Abstract
A series of fluorescent pH probes based on the spiro-cyclic rhodamine core, aminomethylrhodamines (AMR), was synthesized and the effect of cycloalkane ring size on the acid/base properties of the AMR system was explored. The study involved a series of rhodamine 6G (cAMR6G) and rhodamine B (cAMR) pH probes with cycloalkane ring sizes from C-3 to C-6 on the spiro-cyclic amino group. It is known that the pKa value of cycloalkylamines can be tuned by the different ring sizes in accordance with the Baeyer ring strain theory. Smaller ring amines have lower pKa value, i.e. they're less basic, such that the relative order in cycloalkylamine basicity is: cyclohexyl>cyclopentyl>cyclobutyl>cyclopropyl. Herein, it was found that the pKa values of the cAMR and cAMR6G systems can also be predicted by Baeyer ring strain theory. The pKa values for the cAMR6G series were shown to be higher than the cAMR series by a value of approximately 1.
Keywords: fluorescent, rhodamine, pH probes, cycloalkylamine, Baeyer ring strain
Introduction
The pH values of biological systems are associated with a variety of important processes, such as organelle acidification, posttranslational processing of secretory proteins, cleavage of prohormones, ATP synthesis etc. [1,2]. Normal physiological conditions exist in neutral to slightly basic pH (7–7.4) range and deviations toward slightly acidic pH (6.8-6.2) may represent an irregular growth caused by cancer or other diseases [3,4]. Analytical measurements of pH are routinely carried out using electrochemical measurements. However, these methods are not always ideal and certain situations may require an alternative approach. For example, conventional electrochemical methods are generally not suited for measuring biological pH because of the potential to cause disturbance to the system. Non-invasive techniques, such as fluorescence, serve as an alternative to these more analytically challenging environments.
A large number of publications have reported on various fluorophores that are being used as pH probes, many of which are commercially available [5]. In some recent examples, the rhodamine spiro-cyclic amide scaffold was used in the development of fluorescent probes. This scaffold is non-fluorescent and does not exhibit absorption of visible light in the spiro-cyclic form; however, upon protonation, the spiro-cyclic ring opens, consequently restoring the absorption and fluorescence properties of the rhodamine fluorophore [6–11]. Although many probes have been built around this scaffold, the effect of substituents on the amide nitrogen in the ring-opening process (i.e. the pKa) is still being explored. In two of the most thorough studies of the substituents effect on this system, Feng and co-workers have shown that steric hindrance around the spiro-cyclic ring results in a higher pKa [10], while Harbron and coworkers have shown that the pKa is seemingly unaffected by the normal electronic effects on basicity, e.g. electron donating or electron withdrawing groups [11].
Our efforts in this area focus on the effect of substituents on the amino nitrogen in the ring-opening process. Our group and others have developed a structurally analogous scaffold, the aminomethylrhodamines or rhodaminedeoxylactams as fluorescent probes for pH, which have a pKa value that is strongly dependent on the electronic properties affecting the amino group [12–15]. This approach has allowed us to rationally design rhodamine pH probes where the photophysical response to pH is easily predicted by correlation to the amine's pKa. To expand on this effect and to develop fluorescent probes in the neutral pH region, we decided to utilize a series of cycloalkylamines whose pKa values are affected by Baeyer ring strain. The Baeyer ring strain effect was described by Perrin et al. who demonstrated that the pKa values of cycloalkylamines were dependent on the ring size, such that the pKa value of cyclo – propylamine<butylamine<pentylamine< cyclohexylamine [16]. This observation was explained by the decreased bond angles in smaller rings resulting in an increase in p-character, which can withdraw electron density from the nitrogen lone pair. We explored this effect in the AMR series by synthesizing a series of cycloalkyl-aminomethylrhodamines (cAMR) with various cycloalkane ring sizes and evaluate the pH dependent properties of these systems using ultraviolet visible (UV-Vis) and fluorescence spectroscopy. We expected these cAMR derivatives to take on a similar non-fluorescent spiro-cyclic ring structure at high pH and open reversibly under increasingly acidic conditions with the pKa values being determined by the cycloalkylamine ring size. Herein we report our findings on a series of cAMR and cAMR6G derivatives and their pH dependent properties.
Materials and Methods
General chemicals were purchased from Acros organics, Aldrich, and Merck. DMF, acetone, chloroform and ethyl acetate were reagent grade and used without further purification unless otherwise mentioned. Methylene chloride and THF were purified using a M-Braun solvent purification system. Analytical thin layer chromatography (TLC) was conducted on pre-coated TLC plates, alumina (neutral), layer thickness 0.25 mm, manufactured by Sigma-Aldrich. Column chromatography was performed on a Biotage isolera 4 with an ultraviolet detector. 1H NMR and 13C NMR spectra were recorded on a Mercury 400 MHz instrument using deuterated chloroform (CDCl3). Chemical shifts are reported in delta (δ) parts per million (ppm). Splitting patterns are abbreviated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad.
Mass spectra (MS) were measured on a Quattro II quadrupole-hexapole-quadrupole (QHQ) mass spectrometer. Absorbance data were obtained using a Cary 100 (Varian) UV-Vis spectrometer. Fluorescence spectra were recorded on a Fluorolog 3 (Horiba JobinYvon).
The following procedure was used for the spectroscopic analysis of the cAMR pH probes. In a 200 mL beaker, 50 mL of either a 2.5 µM (fluorescence) or a 5 µM (UV-vis) solution of the corresponding sensor in 0.10 M sodium phosphate buffer and 0.5% DMSO was stirred in open air for approximately 0.5 hours allowing the solution to become saturated with oxygen. cAMR6G solutions were similarly prepared, with the exception that a 2.5 µM concentration was used for both fluorescence and UV-vis. Using a digital pH meter (Accumet AB15) equipped with a glass electrode (Accumet, pH/ATC calomel) the pH was monitored and adjusted to acidic or basic conditions using small aliquots of conc. HCl or a 4.0 M NaOH solution, respectively. The pH was allowed to stabilize for ~1 minute and then 3 mL of the solution was added to a quartz cuvette for analysis.
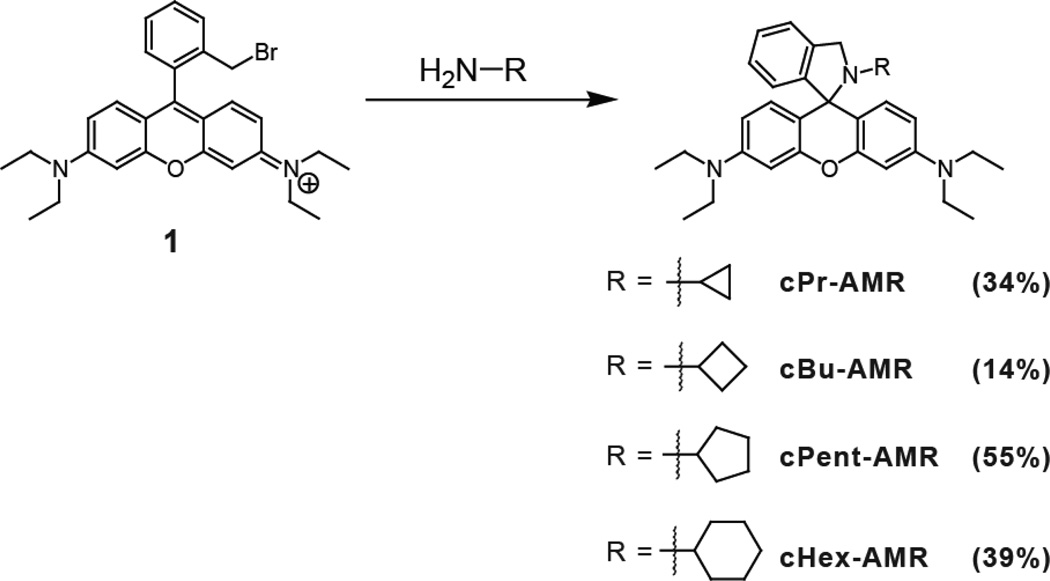
Compound 1 was synthesized according to a previously reported procedure [12]. A general procedure for preparing cPr-AMR, cBu-AMR, cPnt-AMR and cHex-AMR was used (Scheme 1). To a solution of Rhodamine B intermediate 1 (300 mg, 0.57 mmol) in dichloromethane (20 mL) was added the corresponding amine (1 mL). The reaction was stirred at room temperature overnight. Evaporation of the solvent offered the crude product. Purification of the product can be carried out using hexanes:EtOAc (90:10) on a Biotage amine cartridge.
Scheme 1.
Synthesis of AMR series.
2-cyclopropyl-3'-N,3'-N,6'-N,6'-N-tetraethyl-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3',6'-diamine (cPr-AMR)
Yield 91 mg (34 %); RF = 0.46 in 90:10 Hex:EtOAc. 1H NMR (400 MHz, CDCl3) δ 7.31 (d, J = 7.2 Hz, 1H), 7.29 – 7.23 (m, 1H), 7.20 (dd, J = 8.8, 5.1 Hz, 1H), 6.97 (d, J = 7.5 Hz, 1H), 6.59 (d, J = 8.7 Hz, 2H), 6.39 (d, J = 2.6 Hz, 2H), 6.30 (dt, J = 13.0, 6.5 Hz, 2H), 4.18 (s, 2H), 3.50 – 3.17 (q, 8H), 1.64 – 1.38 (m, 1H), 1.29 – 0.91 (t, 12H), 0.27 – 0.07 (m, 2H), 0.08 – −0.13 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 4.82, 12.70, 29.31, 44.34, 56.93, 67.82, 76.74, 77.06, 77.38, 97.63, 107.32, 112.34, 121.70, 124.66, 126.80, 127.41, 129.99, 140.04, 147.87, 148.75, 153.61. Mass (Quattro II) LRMS (ESI) m/z Found 468.4 M+H, calculated: 467.3.
2-cyclobutyl-3'-N,3'-N,6'-N,6'-N-tetraethyl-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3',6'-diamine (cBu-AMR)
Yield 38 mg (14 %); RF = 0.45 in 90:10 Hex:EtOAc. 1H NMR (400 MHz, CDCl3) δ 7.39 – 7.31 (m, 1H), 7.30 – 7.22 (m, 1H), 7.22 – 7.14 (m, 1H), 6.98 – 6.84 (m, 1H), 6.70 – 6.61 (m, 2H), 6.40 – 6.35 (m, 2H), 6.33 (dd, J = 8.8, 2.6 Hz, 2H), 4.41 – 3.92 (m, 2H), 3.50 – 3.21 (q, 8H), 3.21 – 2.95 (m, 1H), 1.94 – 1.66 (m, 2H), 1.66 – 1.51 (m, 2H), 1.48 – 1.31 (m, 2H), 1.30 – 1.00 (t, 12H). 13C NMR (101 MHz, CDCl3) δ 12.70, 12.74, 12.81, 15.87, 27.88, 27.99, 28.07, 44.33, 52.07, 52.75, 52.78, 66.92, 76.74, 77.06, 77.37, 97.48, 97.53, 97.60, 107.36, 107.46, 112.35, 121.82, 124.49, 126.64, 127.38, 130.49, 139.41, 147.89, 149.32, 153.04. Mass (Quattro II) LRMS (ESI) m/z Found 482.4 M+H, calculated: 481.3.
2-cyclopentyl-3'-N,3'-N,6'-N,6'-N-tetraethyl-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3',6'-diamine (cPnt-AMR)
Yield 155 mg (55 %); RF = 0.47 in 90:10 Hex:EtOAc. 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 7.3 Hz, 1H), 7.20 (t, J = 7.2 Hz, 1H), 7.12 (t, J = 7.4 Hz, 1H), 6.81 (d, J = 7.5 Hz, 1H), 6.69 (d, J = 8.6 Hz, 2H), 6.40 – 6.22 (m, 4H), 4.52 – 4.18 (m, 2H), 3.64 – 3.13 (q, 8H), 2.86 – 2.62 (m, 1H), 1.60 (s, 2H), 1.44 (s, 4H), 1.38 – 1.04 (m, 14H). 13C NMR (101 MHz, CDCl3) δ 12.73, 23.42, 30.99, 44.28, 55.41, 60.09, 67.50, 76.73, 77.05, 77.37, 97.55, 107.37, 113.15, 121.55, 124.59, 126.39, 127.31, 130.92, 138.55, 147.81, 150.91, 152.58. Mass (Quattro II) LRMS (ESI) m/z Found 496.4 M+H, calculated: 495.3.
2-cyclohexyl-3'-N,3'-N,6'-N,6'-N-tetraethyl-2,3-dihydrospiro[isoindole-1,9' -xanthene]-3',6'-diamine (cHex-AMR)
Yield 113 mg (39 %); RF = 0.50 in 90:10 Hex:EtOAc. 1H NMR (400 MHz,CDCl3) δ 7.30 (d, J = 7.4 Hz, 1H), 7.19 (t, J = 7.4 Hz, 1H), 7.11 (t, J = 7.4 Hz, 1H), 6.79 (d, J = 7.6 Hz, 1H), 6.70 (d, J = 8.7 Hz, 2H), 6.36 – 6.22 (m, 4H), 4.33 (d, J = 19.6 Hz, 2H), 3.57 – 3.12 (q, 8H), 2.48 (m, J = 10.8, 7.2, 3.7 Hz, 1H), 1.65 – 1.45 (m, 2H), 1.47 – 1.31 (m, 3H), 1.28 (d, J = 22.0 Hz, 1H), 1.25 – 1.09 (t, 12H), 1.10 – 0.78 (m, 4H). 13C NMR (101 MHz, CDCl3) d 12.70, 25.73, 26.11, 32.02, 44.31, 52.18, 55.00, 67.00, 76.71, 77.03, 77.35, 97.63, 107.54, 113.91, 121.64, 124.45, 126.20, 127.19, 130.84, 138.52, 147.73, 150.68, 152.37. Mass (Quattro II) LRMS (ESI) m/z Found 510.5 M+H, calculated: 509.3.
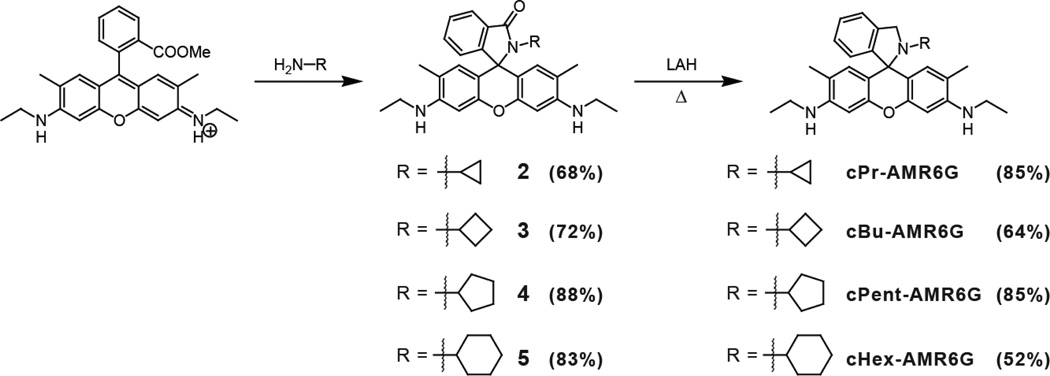
A general procedure for preparing the rhodamine 6G amides 2, 3, 4, and 5 was used (Scheme 2). In a 25 mL RBF, 750 mg (1.56 mmol) of rhodamine 6G was dissolved in 10 mL of DMF. To this solution, 1 mL of the corresponding amine was added and the solution was stirred until the color faded from a dark purple to a light pink. The product was precipitated by transferring the DMF solution into a beaker containing 50 mL of ice water. The product was filtered and washed with copious amounts of cold water. After air drying for several hours, the product was recrystallized using hexanes:chloroform mixed solvent.
Scheme 2.
Synthesis of AMR6G series.
2-cyclopropyl-3'-N,6'-N-diethyl-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3',6'-diamine (2)
Yield 485 mg (68 %). 1H NMR (400 MHz, CDCl3) δ 7.90 (dd, J = 6.0, 2.7 Hz, 1H), 7.45 - 7.38 (m, 2H), 7.01 (dd, J = 6.0, 2.7 Hz, 1H), 6.35 (s, 2H), 6.20 (s, 2H), 3.50 (s, 2H), 3.21 (q, J = 7.1 Hz, 4H), 2.13 -2.05 (m, 1H), 1.90 (s, 6H), 1.32 (t, J = 7.1 Hz, 6H), 0.42 (d, J = 5.6 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 169.38, 153.56, 151.63, 147.22, 132.50, 131.32, 128.23, 127.91, 123.71, 122.69, 117.74, 106.99, 96.50, 65.68, 38.36, 22.91, 16.72, 14.71, 3.81. Mass (Quattro II) LRMS (ESI) m/z Found 454.2 M+H, calculated: 453.24.
2-cyclobutyl-3'-N,6'-N-diethyl-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3',6'-diamine (3)
Yield 529 mg (72 %). 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 6.3 Hz, 1H), 7.46 - 7.29 (m, 2H), 6.92 (dd, J = 6.0, 1.9 Hz, 1H), 6.36 (s, 2H) 6.35 (s, 2H), 3.73 (p, J = 10.0 Hz, 1H), 3.48 (s, 2H), 3.21 (q, J = 7.1 Hz, 4H), 2.88 - 2.66 (m, 2H), 1.91 (s, 6H), 1.74 (m, 1H), 1.50 - 1.24 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 169.17, 154.09, 151.26, 147.17, 132.22, 131.04, 128.07, 127.71, 123.28, 122.57, 117.68, 106.97, 96.67, 65.36, 48.40, 38.38, 28.03, 16.68, 16.06, 14.76. Mass (Quattro II) LRMS (ESI) m/z Found 468.2 M+H, calculated: 467.26.
2-cyclopentyl-3'-N,6'-N-diethyl-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3',6'-diamine (4)
Yield 661mg (88 %) 1H NMR (400 MHz, CDCl3) δ 7.92 - 7.80 (m, 1H), 7.37 (pd, J= 7.3, 1.4 Hz, 2H), 6.94 (dd, J= 6.3, 1.4 Hz, 1H), 6.39 - 6.33 (m, 4H), 3.48 (s, 2H), 3.38-3.30 (m, 1H), 3.21 (q, J= 7.1 Hz, 4H), 2.29 - 2.09 (m, 2H), 1.91 (s, 6H), 1.83 - 1.68 (m, 2H), 1.45-1.18 (m, 10H). 13C NMR (101 MHz, CDCl3) δ 14.76, 16.68, 24.57, 28.89, 38.38, 54.73, 65.84, 76.77, 77.09, 77.41, 96.56, 106.44, 117.57, 122.36, 123.45, 127.72, 128.60, 131.76, 131.97, 147.16, 151.47, 153.93, 167.65. Mass (Quattro II) LRMS (ESI) m/z Found 482.4 M+H, calculated: 481.3.
2-cyclohexyl-3'-N,6'-N-diethyl-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3',6'-diamine (5)
Yield 647 mg (83 %). 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 6.6 Hz, 1H), 7.50 - 7.33 (m, 2H), 6.95 (d, J = 6.2 Hz, 1H), 6.34 (s, 2H), 6.29 (s, 2H), 3.48 (s, 2H), 3.21 (q, J = 7.0 Hz, 4H), 2.98 - 2.74 (m, 1H), 2.10 (m, 2H), 1.90 (s, 6H), 1.61 - 1.37 (m, 3H), 1.33 (t, J = 7.1 Hz, 6H), 1.22 - 0.75 (m, 5H). 13C NMR (101 MHz, CDCl3) δ 150.74, 150.68, 146.03, 138.43, 130.87, 127.24, 126.17, 124.49, 121.65, 116.69, 114.37, 96.33, 67.15, 54.93, 52.16, 38.53, 32.02, 26.09, 25.76, 16.96, 14.89. Mass (Quattro II) LRMS (ESI) m/z Found 496.3 M+H, calculated: 495.3.
A general procedure for cPr-AMR6G, cBu-AMR6G, and cHex-AMR6G was used (Scheme 2). In a 50 mL round bottom flask, 100 mg of the rhodamine amide was dissolved in 30 mL of dry THF. To this solution, 150 mg of LiAlH4 was slowly added and the mixture was refluxed for 18–48 hours. After refluxing, the reaction was quenched by slowly adding 0.5 mL of water, followed by 1 mL of 15% NaOH. The mixture was filtered and the filtrate dried using MgSO4. The THF was removed under reduced pressure and the crude was dissolved in DCM and transferred to a separatory funnel. The product was extracted from the organic layer using 10% HCl. The acid layer was washed using DCM (3 × 20 mL), then made basic using 3.0 M NaOH. The product was isolated by extraction using DCM (3 × 20 mL) and the combined organic layers were washed with brine (2 × 20 mL) then dried over MgSO4. The solvent was removed under reduced pressure. No further purification was necessary.
2-cyclopropyl-3',6'-bis(ethylamino)-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3-one (cPr-AMR6G)
Reflux for 18 hours. Yield 82.3 mg (85 %). 1H NMR (400 MHz, CDCl3) δ 7.33 (d, J = 7.4 Hz, 1H), 7.28 (d, J = 7.8 Hz, 1H), 7.23 - 7.17 (m, 1H), 6.93 (d, J = 7.5 Hz, 1H), 6.40 (s, 2H), 6.35 (s, 2H), 4.18 (s, 2H), 3.37 (s, 2H), 3.21 (m, 4H), 1.96 (s, 6H), 1.41 (m, 1H), 1.32 (t, J = 7.1 Hz, 7H), 0.09 (m, 2H), 0.01 - −0.08 (m, 2H). 13C NMR (101 MHz, CDCl3) 151.95, 148.78, 146.22, 139.89, 129.99, 127.48, 126.78, 124.72, 121.73, 116.63, 112.69, 96.34, 67.99, 56.97, 38.51, 29.31, 16.98, 14.90, 4.76. Mass (Quattro II) LRMS (ESI) m/z Found 440.2 M+H, calculated: 439.21.
2-cyclobutyl-3',6'-bis(ethylamino)-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3-one (cBu-AMR6G)
Reflux for 24 hours. Yield 62.1 mg (64 %). 1H NMR (400 MHz, CDCl3) δ 7.34 (d, J = 7.4 Hz, 1H), 7.26 (d, J = 7.4 Hz, 1H), 7.17 (t, J = 7.4 Hz, 1H), 6.85 (d, J = 7.6 Hz, 1H), 6.43 (s, 2H), 6.31 (s, 2H), 4.16 (s, 2H), 3.37 (s, 2H), 3.20 (bq, 4H), 3.01 (m, 1H), 1.96 (s, 6H), 1.81 - 1.65 (m, 2H), 1.58 - 1.44 (m, 2H), 1.39 (m, 2H), 1.31 (t, J = 7.1 Hz, 6H). 13C NMR (101 MHz, CDCl3) Mass (Quattro II) LRMS (ESI) m/z Found 454.3 M+H, calculated: 453.3.
2-cyclopentyl-3',6'-bis(ethylamino)-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3-one (cPnt-AMR6G)
Reflux for 48 hours. Yield 83 mg (85 %). RF= 0.26 in 90:10 Hex:EtOAc on amine TLC. 1H NMR (400 MHz, CDCl3) δ 7.31 (d, J= 7.4 Hz, 1H), 7.22 (t, J= 7.3 Hz, 1H), 7.12 (t, J= 7.4 Hz, 1H), 6.76 (t, J= 14.8 Hz, 1H), 6.58 - 6.45 (m, 2H), 6.38 - 6.24 (m, 2H), 4.33 (d, J= 20.1 Hz, 2H), 3.36 (s, 1H), 3.20 (dt, J= 7.0, 4.9 Hz, 4H), 2.72 (dd, J= 15.5, 6.5 Hz, 1H), 2.07 - 1.83 (s, 6H), 1.38 - 1.04 (m, 14H). 13C NMR (101 MHz, CDCl3) δ 14.91, 17.00, 23.36, 23.38, 31.02, 38.51, 55.54, 60.11, 67.64, 76.75, 77.07, 77.38, 96.33, 113.64, 113.66, 116.63, 116.65, 121.58, 124.65, 126.37, 127.38, 130.92, 138.47, 146.16, 146.18, 150.93. Mass (Quattro II) LRMS (ESI) m/z Found 468.4 M+H, calculated: 467.3.
2-cyclohexyl-3',6'-bis(ethylamino)-2,3-dihydrospiro[isoindole-1,9'-xanthene]-3-one (cHex-AMR6G)
Reflux for 48 hours. Yield 50.4 mg (52 %). 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 7.4 Hz, 1H), 7.20 (t, J = 7.3 Hz, 0H), 7.11 (t, J = 7.5 Hz, 1H), 6.76 (d, J = 7.5 Hz, 1H), 6.52 (s, 2H), 6.29 (s, 2H), 4.37 (s, 2H), 3.35 (s, 2H), 3.20 (b, 4H), 2.45 (m, 1H), 1.95 (s, 6H), 1.53 - 1.24 (m, 11H), 1.22 - 0.89 (m, 5H). 13C NMR (101 MHz, CDCl3) δ 150.74, 146.03, 138.44, 130.87, 127.24, 126.17, 124.49, 121.65, 116.69, 114.37, 96.33, 67.15, 54.93, 52.16, 38.53, 32.02, 25.76, 16.96, 14.89. Mass (Quattro II) LRMS (ESI) m/z Found 482.3 M+H, calculated: 481.31.
Results and Discussions
Both rhodamine B and rhodamine 6G AMR derivatives were investigated in this study. Rhodamine B analogs were synthesized by reacting a previously reported rhodamine intermediate (compound 1), containing a methylbromide at the 2' position, with the corresponding cycloalkylamine providing the corresponding cycloalkyl-AMR compounds: cyclopropyl (cPr-AMR), cyclobutyl (cBu-AMR), cyclopentyl (cPntAMR), and cyclohexyl (cHex-AMR) (Scheme 1). Rhodamine 6G derivatives were synthesized by direct formation of an amide with the corresponding cycloalkylamine and subsequent reduction using LAH to form the following cycloalkylamines: cyclopropyl (cPr-AMR6G), cyclobutyl (cBu-AMR6G), cyclopentyl (cPnt-AMR6G), and cyclohexyl (cHex-AMR6G) (Scheme 2). Each molecule was characterized by 1H, 13C NMR and mass spectrometry. In the 13C NMR spectrum, a characteristic quaternary carbon ~60 ppm corresponding to the C-N bond was used to verify the spiro-cyclic structure of the AMR series. Upon characterization, each molecule's pH dependent photophysical properties were investigated by UV-vis and fluorescence.
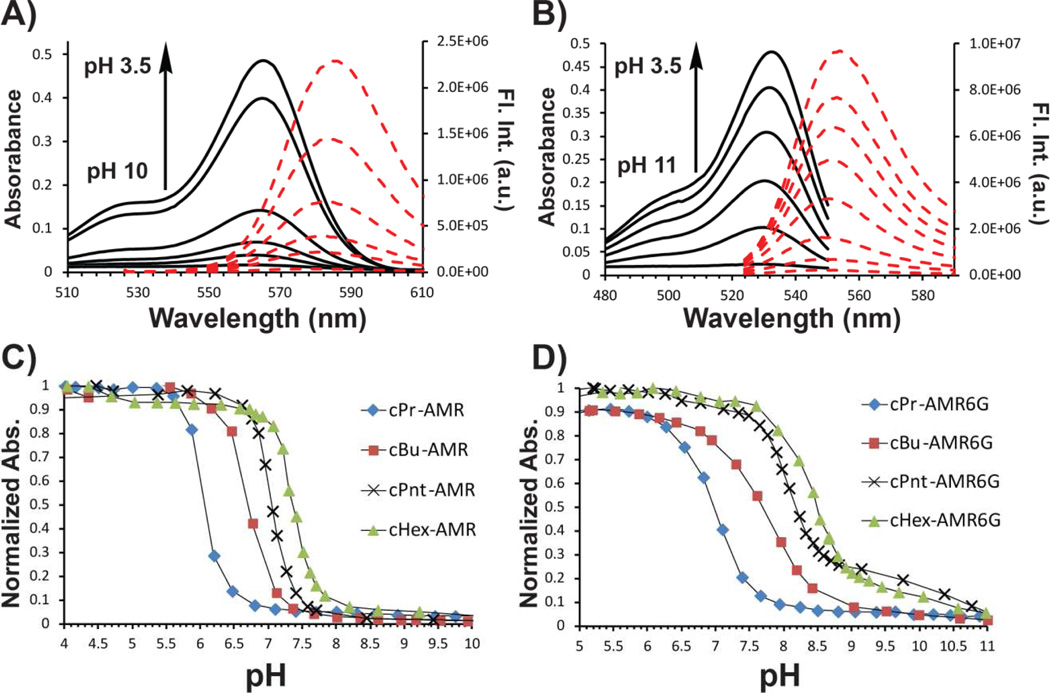
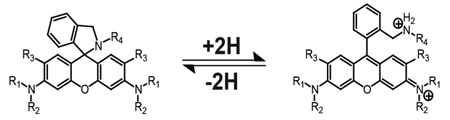
Titrations were carried out on either a 5 µM or 2.5 µM solution of cAMR derivative in phosphate buffer solution for UV-vis and fluorescence respectively. Small aliquots of acid (HCl) or base (NaOH) were added to change the pH of the solution. Both cAMR and cAMR6G derivatives are colorless and non-fluorescent under basic conditions, which is indicative of the spiro-cyclic form of rhodamines. Under neutral or slightly acidic conditions, the characteristic rhodamine color is observed from either rhodamine B (pink) or rhodamine 6G (yellow/green) and the solution becomes highly fluorescent. The mechanism of the ring-opening process involves initial protonation on the nitrogen and subsequent opening of the spiro-cyclic ring. Further protonation occurs but is not observed as a separate event by either UV-vis absorption or fluorescence. This is consistent with previous AMR derivatives [12] but differs from the AnMR series where two separate events were observed in the fluorescence and UV-vis absorption measurements upon decreasing the pH of the solution [14]. An equilibrium constant between the spiro-cyclic form and the open-form can be approximated at half-max of the pH dependent absorbance curve, which we will conveniently refer to as the pKa of the individual cAMR derivative. From the pH titrations curves, a clear trend based on the size of the cycloalkylamine ring is observed, which is consistent with Perrin's et al. studies on the effect of the Baeyer ring strain on cycloalkylamines [16]. Specifically, the pKaof both cAMR and cAMR6G derivatives have the following trend: cPr<cBu<cPnt<cHex, which correlates with the theory that the smaller rings deviate more from the tetrahedral angle of 109° causing a more strained molecule. The decreased bond angle of the cycloalkane group increases the p-character of the bonds, resulting in electron density being pulled from the nitrogen. In other words, the increase in ring strain of the cycloalkyl group on the amine causes it to be less basic. Similarly to what was observed by Feng and co-workers in the rhodamine spiro-cyclic amide series [10], the cAMR6G series was found to have a higher pKa than the cAMR series when comparing analogous cycloalkane derivatives. Interestingly, the difference in the pKa values is approximately one pH unit between the cAMR6G series and the cAMR analogs. A plausible explanation is that the two methyl groups on the rhodamine core in the cAMR6G series create a sterically crowded environment making the molecule less thermodynamically stable. This steric effect may cause the pKa values of the series to be higher. While analyzing the photophysical properties of this system, no apparent trend in the quantum yield or molar absorptivity was observed within each series. However, the quantum yields of the cAMR6G series were higher than the cAMR series, which was expected based on the known lower quantum yield associated with rhodamine B.
Conclusions
In conclusion, we have reported a series of fluorescent pH sensors, which can be tuned to pH ranges of biological interest by introducing various sized cycloalkane rings to the amino group of the AMR scaffold. These fluorescent pH probes were easily synthesized from their rhodamine B and rhodamine 6G parent molecules. The order of cAMR basicity was found to be dependent on the ring size with the following order: cPr<cBu<cPnt<cHex. Overall, the cAMR6G derivatives were found to have higher pKas than their AMR analogs, which is attributed to a more sterically hindered spiro-cyclic structure. Thus, the pH dependent properties of the AMR system can be tuned through a combination of electronic and steric effects. Due to the low quantum yield of the cAMR system, it is unlikely that series could be used in biological applications; however, the exceptional photophysical properties of the cAMR6G series and the pH ranges in which they are responsive make them ideal for a variety of biological applications.
Supplementary Material
Figure 1.
Fluorescence and UV-vis spectra during pH titrations of a 5 M solution of cPr-AMR (A) or cPr-AMR6G (B) in 0.10 M phosphate buffer solution and 1% DMSO. Titration curves of the AMR (C) and AMR6G (D) series.
Table 1.
Spectroscopic and pKa data of the cAMR and cAMR6G series. Quantum yields were measured relative rhodamine B or rhodamine 6G in ethanol.
 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | pKa | Mol.Abs. | Φ |
| cPr-AMR | Et | Et | H | 6.1 | 96,200 | 0.09 | |
| cBu-AMR | Et | Et | H | 6.7 | 89,100 | 0.05 | |
| cPnt-AMR | Et | Et | H | 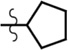 |
7.1 | 92,700 | 0.05 |
| cHex-AMR | Et | Et | H | 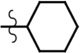 |
7.4 | 107,500 | 0.09 |
| cPr-AMR6G | Et | H | Me | 7.0 | 114,000 | 0.68 | |
| cBu-AMR6G | Et | H | Me | 7.7 | 53,100 | 0.68 | |
| cPnt-AMR6G | Et | H | Me | 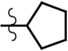 |
8.2 | 76,100 | 0.68 |
| cHex-AMR6G | Et | H | Me | 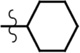 |
8.5 | 79,600 | 0.48 |
Acknowledgements
The authors gratefully acknowledge support fully or in part from the National Institutes of Health (NIH-1R15GM080721-01A1), the National Science Foundation (NSF-CHEM-0719185). The authors also thank the Department of Chemistry and Biochemistry, Southern Illinois University for partial support of this research.
References
- 1.Martinez GA, Kane P. Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J Biol Chem. 2008;283:20309–20319. doi: 10.1074/jbc.M710470200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci. 1998;95:6803–6808. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Research. 2006;66:6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- 4.Prescott DM, Charles HC, Poulson JM, Page RL, Thrall DE, Vujaskovic Z, Dewhirst MW. relationship between intracellular and extracellular pH in spontaneous canine tumors. Clin Cancer Res. 2000;6:2501–2505. [PubMed] [Google Scholar]
- 5.Han J, Burgess K. Fluorescent indicators for intracellular pH. Chem Rev. 2010;110:2709–2728. doi: 10.1021/cr900249z. [DOI] [PubMed] [Google Scholar]
- 6.Tian M, Peng X, Fan J, Wang J, Sun S. A fluorescent sensor for pH based on rhodamine fluorophore. Dyes and Pigm. 2012;95:112–115. [Google Scholar]
- 7.Hu Q, Li M, Liu M, Zhuang W, Li G. A highly sensitive fluorescent acidic pH probe based on rhodamine B diethyl-2-aminobutenedioate conjugate and its application in living cells. Dyes and Pigm. 2013;96:71–75. [Google Scholar]
- 8.Lv H, Liu J, Zhao B, Miao J. Highly selective and sensitive pH-responsive fluorescent probe in living Hela and HUVEC cells. Sens. Actuators, B. 2013;177:956–963. [Google Scholar]
- 9.Wang J, Yang Q, Song H, Zhang W. A fluorescent probe of N′-formyl-rhodamine B hydrazide: structure and spectral properties of protonation behavior. Org Biomol Chem. 2012;10:7677–7680. doi: 10.1039/c2ob26288f. [DOI] [PubMed] [Google Scholar]
- 10.Yuan L, Lin W, Feng Y. A rational approach to tuning the pKa values of rhodamines for living cell fluorescence imaging. Org Biomol Chem. 2011;9:1723–1726. doi: 10.1039/c0ob01045f. [DOI] [PubMed] [Google Scholar]
- 11.Czaplyski W, Purnell GE, Roberts CA, Allred RM, Harbron EJ. Substituent effects on the turn-on kinetics of rhodamine-based fluorescent pH probes. Org Biomol Chem. 2014;12:526–533. doi: 10.1039/c3ob42089b. [DOI] [PubMed] [Google Scholar]
- 12.Best QA, Xu R, McCarroll ME, Wang L, Dyer DJ. Design and Investigation of a Series of Rhodamine-Based Fluorescent Probes for Optical Measurements of pH. Org Lett. 2010;12:3219–3221. doi: 10.1021/ol1011967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Song Y, Yang Y, Yang L, Huang X, Han J, Han S. Rhodamine-deoxylactam functionalized poly[styrene-alter-(maleic acid)]s as lysosome activatable probes for intraoperative detection of tumors. Chem Sci. 2012;3:2941–2948. [Google Scholar]
- 14.Best QA, Liu C, van Hoveln PD, McCarroll ME, Scott SN. Anilinomethylrhodamines: pH sensitive probes with tunable photophysical properties by substituent effect. J Org Chem. 2013;7:10134–10143. doi: 10.1021/jo401323g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bender A, Woydziak ZR, Fu L, Branden M, Zhou Z, Ackley BD, Peterson BR. Novel Acid-Activated Fluorophores Reveal a Dynamic Wave of Protons in the Intestine of Caenorhabditis elegans. ACS Chemical Biology. 2013;8:636–642. doi: 10.1021/cb300396j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrin CL, Fabian MA, Rivero IA. Basicities of cycloalkylamines: Baeyer strain theory revisited. Tetrahedron. 1999;55:5773–5780. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.