Abstract
Objective
We mapped human ventricular fibrillation (VF) to define mechanistic differences between episodes requiring defibrillation versus those that spontaneously terminate.
Background
VF is a leading cause of mortality, yet episodes may also self-terminate. We hypothesized that the initial maintenance of human VF is dependent upon the formation and stability of VF rotors.
Methods
We enrolled 26 consecutive patients (age 64±10 years, n=13 with LV dysfunction) during ablation procedures for ventricular arrhythmias, using 64-electrode basket catheters in both ventricles to map VF prior to prompt defibrillation per IRB-approved protocol. Fifty-two inductions were attempted and 36 VF episodes were observed. Phase analysis was applied to identify bi-ventricular rotors in the first 10 seconds or until VF terminated, whichever came first (11.4±2.9 seconds to defibrillator charging).
Results
Rotors were present in 16 of 19 patients with VF, and in all patients with sustained VF. Sustained, but not self-limiting VF, was characterized by greater rotor stability: (1) rotors were present in 68±17% of cycles in sustained versus 11±18% of cycles in self-limiting VF (p<0.001); with (2) maximum continuous rotations greater in sustained (17±11, range 7–48) versus self-limiting VF (1.1±1.4, range 0–4, p<0.001). Additionally, biventricular rotor locations in sustained VF were conserved across multiple inductions (7/7 patients, p=0.025).
Conclusions
In patients with and without structural heart disease, the formation of stable rotors identifies individuals whose VF requires defibrillation from those in whom VF spontaneously self-terminates. Future work should define the mechanisms that stabilize rotors and evaluate whether rotor modulation may reduce subsequent VF risk.
Keywords: arrhythmia mechanisms, electrical rotors, electrophysiology, ventricular fibrillation
Introduction
Ventricular fibrillation (VF) is a common, life-threatening arrhythmia and a major cause of the 700,000 cases of sudden cardiac death in the US and Europe annually (1). Although our understanding of VF mechanisms continues to improve (2), we still do not fully understand the mechanistic differences between VF episodes which perpetuate and those which spontaneously terminate (3).
Superficially, VF appears to be random and disorganized. However, significant progress has been made to identify deterministic features within VF (4,5). Detailed epicardial mapping suggests the co-existence of electrical rotors and disorganized activity in induced VF in patients with preserved ventricular function during open heart surgery (6). However, the importance of rotors and other propagation patterns to the maintenance of human VF remains uncertain. VF rotors have been studied in the context of ischemia (7) and scar (8) using animal models and explanted human hearts, yet these studies have not explained why some VF episodes require defibrillation while others self-terminate without consequence.
Prior work has shown the presence of rate gradients (9) in sustained VF, supporting the concept of spatial preferences for VF drivers. Subsequent work evaluating surface ECG patterns found evidence for repetitive spatial paths of VF sources (10). More recent studies have shown evidence that electrical rotors predominantly associate with areas of scar (11). Based upon these data, we hypothesized that greater electrical rotor stability would predict the perpetuation of early human VF and its progression to sustained VF.
Methods
Patient Enrollment
In this prospective study of the relationship between VF rotors and duration, we enrolled consecutive patients presenting for ventricular arrhythmia ablation at the University of California San Diego and VA San Diego Healthcare System. The protocol was approved by a joint UCSD/VA institutional review board (IRB), and all patients provided written, informed consent after a full discussion of risks and potential benefits. Exclusion criteria included the presence of ventricular thrombus, hemodynamic instability precluding the safe induction of VF, and unrevascularized coronary ischemia.
Antiarrhythmic drugs (mexilitine=2, amiodarone=1, dronedarone=1, and sotalol=6) were discontinued greater than 5 half-lives (6 weeks for amiodarone) prior to electrophysiology study. LV function was assessed by transthoracic echocardiography prior to the procedure.
Study Protocol
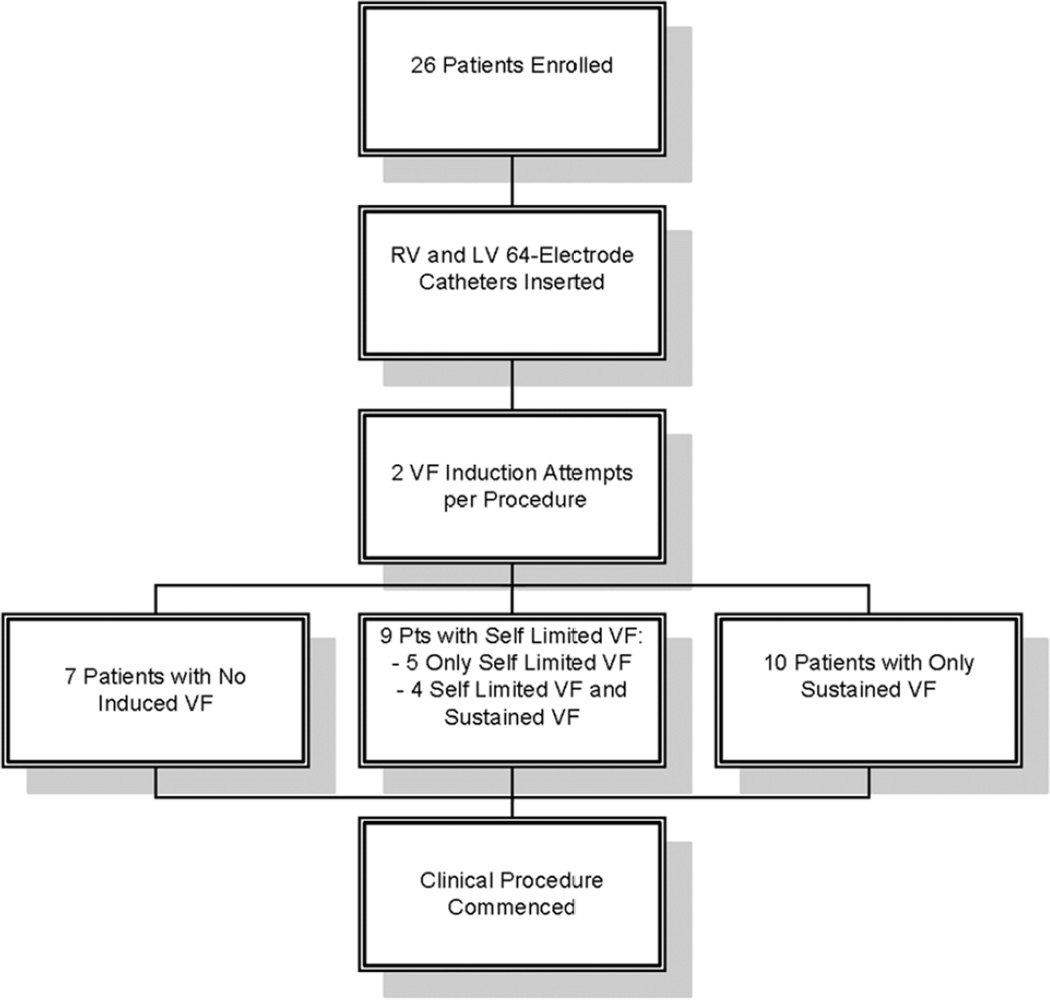
The study protocol is summarized in figure 1. Patients were intubated, ventilated and maintained under a consistent general anesthetic protocol. A decapolar catheter was placed in the coronary sinus, and a quadripolar catheter was placed in the right ventricle (RV) for VF induction. Invasive arterial pressure and vital signs were monitored continuously throughout the case.
Figure 1. Study Protocol.
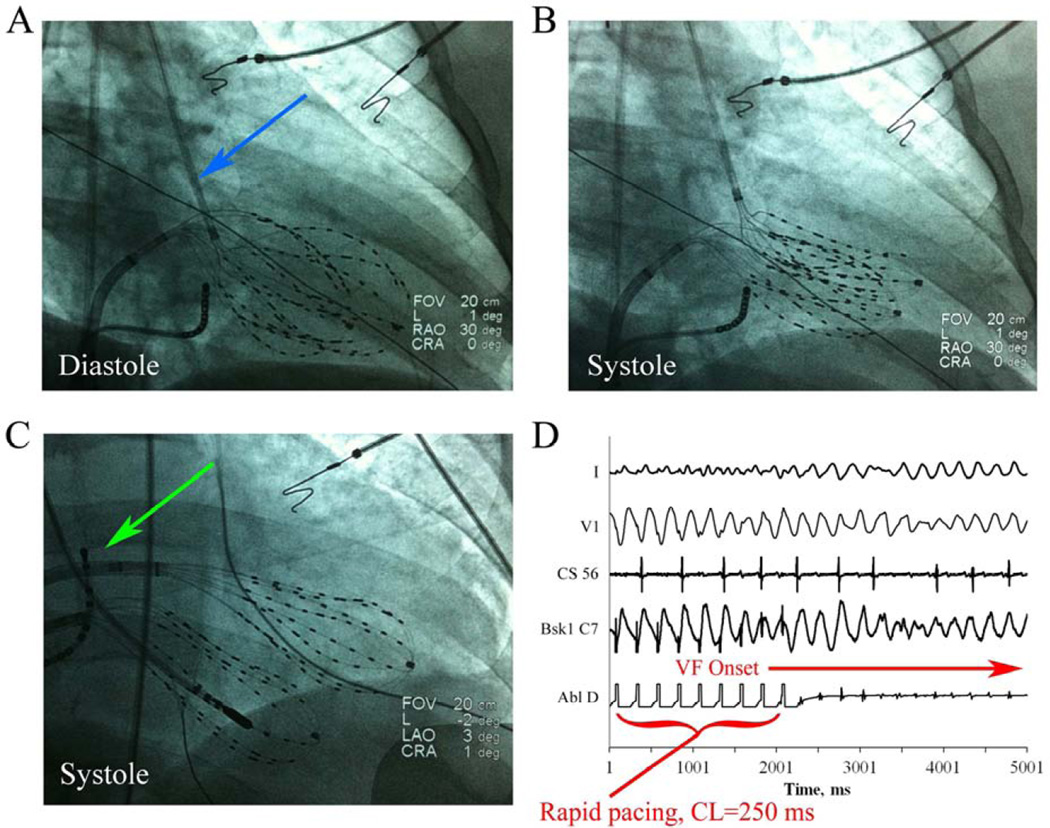
Basket catheters 64-electrode, Constellation, Boston Scientific, Natick, MA) were advanced for simultaneous recording into the RV and left ventricle (LV) either by retrograde aortic (figure 2A–B) or transseptal (figure 2C) approaches, as best suiting the clinical procedure. Basket catheter contact was evaluated by: (1) evaluating fluoroscopic basket catheter morphology to ensure uniform deformation by cineangiography (figure 2A–C), (2) imaging with intracardiac ultrasound, and (3) ensuring that electrogram amplitude both at baseline and during VF was acceptable. Electrodes with noisy or low amplitude signals (<0.5 mV) were excluded from analysis and the corresponding areas on phase mapping left blank; on average 10±7 electrodes (7.8%) were excluded in each case due to suboptimal contact or noise.
Figure 2. Biventricular mapping and VF induction.
Right anterior oblique fluoroscopy of biventricular baskets during diastole (A) and systole (B) in a 51 year old patient with a normal LV EF, mild RV dysfunction, and symptomatic VT and PVCs. The LV basket was advanced via the retrograde aortic approach (blue arrow). (C) Antero-posterior fluoroscopy showing biventricular baskets in a 68 year old patient during systole in which the LV basket was advanced via transseptal catheterization (green arrow). (D) VF induction by protocol-driven rapid pacing (250 msec) showing surface ECG (I and V1) and intracardiac electrograms (CS56, LV basket C7, and ablation distal).
VF Induction
Following baseline programmed ventricular stimulation, rapid pacing was performed for 15 seconds, followed by a 1 minute recovery period, for each cycle length (CL) of 350, 300, 250, then decrementing by 10 msec until VF induction (figure 2D) or 2:1 capture (minimum CL 170 msec) per protocol, similar to prior work (12). As soon as VF was induced, defibrillator charging commenced, and VF was recorded during this charging period. VF was defibrillated as soon as charging was complete (11.4±2.9 seconds; range 8–15 seconds). After a 5 minute waiting interval, a second episode of VF was induced in each patient either with a second burst pacing induction or 3.2 seconds of rapid pacing followed by a 2 Joule T-wave shock (in patients with ICDs). VF was defined as varying ECG morphology with a rate >220 beats per minute as previously defined (8). Following the second attempted VF induction, the clinical procedure was commenced in routine fashion.
Electrogram Analysis
Unipolar electrograms (Bard Pro, Billerica, MA) were recorded at 1000 Hz and filtered from 0.05 to 500 Hz. Multipolar basket electrograms were analyzed offline using software that we have developed and described previously (13) and optimized for VF analysis, using phase analysis (14) of unipolar electrograms (6), within physiologic constraints (15,16). Data were analyzed for the first 10 seconds of VF or until termination, whichever came first.
Rotational activity was identified as a phase singularity formed at the intersection of depolarization and repolarization isolines (4) consisting of at least 1 rotation (figure 3). Rotors were defined as regions of rotational activity that controlled surrounding activation, and the criteria for numbers of rotations in human VF were derived in this study. Regions of centrifugal propagation without rotation were defined as focal activation (figure 4A–B). Continuous, disorganized ventricular activation without a clear rotational or focal activation (‘fibrillatory conduction’; figure 4C–D) was also documented.
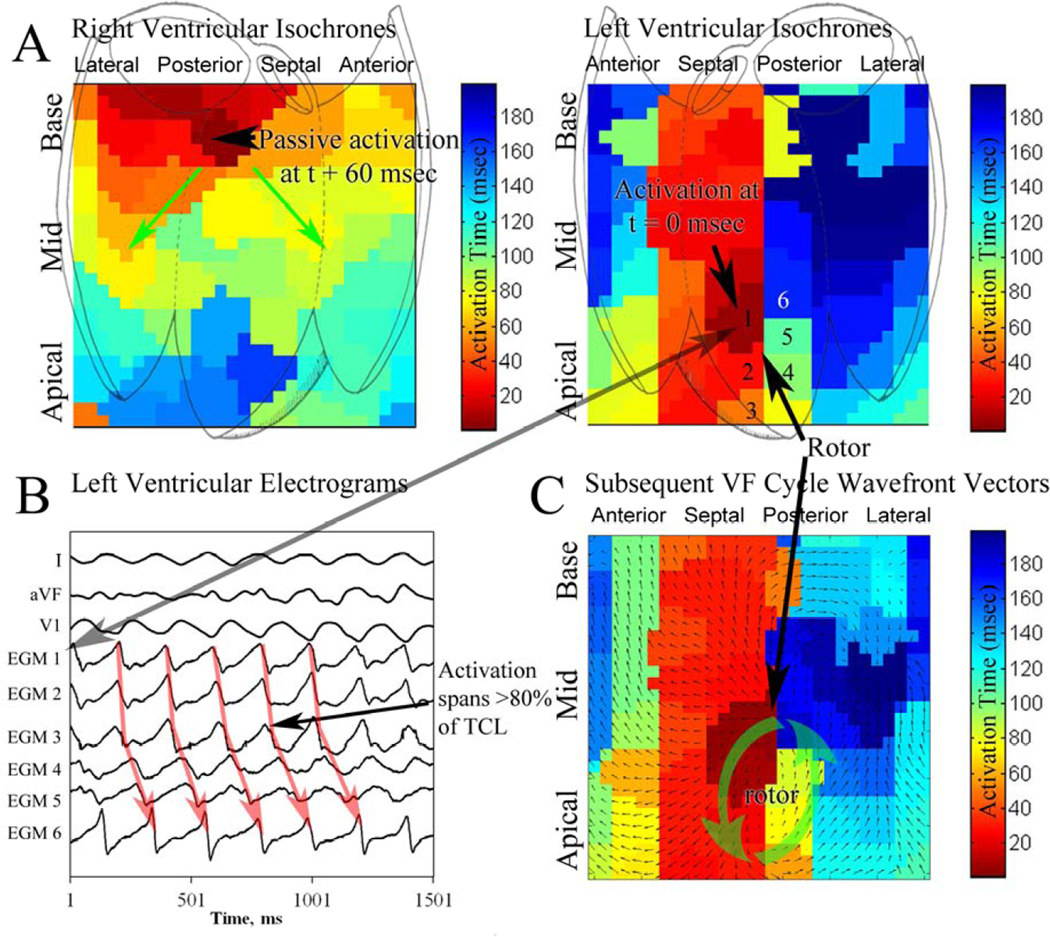
Figure 3. Biventricular VF analysis showing LV rotor.
(A) Isochronal analysis of the RV and LV during ventricular fibrillation in a 73 year old patient, EF 25%, presenting for drug-refractory ventricular tachycardia. LV isochrones show a rotor (CL 220 msec) in the septal LV. This rotor persisted for 15 continuous rotations; rotor activity was seen in 72% of all VF cycles in this patient. (B) Basket electrograms during VF, numbered (1–6) near the rotor core. Note that activation spans <80% of the VF cycle length. (C) Wavefront vector analysis of the subsequent VF cycle, showing consistent rotation about the core with radial activation of distant tissue.
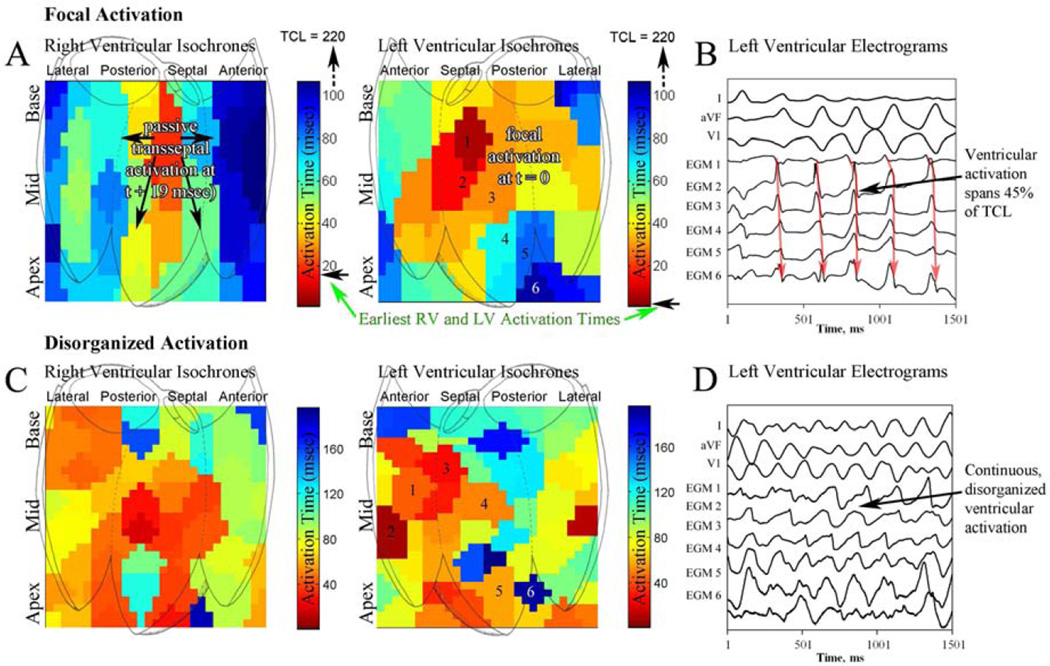
Figure 4. Focal and disorganized activation patterns during VF.
(A) Biventricular isochronal analysis of ventricular fibrillation in a 68 year old patient with idiopathic cardiomyopathy, ejection fraction of 32%, showing a focal source in the anteroseptal LV with passive activation of the RV beginning 19 msec after LV activation. (B) Electrograms at increasing distance from the focal source. Note that LV endocardial activation spans approximately 45% of the VF cycle length. (C) Disorganized biventricular activation without a stable rotor during VF in a 52 year old patient with a normal EF. (D) Intracardiac electrograms are varying and chaotic, spanning the VF cycle.
Measuring Rotor Prevalence and Stability
We quantified the prevalence of rotational activity as the percent of VF cycles showing such activity, with stability quantified as the maximum number of consecutive revolutions of electrical activity within a region bounded by 2 electrodes in each axis. We performed receiver-operating characteristic (ROC) analysis to determine criteria for prevalence and stability which functionally separated sustained from self-limiting episodes of VF.
Modeling Endocardial Recording of Non-Endocardial VF Sources
To explore the endocardial projection of non-endocardial VF sources, we created a 3-dimensional computational model of a hairpin-shaped rotor filament with both ends terminating on the epicardium. The Barkley model (17) was implemented on 200×100×100 grid, and the filament was initiated as previously described (18). Additional details may be found in the online supplement.
Statistical Methods
Continuous variables are expressed as mean±standard deviation. The Student t test was used to compare continuous variables; the Fisher exact test was used to compare nominal variables. The ROC cutpoints were determined by optimization of the Youden index. The relationship between rotor stability and ejection fraction (EF) was calculated using Pearson correlation. Subject-wise and episode-wise statistics are indicated. For episode-wise comparisons, repeated measures analysis of variance was used to determine differences between self-limited and sustained VF episodes. The Bonferroni correction was applied for planned multiple comparisons. The paired t-test was used in the analysis of patients with both sustained and self-limited VF. Statistics were calculated using SPSS 19 (IBM, Somers, NY, USA).
Results
We enrolled 26 patients (13 with LV EF < 50%, age 64±10 y); demographics are shown in table 1. There were no thromboembolic complications or other adverse events during the study.
Table 1.
Study Demographics
| Characteristics | Preserved EF | LV Dysfunction | p Value |
|
|---|---|---|---|---|
| n | 10 | 12 | - | |
| Age (years) ±SD | 62±13 | 67±7 | 0.27 | |
| Left atrial diameter, mm±SD | 36±5 | 45±12 | 0.08 | |
| Left ventricular EF (%) | 65±8 | 33±8 | 0.001 | |
| Hypertension (%) | 6(60) | 10(83) | 0.35 | |
| Diabetes mellitus (%) | 3(30) | 3(25) | 1 | |
| Hyperlipidemia (%) | 8(80) | 10(83) | 1 | |
| Coronary disease (%) | 6(60) | 6(50) | 0.69 | |
| Prior Myocardial Infarction (%) | 3(30) | 4(33) | 1 | |
| Prior PCI (%) | 3(30) | 4(33) | 1 | |
| CABG (%) | 1(10) | 3(25) | 0.59 | |
| COPD (%) | 1(10) | 1(8.3) | 1 | |
| Medications | ||||
| Beta-Blocker (%) | 7(70) | 11(92) | 0.29 | |
| ACEI/ARB (%) | 4(40) | 10(83) | 0.07 | |
| Digoxin (%) | 1(10) | 4(33) | 0.32 | |
| Calcium Channel Blockers (%) | 1(10) | 3(25) | 0.59 | |
| Mexilitine (%) | 0 | 1(8) | 1 | |
| Amiodarone (%) | 0 | 0 | 1 | |
| Sotalol (%) | 1(10) | 5(42) | 0.16 | |
| Dofetilide (%) | 0 | 0 | 1 | |
| Coumadin (%) | 2(20) | 5(42) | 0.38 | |
| Aspirin (%) | 5(50) | 4(33) | 0.67 | |
| Statin (%) | 7(70) | 7(58) | 0.68 | |
VF Induction
A total of 52 VF induction attempts were performed per IRB-approved protocol resulting in 36 episodes of VF (CL 210±26). Other VF induction attempts yielded monomorphic ventricular tachycardia (VT, n=8) and no ventricular arrhythmia (n=8) and were excluded from analysis. There were no significant differences in cycle length between pacing-induced and shock-induced VF (see online supplement for additional details and results). Of VF episodes, 21 lasted ≥8 seconds (“sustained VF”) and required defibrillation (duration 11.4±2.9 seconds), and 15 were self-limited (duration 3.9±1.4 seconds).
The demographics of patients with sustained and self-limited VF are shown in table 2. Cycle length was similar for sustained VF (203±25 msec) and self-limited VF (216±21 msec, p=0.08). Patients with self-limited VF had higher LV EF than those with sustained VF. Ischemic cardiomyopathy was more common in patients with sustained VF (50%) than without (0%, p=0.03).
Table 2.
Demographics of Patients with Sustained and Self-Limited VF
| Characteristics of Pts with VF |
Self-Limited VF | Sustained VF | p Value |
|
|---|---|---|---|---|
| n | 9 | 10 | - | |
| Age (years) ±SD | 64±8 | 67±7 | 0.37 | |
| Left atrial diameter, mm±SD | 39±9 | 44±13 | 0.45 | |
| Left ventricular EF (%) | 52±14 | 32±9 | 0.002 | |
| Hypertension (%) | 8(89) | 8(80) | 1 | |
| Diabetes mellitus (%) | 3(33) | 2(20) | 0.63 | |
| Hyperlipidemia (%) | 7(78) | 9(90) | 0.58 | |
| Coronary disease (%) | 5(56) | 6(60) | 1 | |
| Prior Myocardial Infarction (%) | 2(22) | 5(50) | 0.35 | |
| Prior PCI (%) | 5(56) | 2(20) | 0.17 | |
| CABG (%) | 2(22) | 4(40) | 0.63 | |
| COPD (%) | 0 | 2(20) | 0.47 | |
| Cardiomyopathy, EF<50% | 3(33) | 10(100) | 0.003 | |
| Etiology: Ischemic CMP | 0 | 5(50) | 0.03 | |
| Etiology: Nonischemic CMP | 3(33) | 5(50) | 0.65 | |
| Medications | ||||
| Beta-Blocker (%) | 7(78) | 9(90) | 0.58 | |
| ACEI/ARB (%) | 6(67) | 8(80) | 0.63 | |
| Digoxin (%) | 0 | 4(40) | 0.09 | |
| Calcium Channel Blockers (%) | 1(11) | 2(20) | 1 | |
| Mexilitine (%) | 0 | 2(20) | 0.47 | |
| Amiodarone (%) | 0 | 1(10) | 1 | |
| Dronedarone (%) | 0 | 0 | 1 | |
| Sotalol (%) | 0 | 5(50) | 0.03 | |
| Dofetilide (%) | 0 | 0 | 1 | |
| Warfarin (%) | 1(11) | 5(50) | 0.14 | |
| Aspirin (%) | 5(56) | 4(40) | 0.66 | |
| Statin (%) | 7(78) | 6(60) | 0.63 | |
Rotors in VF
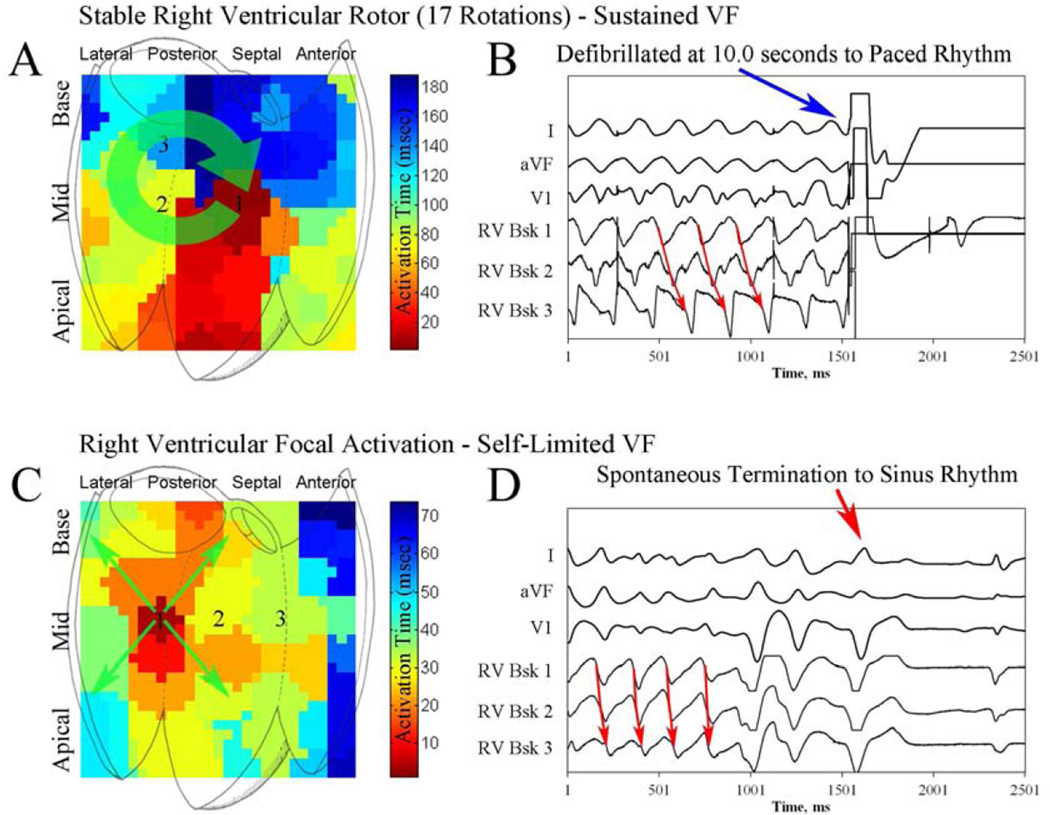
Localized sites of rotational activation were seen in 16 of 19 patients with VF (89%), and in all patients with sustained VF (10/10, 100%), in whom sustained rotors of longer prevalence and stability were found. Figure 3A and online movie 1 show a LV counterclockwise rotor during induced VF in a 73 year old patient with an EF of 23% presenting for first VT ablation. This rotor was mapped over 15 rotations; VF required defibrillation to terminate. Electrograms showing sequential activation around a core, spanning >80% of the VF cycle are shown in figure 3B. Vector analysis of the subsequent VF cycle (3C) shows stable activation around the core with wavefront spread to more distant tissue, controlling ventricular activation. In figure 3A, right ventricular activation is passive, consistent with transseptal conduction.
Spatial Conservation of Stable Rotors over Multiple VF Inductions
Stable VF rotors in sustained VF were conserved over multiple inductions: there were 7 patients in whom 2 episodes of sustained VF were induced. In each, rotor sites were conserved within 1 electrode radius (7/7 patients, p=0.023). Online movies 2 and 3 show sequential VF episodes in a 68 year old patient with recurrent VT in which the rotor recurs in the posteroseptal LV. In contrast, focal sources locations were infrequently conserved (2/10 patients with conserved focal source sites, p=NS).
Differences in Rotor Prevalence between Sustained and Self-Limited VF
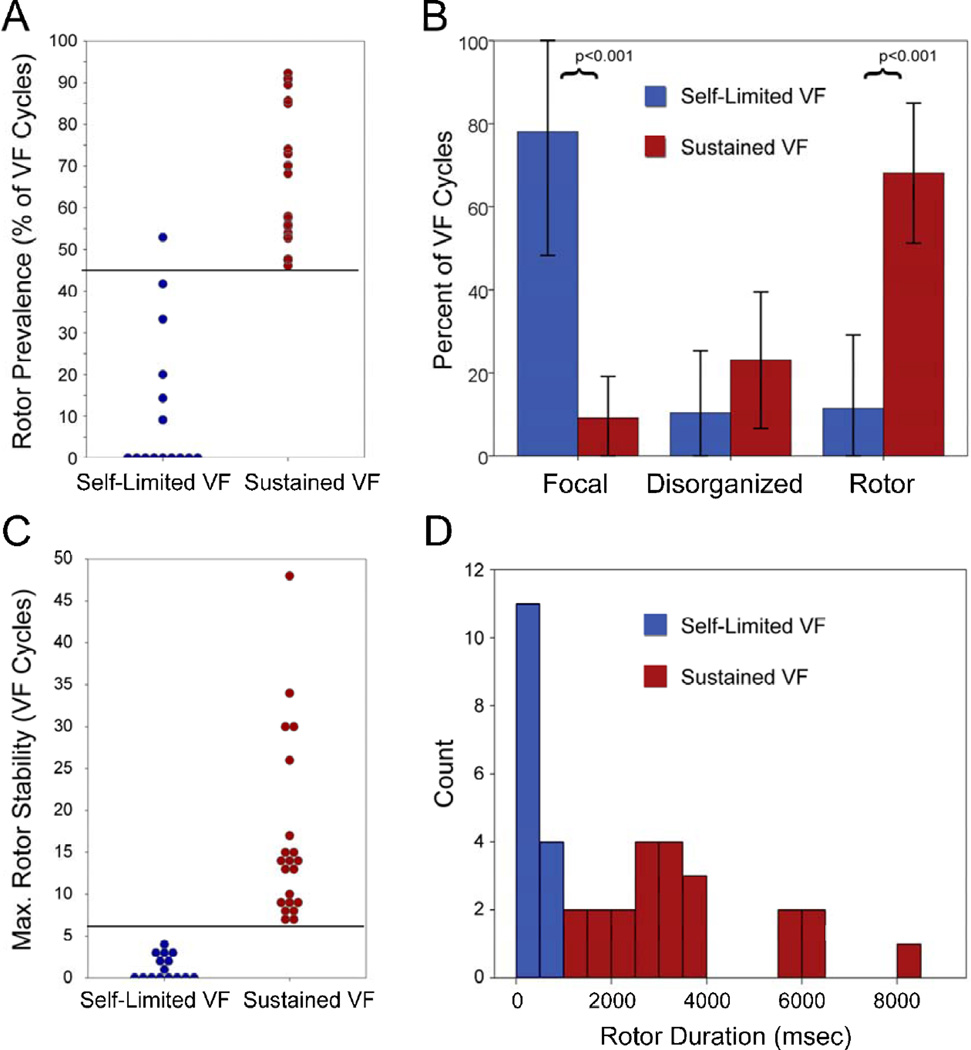
Rotors were more prevalent in sustained VF; they were present for 68±17% of VF cycles in sustained VF versus 11±17% in self-limited VF (p<0.001). ROC analysis for rotor prevalence and VF outcome demonstrate that a cutoff of ≥45% of VF cycles showing rotors separated sustained from self-limited VF with 100% sensitivity and 93% specificity (figure 5A).
Figure 5. Activation patterns in sustained versus self-limited VF.
(A) Rotor prevalence for each VF episode; prevalence ≥45% separates sustained from self-limited VF with 100% sensitivity and 93% specificity. (B) Rotor, disorganized and focal activation prevalence for the study. Rotor and focal activation prevalence were different between sustained and self-limited VF. (C) Maximum rotor stability (consecutive rotations) for each episode: sustained and selflimited VF populations do not overlap. (D) Histogram of temporal rotor stability (milliseconds) for sustained and self-limited VF. When rotors persisted <1 second, VF perpetuated and required defibrillation.
Focal and Disorganized Activation Patterns in VF
Figure 4A–B and movie 4 show an example of focal activation in a 68 year old patient with idiopathic cardiomyopathy (LV EF 32%), located in the anteroseptal LV during VF (CL 222 msec). Figure 4B shows basket electrograms with activation spanning only 45% of the VF cycle. VF terminated spontaneously after 4 seconds. Figure 4C–D and movie 5 show disorganized activation in a 52 year old patient with frequent, symptomatic PVCs and an EF of 69% during VF. Basket electrograms show disorganized activation spanning each VF cycle.
Figure 5B shows the prevalence of rotors and alternative activation patterns for all VF episodes. Notably, focal activity comprised a greater proportion of VF cycles in self-limited VF (78±29%) versus sustained VF (9±9%, p<0.001). Unlike rotors, focal sources were infrequently spatially conserved; 2 of 12 focal sources (17%, p=NS) were located within 1 inter-electrode separation between VF episodes. Disorganized activation (fibrillatory conduction) was similarly prevalent between self-limited VF (23±16%) and sustained VF (10±15%, p=0.1).
Differences in Rotor Stability between Sustained and Self-Limited VF
Rotors in sustained VF persisted for more consecutive VF cycles (17±11 cycles, range 7–48) than rotors in self-limited VF (1.1±1.4 cycles, range 0–4, p<0.001). Figure 6A–B shows a sustained VF episode in which the rotor completes 17 rotations. This VF episode required defibrillation (fig 6B). Figure 6C shows self-limited VF with focal activation and no rotor. A cutoff of 7 consecutive rotations effectively separates sustained and self-limited VF episodes (fig 5C). Figure 5D shows temporal rotor stability for sustained and self-limited VF.
Figure 6. Right ventricular rotor and focal activation during VF.
(A) Isochronal analysis of VF showing a right ventricular rotor in a 79 year old patient with ischemic cardiomyopathy. Maximum rotor stability was 17 rotations, and rotor prevalence was 91%. (B) This VF episode was defibrillated (right side) 10 seconds after induction. (C) VF isochronal analysis demonstrating a right ventricular focal source in a 68 year old patient with preserved left ventricular function. (D) VF terminated spontaneously.
Patients with both Sustained and Self-Limited VF
There were 4 patients in whom sustained and self-limited VF were observed in separate episodes. Similar to the overall population, rotors were more prevalent (50±14% vs 8±15% of rotations, p=0.032) and more stable (15±10 vs 2±2 consecutive rotations, p=0.04) in sustained vs self-limited VF, respectively, for this subgroup.
Rotor Stability, Ventricular Substrate and Location
We found that VF rotor duration was negatively correlated with LV function (correlation coefficient 0.58, p=0.037). Although the majority of rotors and focal sources were found in the left (67%) versus right ventricle (33%), the difference was not statistically significant.
Endocardial Mapping of Non-Endocardial VF Sources
In our simulations, non-endocardial rotors presented endocardially as focal activation. Additional details may be found in the online supplement.
Discussion
There are three main findings of the present study. First, rotational activation is common in human VF, and stable rotors are common in sustained VF requiring defibrillation, which is most likely to result in clinical sequelae. Second, rotors lie in conserved areas for successive VF inductions, suggesting that rotors form in regions of favorable structural and functional substrate. Third, focal activation without stable rotors is more prevalent in self-limiting VF, and may thus reflect absence of this substrate. Importantly, these differences are seen in the subset of patients in whom both sustained and self-limited VF episodes were induced, and thus these findings are independent of substrate. These findings motivate studies to define the mechanisms underlying rotor formation, to explore the feasibility of localized therapies such as ablation, pre-emptive pacing, or cellular therapy to prevent VF.
The Importance of Rotors to VF Perpetuation
Multiple mechanisms have been observed in ongoing VF: sustained rotors (4,5) and focal sources (2) in canine ventricles; transient rotors and multiple wavelets in human ventricles (6). A central question is whether these mechanisms differ between VF that terminates spontaneously, and episodes which progress to sustained VF and result in symptoms, syncope, and sudden death.
In this work, we studied a spectrum of patients with ventricular arrhythmias. We found that rotor prevalence ≥45% of mapped cycles and stability ≥7 cycles identified VF that was sustained and required cardioversion from episodes that terminated spontaneously. While such cutoffs are somewhat arbitrary, since the mechanisms for and risk of VF likely form a spectrum, they re-emphasize that the formation of stable rotors is important to VF maintenance. Perhaps the most compelling support for the importance of stable rotor formation comes from the subgroup with both sustained and self-limited episodes. Despite identical substrate between episodes, significant differences in rotor stability were observed between the episode that required cardioversion and the episode that spontaneously terminated. Thus rotor stabilization is at least a hallmark of, and possibly a critical step in the transition of early VF to sustained VF.
Notably, the average rotor prevalence and stability in sustained VF was ~70% and 17 rotations, respectively. These values differ from prior work, but important differences must be considered. First, many prior studies used animal models (4,9,19), explanted human hearts supported by Langendorf-perfusion (8,11), or subjects undergoing open heart surgery (6,7). As shown by Qin and colleagues, such techniques may alter VF mechanisms (20). Our study employed multielectrode mapping via a percutaneous approach in patients, more closely approximating physiologic conditions. Additionally, we sampled a large proportion of the endocardium of both ventricles, including both sides of the interventricular septum, using biventricular basket catheters. Such catheters have previously been used to study VF in animal models (21–23), and patients (24). Second, we quantified ventricular activation patterns in early VF. Different mechanisms may predominate later in VF due to progression of ischemia and electrical remodeling.
Structural Determinants of VF Rotor Sites
Prior work has shown the dependence of rotors upon myocardial scar (8). A subsequent study supports the observation that rotors tend to more frequently localize at diseased substrate (11). Our work is in agreement with these findings, noting that the majority of sustained VF episodes, with more stable rotors, were found in patients with LV dysfunction. Ischemic cardiomyopathy, in particular, was more common in patients with sustained VF. However, the presence of structural heart disease or EF alone, were imperfect predictors of sustained VF; several patients without LV dysfunction had VF progress to sustained, clinically significant episodes.
Importantly, we found that sustained VF rotor locations were conserved between episodes. Such spatial conservation implies that structural factors or fixed spatial distributions of functional gradients determine rotor locations. In a separate case report (25), we have previously described a patient in whom VT ablation incidentally coincided with the primary VF rotor site. He then presented with recurrence of VT several months later, and again consented to the study protocol, which was then unable to induce VF, only monomorphic VT. Based on these findings, future studies should examine if targeted intervention may reduce the probability of sustained VF (19).
Future Directions: Rotor Sites As Therapeutic Targets
As with other arrhythmias, intervention in VF is possible at multiple points, including initiating triggers and sustaining sites. Previously, ablation of Purkinje-related premature ventricular contraction (PVC) triggers has been shown to decrease VF episodes in studies of patients without structural heart disease (26). However, the role of triggers in initiating VF in patients with structural heart disease is less clear (27), while sustaining mechanisms for VF are acknowledged as the predominant driver for sudden death in all patients. Inspired by animal models showing stable VF rotors (4), and by recent work in which stable atrial fibrillation rotors sites were successfully identified and ablated in real time (28), we hypothesize that VF rotors may be suitable targets for ablation to decrease ICD shocks for patients with recurrent VF.
Limitations
First, this study was limited by the spatial resolution of the electrode spacing (4–5 mm interelectrode, 10 mm interspline) of the basket catheters. However, from wavelength considerations, the minimum APD in human ventricle is ~140 ms (15) and minimum conduction velocity approximately 40 cm/second (16), providing a minimum circumference of approximately 7 cm (2 cm diameter). Thus, resolution should be sufficient to resolve such rotors. Second, only the endocardium was mapped. Based upon our simulation studies and others (29), endocardial mapping may misclassify non-endocardial rotors as focal sources, and thus underestimate the true prevalence of VF rotors. However, only a minority (17%) of mapped focal sources in this study displayed characteristics consistent with rotors. Furthermore, prior work in explanted human hearts has shown the prevalence of intra-myocardial rotors is low (8), and animal models of VF have shown that endocardial intervention altered VF inducibility (19), consistent with the hypothesis that the endocardium is important for early VF maintenance. As a result, we believe that endocardial mapping in this study may underdetect <20% of rotors in early VF. Third, we enrolled a heterogeneous group of patients by design, using protocol-driven VF inductions to examine mechanistic differences in outcome. That differences between sustained and self-limited VF were consistent across patients regardless of LV function and induction type supports the generalizability of our findings. Fourth, VF was induced by rapid pacing and shock-on-T, and differences in ischemic time may have influenced VF mechanisms. However, the total difference in ischemic time (~11 seconds) was significantly below the 30 second threshold for ischemia to significantly alter VF (7). Furthermore, we were unable to identify differences in VF rate, regularity, number of rotors, or rotor duration (see online supplement) between these induction methods. Fifth, for practical reasons we could study only early VF in this procedural model. However, early VF is of critical importance because patients may become symptomatic and experience syncope or ICD shocks during early VF, and because early VF initiates the cascade leading to sustained VF. Sixth, we did not routinely ablate rotor sites and retest VF inducibility to prove that such sites are critical for the maintenance of VF as we have for atrial rotor sites (30), although we have reported a case (31) in which that did occur, and VF was subsequently not inducible with the study pacing protocol. Future studies of VF rotor ablation are planned. Seventh, artifact during VF may have created the appearance of rotors. However, such artifact was not seen during rapid pacing prior to VF. Finally, the sample size of the study is small, which may limit the generalizability of our findings.
Conclusions
Rotor prevalence and stability separate sustained and self-limited VF. Rotor sites in sustained VF are conserved, and rotor stability is inversely correlated with LV function, implicating a dependence on a localized pro-arrhythmic substrate. Future studies should determine the conditions under which stable rotors form, and whether such sites may be safely modulated in humans to reduce subsequent VF risk.
Supplementary Material
Acknowledgements
We are indebted to Kathleen Mills, BA for coordinating this study. We also wish to thank Donna Cooper, RN, Elizabeth Greer, RN, Stephanie Yoakum, RNP, Ken Hopper, CVT, Tony Moyeda, CVT, Judy Hildreth, RN, and Cherie Jaynes, RN for their assistance with the clinical portion of this study.
Funding Sources: Dr. Krummen has received grant support from the American Heart Association (10 BGIA 3500045) and NIH (HL 83359). Dr. Narayan has received grant support from the NIH (HL 83359, HL103800) and Doris Duke Foundation.
Dr. Krummen has served as a consultant to InsilicoMed, and has received fellowship program support from Biotronik, Boston Scientific, Medtronic, St. Jude Medical, and Biosense-Webster. Dr. Rappel has intellectual property interest in Topera Medical. Dr. Narayan has intellectual property and ownership interest in Topera Medical, and has served as a consultant to Biotronik, Medtronic, St. Jude Medical, and Topera. He has received fellowship program support from Biotronik, Boston Scientific, Medtronic, St. Jude Medical, and Biosense-Webster.
Abbreviations
- CL
Cycle Length
- EF
Ejection Fraction
- ICD
Implantable Cardioverter Defibrillator
- IRB
Institutional Review Board
- LV
Left Ventricle
- ROC
Receiver Operating Characteristic
- RV
Right Ventricle
- VT
Ventricular Tachycardia
- VF
Ventricular Fibrillation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Hayase, Dr. Morris, Dr. Ho, Ms. Smetak, and Mr. Clopton have no disclosures.
References
- 1.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. Jama. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Zheng X, Dosdall DJ, Huang J, Ideker RE. Different types of long-duration ventricular fibrillation: can they be identified by electrocardiography. Journal of electrocardiology. 2012;45:658–659. doi: 10.1016/j.jelectrocard.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair K, Umapathy K, Downar E, Nanthakumar K. Aborted sudden death from sustained ventricular fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2008;5:1198–1200. doi: 10.1016/j.hrthm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 5.Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992;355:349–351. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- 6.Nash MP, Mourad A, Clayton RH, et al. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation. 2006;114:536–542. doi: 10.1161/CIRCULATIONAHA.105.602870. [DOI] [PubMed] [Google Scholar]
- 7.Bradley CP, Clayton RH, Nash MP, et al. Human ventricular fibrillation during global ischemia and reperfusion: paradoxical changes in activation rate and wavefront complexity. Circulation. Arrhythmia and electrophysiology. 2011;4:684–691. doi: 10.1161/CIRCEP.110.961284. [DOI] [PubMed] [Google Scholar]
- 8.Nair K, Umapathy K, Farid T, et al. Intramural activation during early human ventricular fibrillation. Circulation. Arrhythmia and electrophysiology. 2011;4:692–703. doi: 10.1161/CIRCEP.110.961037. [DOI] [PubMed] [Google Scholar]
- 9.Zaitsev AV, Guha PK, Sarmast F, et al. Wavebreak formation during ventricular fibrillation in the isolated, regionally ischemic pig heart. Circulation research. 2003;92:546–553. doi: 10.1161/01.RES.0000061917.23107.F7. [DOI] [PubMed] [Google Scholar]
- 10.Narayan SM, Bhargava V. Temporal and spatial phase analyses of the electrocardiogram stratify intra-atrial and intra-ventricular organization. IEEE Trans Biomed Eng. 2004;51:1749–1764. doi: 10.1109/TBME.2004.827536. [DOI] [PubMed] [Google Scholar]
- 11.Jeyaratnam J, Umapathy K, Masse S, et al. Relating spatial heterogeneities to rotor formation in studying human ventricular fibrillation. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society; IEEE Engineering in Medicine and Biology Society. Conference 2011; 2011. pp. 231–234. [DOI] [PubMed] [Google Scholar]
- 12.Cao JM, Qu Z, Kim YH, et al. Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing: importance of cardiac restitution properties. Circulation research. 1999;84:1318–1331. doi: 10.1161/01.res.84.11.1318. [DOI] [PubMed] [Google Scholar]
- 13.Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. Journal of cardiovascular electrophysiology. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray MA, Wikswo JP. Considerations in phase plane analysis for nonstationary reentrant cardiac behavior. Physical review. E, Statistical, nonlinear, and soft matter physics. 2002;65:051902. doi: 10.1103/PhysRevE.65.051902. [DOI] [PubMed] [Google Scholar]
- 15.Narayan SM, Franz MR, Lalani G, Kim J, Sastry A. T-wave alternans, restitution of human action potential duration, and outcome. J Am Coll Cardiol. 2007;50:2385–2392. doi: 10.1016/j.jacc.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Narayan SM, Bayer JD, Lalani G, Trayanova NA. Action potential dynamics explain arrhythmic vulnerability in human heart failure: a clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol. 2008;52:1782–1792. doi: 10.1016/j.jacc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkley D. A Model for Fast Computer-Simulation of Waves in Excitable Media. Physica D. 1991;49:61–70. [Google Scholar]
- 18.Dutta S, Steinbock O. Steady motion of hairpin-shaped vortex filaments in excitable systems. Physical Review E. 2010;81 doi: 10.1103/PhysRevE.81.055202. [DOI] [PubMed] [Google Scholar]
- 19.Pak HN, Kim GI, Lim HE, et al. Both Purkinje cells and left ventricular posteroseptal reentry contribute to the maintenance of ventricular fibrillation in open-chest dogs and swine: effects of catheter ablation and the ventricular cut-and-sew operation. Circulation journal : official journal of the Japanese Circulation Society. 2008;72:1185–1192. doi: 10.1253/circj.72.1185. [DOI] [PubMed] [Google Scholar]
- 20.Qin H, Kay MW, Chattipakorn N, Redden DT, Ideker RE, Rogers JM. Effects of heart isolation, voltage-sensitive dye electromechanical uncoupling agents on ventricular fibrillation. American journal of physiology. Heart and circulatory physiology. 2003;284:H1818–H1826. doi: 10.1152/ajpheart.00923.2002. [DOI] [PubMed] [Google Scholar]
- 21.Eldar M, Ohad DG, Goldberger JJ, et al. Transcutaneous multielectrode basket catheter for endocardial mapping and ablation of ventricular tachycardia in the pig. Circulation. 1997;96:2430–2437. doi: 10.1161/01.cir.96.7.2430. [DOI] [PubMed] [Google Scholar]
- 22.Everett THt, Wilson EE, Foreman S, Olgin JE. Mechanisms of ventricular fibrillation in canine models of congestive heart failure and ischemia assessed by in vivo noncontact mapping. Circulation. 2005;112:1532–1541. doi: 10.1161/CIRCULATIONAHA.104.521351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dosdall DJ, Osorio J, Robichaux RP, Huang J, Li L, Ideker RE. Purkinje activation precedes myocardial activation following defibrillation after long-duration ventricular fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2010;7:405–412. doi: 10.1016/j.hrthm.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu W, Aiba T, Kurita T, Kamakura S. Paradoxic abbreviation of repolarization in epicardium of the right ventricular outflow tract during augmentation of Brugada-type ST segment elevation. Journal of cardiovascular electrophysiology. 2001;12:1418–1421. doi: 10.1046/j.1540-8167.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- 25.Hayase J, Tung R, Narayan SM, Krummen DE. A case of a human ventricular fibrillation rotor localized to ablation sites for scar-mediated monomorphic ventricular tachycardia. Heart rhythm : the official journal of the Heart Rhythm Society. 2013 doi: 10.1016/j.hrthm.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knecht S, Sacher F, Wright M, et al. Long-term follow-up of idiopathic ventricular fibrillation ablation: a multicenter study. J Am Coll Cardiol. 2009;54:522–528. doi: 10.1016/j.jacc.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 27.Sacher F, Victor J, Hocini M, et al. Characterization of premature ventricular contraction initiating ventricular fibrillation. Archives des maladies du coeur et des vaisseaux. 2005;98:867–873. [PubMed] [Google Scholar]
- 28.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of Atrial Fibrillation by the Ablation of Localized Sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) Trial. Journal of the American College of Cardiology. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berenfeld O, Jalife J. Purkinje-muscle reentry as a mechanism of polymorphic ventricular arrhythmias in a 3-dimensional model of the ventricles. Circ Res. 1998;82:1063–1077. doi: 10.1161/01.res.82.10.1063. [DOI] [PubMed] [Google Scholar]
- 30.Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation) Journal of the American College of Cardiology. 2013;62:138–147. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayase J, Tung R, Narayan SM, Krummen DE. A case of a human ventricular fibrillation rotor localized to ablation sites for scar-mediated monomorphic ventricular tachycardia. Heart rhythm : the official journal of the Heart Rhythm Society. 2013;10:1913–1916. doi: 10.1016/j.hrthm.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.