Abstract
Background
Former sleep studies among non-treatment seeking chronic cocaine users had captured polysomnographic changes for as long as three weeks of abstinence.
Methods
20 cocaine dependent participants, randomized to placebo in an ongoing clinical trial, received 12 days of inpatient substance abuse treatment followed by 6 weeks of outpatient cognitive behavioral therapy. Polysomnographic recording was performed on consecutive nights during the 1st and 2nd inpatient and 3rd and 6th outpatient weeks. Number of days abstinent was determined from thrice weekly urine toxicology and self-report. Polysomnographic sleep was compared between study week 1 and 2, using paired t-tests. Trajectory of total sleep time (TST) was modeled both as a linear and a quadratic function of days abstinent.
Results
Despite reporting an improvement in overall sleep quality, polysomnographic sleep worsened from week 1 to 2. Among all participants, TST and stage 2 sleep time decreased, while REM sleep latency increased. Among participants who began the study with a positive urine test, there was also a decrease in REM and a trend for decreased slow wave sleep. TST compared to number of days abstinent (up to 54 days) was best fit with a quadratic model (p = 0.002), suggesting the possibility of an improvement in total sleep time with extended abstinence.
Conclusions
This is the first polysomnographic characterization of sleep in a large sample of cocaine users in treatment. Present findings confirm earlier results of poor and deteriorating sleep early in abstinence, and raise the possibility of improvement after an extended abstinence.
1. INTRODUCTION
An important feature of cocaine use disorders is the increased vulnerability to relapse that patients undergo during the first weeks of abstinence. This phenomenon has been described in humans (Gawin and Kleber., 1986) in whom cue-induced craving increases after 2–10 weeks of abstinence, and in rodents, displaying not only a delay in the onset of craving, but also its progressive increment over a two months’ period (Grimm et al., 2001).
Sleep dysfunction may be an important contributor to this pattern of relapse. As one of several commonly recognized abstinence/withdrawal symptoms, it may influence important endpoints such as capacity to complete treatment (Kampman et al., 2001). Early descriptions of cocaine abstinence discuss the normalization of sleep as an event that takes place in the transition between “the crash,” or the first week after last cocaine use, and the “withdrawal phase” 2 to 10 weeks after last cocaine use. In addition, early qualitative studies observed improvement in self-reported sleep measures with abstinence (Weddington et al., 1990; Gawin and Kleber, 1986). Contrary to those initial descriptions and subjective reports, polysomnographic studies found worsening of sleep over the first 2–3 weeks of abstinence (for review see Morgan and Malison, 2007), introducing the possibility that persistent sleep abnormalities could have an effect on clinical outcomes, including and in addition to treatment completion.
A connection between sleep and treatment in drug dependence may not be surprising given the intrinsic importance of sleep physiology to brain function and in particular the many overlapping neurotransmitter systems and brain regions that affect sleep. Regulation of sleep and wakefulness involves structures such as the brain stem, hypothalamus, basal forebrain, preoptic area of the hypothalamus, the thalamus and the cortex, and is influenced by the actions of GABA, glutamate, adenosine, nitric oxide, acetylcholine, dopamine, serotonin, norepinephrine, histamine, orexin/hypocretin, and neuropeptide S, among other neurotransmitters (Brown et al., 2012). The action of cocaine on these systems produces several changes that can affect sleep. For instance, blocking uptake of not only dopamine but also serotonin and norepinephrine by cocaine may disrupt the balance between sleep and wakefulness, given that these amines promote wake and suppress sleep (Baumann et al., 1995; Koob and Nestler, 1997). In addition, cocaine intake can also lead to enduring changes in the cholinergic (Heidbreder and Shippenberg, 1996), adenosinergic (Toda et al., 2003), glutamatergic (Kalivas et al., 2003), and GABAergic (Xi et al., 2003) systems, all known to be involved in the regulation of the sleep- wake cycle (Brown et al., 2012).
The changes in sleep and sleep architecture associated with cocaine use and abstinence may be particularly relevant to understanding the potential impact of sleep abnormalities on treatment outcomes. Changes in sleep architecture observed during early withdrawal include shortened sleep onset latency, decreased rapid eye movement (REM) sleep latency, and increased REM sleep time and total sleep time (TST) (Morgan et al., 2006; Morgan et al., 2008). After 2–3 weeks of abstinence, however, TST appears shortened (5–6 hours), latency to sleep onset is delayed (by 10–50 minutes), and REM sleep time is reduced despite decreased REM latency (Morgan and Malison, 2007). Perhaps most striking, however, is the finding that slow-wave sleep (SWS) time appears dramatically reduced compared to age matched-controls (Morgan et al., 2010). This change may be particularly important given the evolutionary value of SWS (Rattenborg, 2006), its preservation despite chronic sleep restriction (Van Dongen et al., 2003) or in response to sleep deprivation (Borbely et al., 1981), the multiple cognitive functions that appear to rely on it (Stickgold et al., 2000), and evidence suggesting that promoting SWS with a medication such as tiagabine can improve cognitive performance in sleep restricted individuals (Walsh et al., 2006).
Unfortunately, there have been relatively few studies of appreciable size that have examined sleep deficits objectively in abstinent cocaine users, owing to the difficulty of such studies. In addition, these studies have been limited in the duration of abstinence in which sleep is measured. For example, previous work from our laboratory on this question has been restricted to fully inpatient studies of not more than 3 weeks (Morgan et al., 2006; Morgan et al., 2008; Morgan et al., 2010), with a mix of treatment and non-treatment seeking participants, and without directed treatment being offered. Here we present objective and self-report sleep measurement from an ongoing, randomized controlled trial for the treatment of cocaine dependence. In particular, we are presenting sleep data from the placebo arm of this trial, which to date constitutes the largest sample of cocaine dependent persons in whom polysomnographic sleep has been measured, and a sample in which extended periods of outpatient abstinence were achieved.
2. METHODS
2.1. Cocaine Dependent Participants
Chronic cocaine users were recruited to participate in an ongoing randomized placebo-controlled trial of modafinil.
In order to be eligible for all study procedures, participants underwent a screening in three phases. The first phase included a telephone interview, assessing whether potential participants were likely to qualify, their desire for treatment and interest in the study, basic demographic questions, and severity of cocaine use. The second phase entailed an in depth medical, psychiatric, and substance abuse screening. For the medical evaluation, an experienced physician obtained a medical history, performed a physical exam, reviewed medical records when available, and reviewed results of basic blood and urine laboratory work and an electrocardiogram. Psychiatric and substance abuse assessment consisted of an unstructured clinical interview performed by a psychiatrist and other instruments to determine additional substance/alcohol use, such as the Substance Use Calendar. Psychiatric interview of this phase was conducted on either an outpatient basis or upon admission to the research unit. The third and final screening phase was conducted upon admission, and included a new urine toxicology screening, a breath alcohol test, and a final recapitulation of drug and alcohol use since outpatient screening or in the past 30 days prior to inpatient admission. On the third inpatient night and as the last step on the screening process, participants underwent a clinical polysomnography in order to identify previously undiagnosed sleep disorders.
This report includes data from all participants who were found to have received placebo (n=20) at a preliminary unblinding point.
Participants were required to meet DSM-IV criteria for cocaine dependence with a duration of at least 2 years, current criteria for dependence as measured by a score ≥ 3 on the Severity of Dependence Scale (Kaye and Darke, 2002), and have a positive urine test for cocaine (benzoylecgonine) during the screening process.
Potential participants were excluded if they had a medical condition that would render study participation unsafe; a chronic primary sleep disorder; a known hypersensitivity to modafinil; current dependence on any drugs other than cocaine or nicotine; lifetime dependence on alcohol, benzodiazepines, or opiates; current, non-substance related Axis I disorder; current use of alcohol in excess of 21 standard drinks/week in the past month or non-zero breathalyzer at screening or study start; or a positive urine toxicology test for opiates, methadone, amphetamines, barbiturates, benzodiazepines, PCP, methaquolone, and propoxyphene at the time of screening, or a positive test for any of those listed plus cannabis at the time of study start. Individuals were also excluded if they were taking psychiatric medications, medications that affect sleep, or medications not safe to take with modafinil; or, if female and of childbearing potential, if they were pregnant, lactating, or unwilling to use contraceptives for the duration of the study.
All individuals reviewed and signed a consent form approved by the local institutional review board, with understanding assessed by a quiz.
2.2. Study Design
The study consisted of a 12-day inpatient stay followed by a six-week outpatient phase with appointments three times per week. Inpatient treatment for substance abuse included both individual and group therapy, and outpatient treatment consisted of weekly, manual guided cognitive behavioral therapy (Kadden et al., 1992), and thrice-weekly urine toxicology screening. Adherence to study participation was promoted through contingency management (Budney and Higgins, 1998; Petry, 2000) wherein keeping appointments and participating in study related duties (but not abstinence) were rewarded. In week 3 and week 6 of the outpatient phase, participants returned for additional inpatient stays of two nights each.
Participants were compensated by check after the end of the study, including $200 for the initial inpatient stay, $50 for each readmission, and $200 for participating in the entire study. They also earned up to $10 in cash each week for returning their empty pill pack, and depending on their adherence to the outpatient portion of the study (not related to abstinence), they earned contingency management prizes adding up to a mean of $234.
2.3. Inpatient Environment
Participants were admitted to an inpatient psychiatry research unit. Participants slept in a single room every night and were not allowed to sleep, nap, or lie in a recumbent position between 7am and 11pm. The unit provided all meals and snacks as part of a caffeine free diet. In addition, fresh air breaks outside of the building were allowed with staff members three times a day, during which time smokers had the opportunity to smoke 1–2 cigarettes.
2.4. Polysomnographic Sleep Measures
Overnight polysomnographic sleep studies (PSG) were conducted eight times total: during the inpatient portion on the nights of days 3, 4, 10, and 11; and during outpatient weeks 3 and 6 on both nights of the 2-day admissions. The first night of each consecutive day set was used as an accommodation night to acclimate the participant to wearing the polysomnographic equipment, while data from the second night was used for analyses and is reported here. For all PSG studies, a strict 11pm to 7am time in bed was followed.
Polysomnography was performed using a TEMEC 8 Channel Universal system (TEMEC Instrument B.V., Kerkrade, the Netherlands) and consisted of two electrographic (EEG) leads (C3-A2 and C4-A1), left and right electrooculogram (EOG) referenced to the opposite mastoid, a two-lead chin electromyogram (EMG), and a two-lead electrocardiogram (ECG).
On the first night of recording (night 3), a more extensive clinical sleep study was carried out (Siesta; Compumedics, Abbotsford, Australia) to screen for sleep disorders. In addition to the above leads, this setup included four more EEG leads (F3-A2, F4-A1, O1-A2, and O2-A1), right and left leg EMGs, finger pulse oximeter, plethysomographic thoracic and abdominal belts, airflow sensor, and snore microphone.
All PSG records were scored according to American Academy of Sleep Medicine guidelines (Iber et al., 2007) by an experienced sleep scorer who was blind to treatment group and study night. We also followed the guidelines with regard to the naming of the sleep stages (N1 = non-REM stage 1; N2 = non-REM stage 2; N3 = non-REM stage 3 [constituting what was previously all slow-wave sleep] and stage R or REM sleep.
Times in REM sleep and in N1, N2, and N3 are reported. Sleep onset latency (SL) was defined as time from “lights out” until the first epoch of sleep and REM latency (RL) was defined as time from sleep onset to the first epoch of REM sleep. TST was calculated by taking the time from sleep onset to the final awakening, minus time awake after sleep onset. Because a strict 8-hour time in bed was implemented, sleep efficiency is not reported separately, but is reflected in TST.
2.5. Questionnaires and Urine Toxicology
Demographics were collected during the initial phone screen. During the in-person screen and study day 1, detailed baseline information was collected including lifetime substance use history and a 30-day timeline follow back of all substance use. Each time participants presented to the unit, they were also queried as to the day of their last cocaine use. Urine was collected and tested for cocaine (benzoylecgonine) as well as opiates, benzodiazepines, methadone, amphetamines, barbiturates, cannabis, PCP, and propoxyphene upon each admission to the unit, on days 1, 4, 8 and 11 of the initial inpatient stay, and three times per week during the outpatient phase. At each outpatient appointment, participants also completed a timeline follow back of all substances used since their last visit. Number of days abstinent at each time point was estimated by corroborating participants’ self-report of last use and timeline follow back data with urine toxicology data. If a cocaine-positive urine conflicted with the participant’s self-report, the number of days abstinent was set to 0 on the day before urine collection.
Subjective reports of sleep quality were obtained every morning while on the inpatient unit, upon awakening. Participants scored “overall sleep quality” on a visual analog scale ranging from 0 to 100 (measured in mm) (Morgan et al., 2006).
2.6. Comparison Data
Baseline sleep data from a historical cohort of healthy control individuals (previously reported in Morgan et al., 2010) is presented for comparison purposes. Briefly, 12 healthy male participants, age 30–50 years, reported to the sleep laboratory for 2 consecutive nights, following a week of maintaining regular sleep habits (as monitored with daily questionnaires and 24-hour actigraphy). Participants were prepared for polysomnographic sleep recording using the TEMEC system. Data from the second night are presented, with the first night serving as an accommodation night.
2.7. Statistical Analyses
Changes in PSG sleep measures were compared between day 4 (week 1) and day 11 (week 2) within cocaine dependent participants using paired t-tests. Analyses were performed on data from all participants and on data from the subset of participants whose urine toxicology screen was positive on admission (n=11). Overall sleep quality scores from days 1–6 (week 1) and days 7–11 (week 2) were averaged for each participant and compared with a paired t-test. Trajectories of TST including data from days 4 and 11 as well as outpatient weeks 3 and 6 were modeled as both a linear and a quadratic function of number of days abstinent at each time point using regression analysis. In these models, days abstinent was first considered as a continuous predictor and then considered as an ordinal variable (1, 2, 3, 4, and 5+ weeks abstinent). Statistical analyses were performed on TST only in order to, for the first time, have a general preliminary idea of sleep changes as participants gain more weeks of abstinence and while they are not living in an inpatient facility. Raw data for other PSG measurements (SL, N3, REM, and RL) was not analyzed statistically, given the small number of participants that attained prolonged abstinence, but these data are included as supplementary material. 1
In order to address the possibility that sleep architectural changes were a reflection of baseline alcohol consumption, number of drinks in the past 30 days was correlated with each PSG measurement, early and late in abstinence.
3. RESULTS
3.1. Demographic Characteristics
Demographic data for all participants is presented in table 1. PSG measurement among all participants showed decreases in TST and N2 sleep time, and a trend toward decreased REM sleep time from week 1 to week 2 (Table 2). Among participants who had urine toxicology that was positive upon admission, similar results were found but the decrease in REM sleep time was statistically significant, and there was a trend toward decreased N3 time. In both analyses there was a statistically significant increase in RL from week 1 to week 2.
TABLE 1.
Baseline and Demographic Characteristics
| Demographic | Value (SD) | Median (Semi- Interquartile Rage) |
|---|---|---|
| Sex (#M: #F) | 16:4 | |
| Age (years) | 45 (7) | |
| Education (years) | 12 (1) | |
| Cocaine use | ||
| Total number of years of use | 25 (7) | |
| Number of days in past 30 days | 14 (8) | |
| Amount in grams in past 30 days | 23 (26) | |
| Number of days from last use to study day 4 | 7.2 (3.7) | |
| Cigarette use (number per day) (N = 14/20) | 10 (6) | |
| Cannabis use (number of joint-equivalents in past 30 days) (N = 3/20) | 2 (1) | |
| Alcohol use (number of drinks in past 30 days) (N = 14/20) | 48 (48) | |
| Pittsburgh Sleep Quality Index | 9 (5) |
TABLE 2.
PSG sleep measurements and sleep quality among all CD participants and subset with positive urine upon admission
| All Participants | Cocaine(+) at Admission | Healthy Comparison Data | |||||
|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | p | Week 1 | Week 2 | p | ||
| Days Abstinent | 6.8 (0.7) | 5.2 (0.2) | |||||
| TST | 392 (11) | 342 (14) | 0.004 | 399 (12) | 327 (19) | 0.002 | 416 (15) |
| N1 | 28 (4) | 34 (6) | >0.1 | 29 (6) | 31 (5) | >0.1 | 242 (12)* |
| N2 | 225 (9) | 191 (10) | 0.003 | 226 (12) | 192 (14) | 0.02 | |
| N3 | 40 (8) | 39 (5) | >0.1 | 47 (12) | 34 (11) | 0.10 | 85 (14) |
| REM | 99 (8) | 82 (6) | 0.08 | 97 (11) | 69 (6) | 0.04 | 87 (5) |
| Sleep Latency | 18 (4) | 22 (4) | >0.1 | 16 (4) | 23 (5) | >0.1 | 14 (2) |
| REM Latency | 35 (6) | 61 (10) | 0.04 | 32 (8) | 64 (12) | 0.02 | 73 (8) |
| Subjective Sleep Quality (0–100) | 74 (3) | 82 (4) | 0.01 | 77 (4) | 85 (4) | 0.05 | 67 (6) |
includes N1 and N2 sleep time
All values given in minutes (s.e.m.) except for subjective sleep quality
Self-reported overall sleep quality also increased significantly in both analyses from week 1 to week 2.
There were no correlations between baseline alcohol use and PSG measurements.
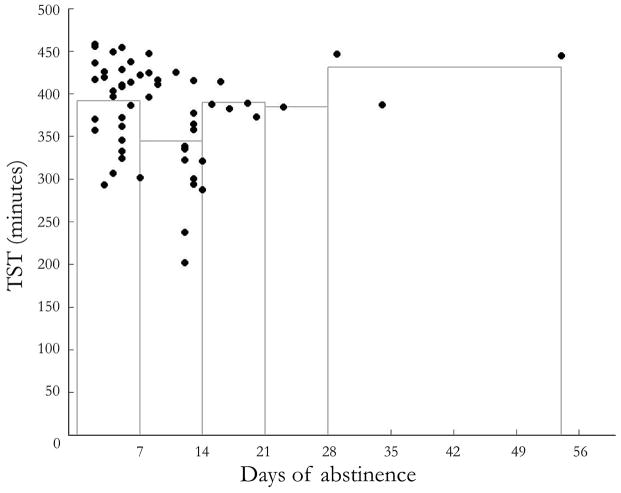
3.2. Trajectory of Total Sleep Time (TST) by abstinent days
Total sleep time over the course of abstinence is depicted in Figure 12. A quadratic model fit the data well when modeling both the raw data (t36=3.37, pquadratic=0.002) and when days abstinent were considered as an ordinal measure ranging from 1 to 5+ weeks abstinent (t22=3.12, pquadratic=0.005). Both models were superior to a linear fit.
Figure 1.
4. DISCUSSION
This is the largest single study to date reporting polysomnographic (PSG) sleep data in chronic cocaine users. This is also the first study examining sleep architecture changes for as long as 54 abstinent days and during outpatient treatment. The results of this study confirm previous PSG findings showing impaired slow wave sleep (N3), reduced REM latency early in abstinence, and deteriorating REM and total sleep time (TST) during the first weeks of abstinence, with concurrent improvement in self-reported sleep quality (“occult insomnia”; Morgan et al., 2006).
The most striking finding, however, is the markedly shortened SWS time observed in persons with cocaine dependence relative to healthy sleepers of a similar age. This deficit was profound – a greater than 50% reduction – during the first two weeks of abstinence. This finding replicates our former studies in cocaine dependence (Morgan et al., 2010) and is similar to what has been reported for several substances such as cocaine and heroin (Schierenbeck et al., 2008), stimulants (Thompson et al., 1995), alcohol (Brower, 2003), and marijuana (Barratt et al., 1974; Bolla et al., 2008). Moreover, this deficit is potentially clinically relevant, as it has been shown to predict relapse among alcohol dependent individuals (Allen et al., 1971; Brower, 2003). Also in line with previous studies (Johanson et al., 1999; Pace-Schott et al., 2005; Morgan et al., 2008; Walsh et al., 2009), we found that REM latency was shortened in the early abstinence period. Given that REM latency is associated with relapse in alcohol dependent patients (Gillin et al., 1994), this finding may prove clinically significant. Also consistent with prior work (Morgan et al., 2008), total REM time decreased from the first to second week of abstinence, particularly among participants who had fewer days of abstinence (confirmed by positive urine toxicology for cocaine at admission) at the time of testing in the first week.
TST was reduced in the second week of abstinence relative to the first, and was more than 60 minutes less than in healthy sleepers in the same environment. The decrease in sleep time and relationship to healthy sleepers concurs with previous work (Morgan et al., 2006; Morgan et al., 2010) and may be associated with consequences of poor sleep, like cognitive impairment (Morgan et al., 2006; Morgan et al., 2008). As cognitive impairments negatively affect retention in outpatient CBT treatment for cocaine dependence (Aharonovich et al., 2006), the decrease in sleep time associated with abstinence may be relevant to clinical outcomes.
Considering that the present study had few participants who maintained abstinence beyond 21 days, any inferences made about TST during this period are tenuous and non-conclusive. Even though the quadratic model as well as visual inspection of the data suggests improvement of TST later in abstinence, this is based on only four data points, and is thus very preliminary. Despite this limitation, this is the first study showing the trajectory of TST based on PSGs obtained during inpatient (study weeks 1 and 2) and outpatient (study weeks 3 and 6) environments. Whether or not sleep improves with extended abstinence is an important question, as it may help differentiate the contribution of pre-existing sleep deficits to risk for cocaine use relative to the effects of chronic cocaine use on sleep. The present findings suggest that, at least for total sleep time, the duration of abstinence that needs to be studied to start answering this question may be 2 months or less.
Another potential limitation pertains to participants who have a negative urine test upon admission and to the categorization of their number of days abstinent. For these individuals, calculation of days abstinent is determined largely by their self-report, which could result in less precise estimates of the length of abstinence. The extent to which this recall bias could have affected accuracy of number of days abstinence is decreased by the fact that all participants who had a negative urine toxicology test upon admission had a positive urine on the screening visit, no more than 30 days before admission.
These findings suggest several avenues of further investigation. Recruitment of additional participants will afford us the opportunity to further clarify our conclusions with regard to prolonged abstinence. With more data it may also become possible to assess more detailed changes in sleep architecture over this time period. For example, it seems likely that given how low SWS time is early in cocaine abstinence, and that SWS remains reduced for the first several months of abstinence in alcohol dependent patients (Ishibashi et al., 1987; Williams et al., 1981), SWS in this population may not recover along with TST. Moreover, past work has shown that individual sleep changes early in abstinence have consequences on cognitive performance (Morgan et al., 2006; Morgan et al., 2008). It remains to be seen to what extent this holds true over the course of abstinence, and whether a relationship exists between potential beneficial effects of modafinil on sleep and cognition. Additional future directions pertain to clinical outcomes such as withdrawal symptoms or likelihood of relapse. It will be important to establish whether sleep changes observed during abstinence are related with clinical outcomes, as has been reported in alcohol dependence (Arnedt et al., 2007). Once such relationships are tested, the next step could be to evaluate causality and/or interactions. The present study is a clinical trial of modafinil, therefore, testing the relationship between this medication on sleep (Morgan et al. 2010) and clinical outcomes of cocaine use will be important in determining whether modifying sleep can affect treatment.
In sum, these results confirm prior observations about sleep changes over the course of abstinence, using objective sleep measurement and the largest sample of chronic cocaine users to date. Additionally, we present data that extends beyond the first three weeks of abstinence, far longer than has been examined in past work. While the finding on TST requires further confirmation, these data comprise the first evidence of a possible objective improvement in sleep with prolonged abstinence.
Supplementary Material
Footnotes
Access to raw data on Trajectory of SL, N3, REM, and RL is available online, as supplementary material.
Access to raw data on Trajectory of SL, N3, REM, and RL is available on line, as supplementary material.
Contributors:
P.T. Morgan designed the study and wrote the protocol. G. A. Angarita and S. V. Canavan contributed equally to this work. G. A. Angarita, S. V. Canavan, E. Forselius, A. Bessette, and P.T. Morgan participated in recruitment, screening, and clinical care/follow up of subjects. G. A. Angarita, S. V. Canavan, B. Pittman, and P.T. Morgan performed statistical analyses and wrote the manuscript.
ClinicalTrials.gov Identifier: NCT01137396
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313–22. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Allen RP, Faillace LA, Wagman A. Recovery time for alcoholics after prolonged alcohol intoxication. Johns Hopkins Med J. 1971;128:158–164. [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. J Addict Dis. 2007;26(4):41–54. doi: 10.1300/J069v26n04_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES, Beaver W, White R. The effects of marijuana on human sleep patterns. Biol Psychiatry. 1974;8(1):47–54. [PubMed] [Google Scholar]
- Baumann MH, Becketts KM, Rothman RB. Evidence for alterations in presynaptic serotonergic function during withdrawal from chronic cocaine in rats. Eur J Pharmacol. 1995;282(1–3):87–93. doi: 10.1016/0014-2999(95)00280-x. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, David PM, Verdejo-Garcia A, Benbrook AR. Sleep disturbance in heavy marijuana users. Sleep. 2008;31(6):901–8. doi: 10.1093/sleep/31.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51(5):483–95. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep med rev. 2003;7 (6):523–39. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RE. Control of Sleep and Wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST. National Institute on Drug Abuse therapy manuals for drug addiction; manual 2. Rockville, Maryland: US Department of Health and Human Services; 1998. A community reinforcement plus vouchers approach: treating cocaine addiction. [Google Scholar]
- Gawin F, Kleber H. Abstinence symptomatology and psychiatric diagnosis in chronic cocaine abusers. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Smith TL, Irwin M, Butters N, Demodena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3-month follow-up. Arch Gen Psychiatry. 1994;51(3):189–97. doi: 10.1001/archpsyc.1994.03950030025003. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412 (6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Shippenberg TS. Evidence for an involvement of muscarinic cholinergic systems in the induction but not expression of behavioral sensitization to cocaine. Synapse. 1996;24(2):182–92. doi: 10.1002/(SICI)1098-2396(199610)24:2<182::AID-SYN10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quen S, editors. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, Ill: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Ishibashi M, Nakazawa Y, Yokoyama T, Yoshiaki K, Yasushi M, Norimasa H, Katsuaki O. Cerebral atrophy and slow wave sleep of abstinent chronic alcoholics. Drug Alcohol Depend. 1987;19 (4):325–32. doi: 10.1016/0376-8716(87)90019-6. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Roehrs T, Schuh K, Warbasse L. The effects of cocaine on mood and sleep in cocaine dependent males. Exp Clin Psychopharmacol. 1999;7 (4):338–346. doi: 10.1037//1064-1297.7.4.338. [DOI] [PubMed] [Google Scholar]
- Kadden R, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R. Cognitive-behavioral coping skills therapy manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1992. [Google Scholar]
- Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D. Glutamate transmission and addiction to cocaine. Ann N Y Acad Sci. 2003;1003:169–75. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, Mulholland EM, Jawad AF, Parikh GA, Mulvaney FD, Weinrieb RM, O’Brien CP. Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict Behav. 2001;15(1):52–9. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- Kaye S, Darke S. Determining a diagnostic cut-off on the Severity of Dependence Scale (SDS) for cocaine dependence. Addiction. 2002;97(6):727–31. doi: 10.1046/j.1360-0443.2002.00121.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9(3):482–97. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Malison RT. Cocaine and sleep: early abstinence. ScientificWorldJournal. 2007;7:223–30. doi: 10.1100/tsw.2007.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep architecture, cocaine and visual learning. Addiction. 2008;103(8):1344–52. doi: 10.1111/j.1360-0443.2008.02233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Pittman B, Stickgold R, Malison RT. Normalizing effects of modafinil on sleep in chronic cocaine users. Am J Psychiatry. 2010;167(3):331–40. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, Hart CL, Clarke D, Morgan A, Hobson JA. Sleep quality deteriorates over a binge – abstinence cycle in chronic smoked cocaine users. Psychopharmacology. 2005;179:873–883. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- Petry NM. A comprehensive guide to the application of contingency management procedures in clinical settings. Drug Alcohol Depend. 2000;58 (1–2):9–25. doi: 10.1016/s0376-8716(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC. Evolution of Slow Wave Sleep and Palliopallial connectivity in mammals and birds: a hypothesis. Brain Res Bull. 2006;69 (1):20–9. doi: 10.1016/j.brainresbull.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of Illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep Med Rev. 2008;12 (5):381–389. doi: 10.1016/j.smrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Brown SB, Hatsukami DK. Association of cocaine withdrawal symptoms with more severe dependence and enhanced subjective response to cocaine. Drug Alcohol Depend. 2003;69(3):273–82. doi: 10.1016/s0376-8716(02)00328-9. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Toda S, Alguacil LF, Kalivas PW. Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens. J Neurochem. 2003;87(6):1478–84. doi: 10.1046/j.1471-4159.2003.02121.x. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Gillin JC, Golshan S, Irwin M. Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short-term abstinence. Biol Psychiatry. 1995;38(12):831–6. doi: 10.1016/0006-3223(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Randazzo AC, Stone K, Eisenstein R, Feren SD, Kajy S, Dickey P, Roehrs T, Roth T, Schweitzer PK. Tiagabine is associated with sustained attention during sleep restriction: evidence for the value of slow-wave sleep enhancement? Sleep. 2006;29(4):433–43. [PubMed] [Google Scholar]
- Walsh SL, Stoops WW, Moody DE, Lin SN, Bigelow GE. Repeated dosing with oral cocaine in humans: assessment of direct effects, withdrawal, and pharmacokinetics. Exp Clin Psychopharmacol. 2009;17:205–216. doi: 10.1037/a0016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HL, Rundell OH., Jr Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol Clin Exp Res. 1981;5:318–25. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, Dax EM, Herning RI, Michaelson BS. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47 (9):861–868. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23(8):3498–505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.