.52-2.03) | 0.88 (0.81-0.94) | 0.92 (0.86-1)b |
| Age ≥ 65 yrs. | < 40 | 7.19 (6.22-8.31) | 0.21 (0.19-0.23) | 0.26 (0.23-0.29) |
| Female sex | Male | 0.86 (0.81-0.9) | 0.96 (0.92-1.01)a | 0.93 (0.89-0.97)c |
| African American race | White | 1 (0.93-1.08)a | 0.77 (0.71-0.82) | 0.45 (0.41-0.48) |
| Other race | White | 0.7 (0.61-0.81) | 1.06 (0.95-1.19)a | 0.68 (0.61-0.77) |
| Hispanic ethnicity | Non-Hispanic | 0.73 (0.66-0.81) | 0.96 (0.89-1.03)a | 0.54 (0.5-0.59) |
| Ischemic heart disease | Absent | 1.69 (1.58-1.81) | 0.61 (0.55-0.67) | 0.58 (0.53-0.65) |
| Diabetes | Absent | 1.52 (1.4-1.64) | 0.69 (0.63-0.76) | 0.53 (0.48-0.59) |
| Peritoneal dialysis | Hemodialysis | 0.66 (0.61-0.72) | 1.34 (1.26-1.42) | 1.21 (1.15-1.28) |
| Preemptive transplant | ||||
| Living donor | Hemodialysis | 0.13 (0.10-0.16)c | - | - |
| Deceased donor | Hemodialysis | 0.21 (0.16-0.29)c | - | - |
| Nephrology care ≤ 12 mo.d | ≥12 | 1.09 (1.02-1.16)c | 0.87 (0.82-0.92) | 0.69 (0.65-0.73) |
| eGFR > 15 mL/min/1.73 m2 | ≤15 | 1.76 (1.57-1.98) | 0.74 (0.65-0.85) | 0.73 (0.63-0.84) |
| BMI ≥ 30 kg/m2 | < 30 | 0.84 (0.79-0.89) | 0.97 (0.92-1.02)a | 0.8 (0.76-0.85) |
| Albumin < 3.5 g/dL | ≥ 3.5 | 1.86 (1.76-1.97) | 0.67 (0.63-0.72) | 0.68 (0.64-0.73) |
| Hemoglobin ≥ 9 g/dL | < 9.0 | 0.87 (0.82-0.92) | 1.22 (1.15-1.29) | 1.19 (1.13-1.26) |
Note: Hazards ratios are adjusted for age, sex, race, and ethnicity and are presented with 95% confidence intervals in parentheses. P ≥ 0.05 unless otherwise indicated. ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy.
0.01 ≤P value < 0.05.
0.001 ≤P value< 0.01.
P value < 0.001.
Information available for 58.9% of the cohort (n = 14,002).
Discussion
We found that, when changes in general population demographics were considered, overall incidence rates of ESRD due to ADPKD failed to decline from 2002 on. Estimated GFR levels at dialysis initiation rose between 2001 and 2010, and it is possible that accurate assessment of eGFR at initiation in patients with ADPKD may reflect this population-wide trend, thereby attenuating larger decreases in incident ESRD. This observation is underscored by our analysis of the two eras, as a higher percentage of patients initiated at eGFR ≤ 15 mL/min/1.73m2 in the earlier era (96.4%, 2001-2005; 92.1%, 2006-2010).
The highest rates of ESRD were in patients aged 40-64 years (17.3), with observed increases in the SIR over the biennia of the study. Mean age at RRT onset remained largely unchanged over the timeframe. Compared with patients whose ESRD was from other causes, patients with ESRD from ADPKD were more likely to be middle-aged, white, and free from comorbid illnesses. In addition, they were much more likely to receive predialysis nephrology care, use preemptive transplant or peritoneal dialysis as the first RRT, and use a fistula for hemodialysis. After starting dialysis, patients with ADPKD were substantially more likely to be listed for renal transplant, to undergo transplant, and to survive. These findings are similar to findings of Perrone et al.,7 who used USRDS data to demonstrate that ESRD survival in patients with ADPKD was superior to survival in nondiabetic controls matched for age, sex, and incident ESRD year (relative risk 0.57, P < 0.001). Prominent associations for death, listing for renal transplant, and transplant in the ADPKD population included higher mortality rates for African American than for white patients, and higher rates of listing for transplant and transplant for white patients. Initiation at a higher eGFR level was associated with increased risk of death and with lower risk of listing for and undergoing renal transplant, and may be related to loss of residual renal function associated with initiation of RRT. Considering that ADPKD is a renal disease for which early detection and access to specialized care should exceed most other types of chronic kidney disease, it was disappointing that less than half of the study population had received a year or more of nephrology care before initiating RRT.
Epidemiologic literature regarding the prevalence and incidence of ESRD due to ADPKD is relatively sparse. A study from Olmsted County (Minnesota, USA), incorporating adjustments for age and sex and including autopsy-proven cases, revealed rates of cystic kidney disease of approximately 2.06 per 100,000 patient-years.8 Davies and colleagues estimated the prevalence of ADPKD in a Welsh registry at approximately 1 per 2459 people, with an estimated overall annual ESRD incidence of 4.8 per million.9 Stengel and colleagues estimated incidence rates of ESRD due to ADPKD in several European countries and found estimated rates of 6.0 and 6.9 per million for 1990-1991 and 1998-1999, with higher rates in men, mirroring our study findings.10 In another study spanning 1983-2000, adjusted incidence rates of ESRD due to ADPKD in Japan increased from 3.4 to 5.6 per million in men and from 2.4 to 4.0 per million in women.11 Using data from the Danish National Registry on Regular Dialysis and Transplantation, Orskov and colleagues12 reported that incidence of ESRD due to ADPKD increased between 1990 and 2007, and survival appeared to improve in the later phase of the study.
Our study has several limitations. It was a retrospective, non-experimental, registry-based study of associations, and cause-and-effect relationships cannot be determined. In addition, ADPKD as a diagnostic code has not been validated. For example, to be certain that cases of ESRD were truly due to ADPKD, it would be desirable to have family histories and diagnostic imaging and genotypic information. Dichotomization of responses on form CMS-2728 may lead to underreporting. This is an important consideration regarding predialysis care delivery, as patients who received some nephrology care, but not more than 6 months, may be incorrectly classified as having received none. Regarding the apparent recent decline in ESRD incidence rates in the US, sequential estimation of the true general population denominator of affected individuals would be needed to rule out an explanatory hypothesis such as the decline in ESRD being due to smaller sizes of affected families, use of agents targeting the renin-angiotensin-aldosterone axis, or better control of blood pressure. Recent work by Patch et al.13 demonstrated a mortality benefit with better blood pressure control in patients with ADPKD in the UK; however, only 32% of deaths were observed in patients with ESRD. If these findings prove to be generalizable, it is possible that we identified only a survival proportion of patients whose disease progressed to ESRD, underscoring the need for an accurate population denominator.
Limitation notwithstanding, this study may include some useful elements. For example, the sample size is large and nationally representative, facilitating precise estimates for rates and risk factors. In addition, as a paradigmatic renal disease that can usually be detected early in its natural history, ADPKD may provide valuable insights into the configuration of renal care delivery applied to a continuum spanning preclinical, clinical, and terminal phases. Finally, given these same characteristics of ADPKD as a paradigmatic renal disease, the mortality hazards for preemptive transplant may help inform debate regarding preemptive transplant as opposed to other modes of RRT.
Supplementary Material
Acknowledgments
Contributions: research idea and study design: S.R., R.F., D.S.; data acquisition: S.R., R.F., D.S.; data analysis/interpretation: S.R., R.F., D.S., C.S., S.C., A.C.; statistical analysis: S.R., R.F., D.S.; supervision or mentorship: R.F. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. S.R. takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
The authors thank Chronic Disease Research Group colleagues Delaney Berrini, BS, for manuscript preparation, and Nan Booth, MSW, MPH, ELS, for manuscript editing.
Support
This study was performed as a deliverable under Contract No. HHSN267200715002C (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland). The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Financial Disclosure
The authors have no conflicts of interest with the study’s subject matter.
Reference List
- (1).Collins AJ, Foley RN, Herzog C, et al. U.S. Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61:A7. doi: 10.1053/j.ajkd.2012.11.031. e1-A7,476. [DOI] [PubMed] [Google Scholar]
- (2).Jouret F, Krzesinski JM. Tolvaptan in autosomal dominant polycystic kidney disease. N Engl J Med. 2013;368:1258–1259. doi: 10.1056/NEJMc1300762. [DOI] [PubMed] [Google Scholar]
- (3).Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Stallone G, Infante B, Grandaliano G, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrol Dial Transplant. 2012;27:3560–3567. doi: 10.1093/ndt/gfs264. [DOI] [PubMed] [Google Scholar]
- (5).U.S. Renal Data System Researcher's Guide to the USRDS Database; Appendix D: Data File Descriptions. 2013 Available at: http://www.usrds.org/2013/rg/C_Data_File_Descriptions_13.pdf. Accessed May 20, 2014.
- (6).U.S. Census Bureau State Intercensal Estimates (2000-2010) 2013 Available at: https://www.census.gov/popest/data/intercensal/state/state2010.html. Accessed May 20, 2014. [Google Scholar]
- (7).Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001;38:777–784. doi: 10.1053/ajkd.2001.27720. [DOI] [PubMed] [Google Scholar]
- (8).Iglesias CG, Torres VE, Offord KP, Holley KE, Beard CM, Kurland LT. Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935-1980. Am J Kidney Dis. 1983;2:630–639. doi: 10.1016/s0272-6386(83)80044-4. [DOI] [PubMed] [Google Scholar]
- (9).Davies F, Coles GA, Harper PS, Williams AJ, Evans C, Cochlin D. Polycystic kidney disease re-evaluated: a population-based study. Q J Med. 1991;79:477–485. [PubMed] [Google Scholar]
- (10).Stengel B, Billon S, van Dijk PC, et al. Trends in the incidence of renal replacement therapy for end-stage renal disease in Europe, 1990-1999. Nephrol Dial Transplant. 2003;18:1824–1833. doi: 10.1093/ndt/gfg233. [DOI] [PubMed] [Google Scholar]
- (11).Wakai K, Nakai S, Kikuchi K, et al. Trends in incidence of end-stage renal disease in Japan, 1983-2000: age-adjusted and age-specific rates by gender and cause. Nephrol Dial Transplant. 2004;19:2044–2052. doi: 10.1093/ndt/gfh317. [DOI] [PubMed] [Google Scholar]
- (12).Orskov B, Sorensen VR, Feldt-Rasmussen B, Strandgaard S. Improved prognosis in patients with autosomal dominant polycystic kidney disease in Denmark. Clin J Am Soc Nephrol. 2010;5:2034–2039. doi: 10.2215/CJN.01460210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Patch C, Charlton J, Roderick PJ, Gulliford MC. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: a population-based study. Am J Kidney Dis. 2011;57:856–862. doi: 10.1053/j.ajkd.2011.01.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.