Abstract
The proteoglycan decorin putatively inhibits cell adhesion and cell migration on various extracellular matrix substrates through interactions with β1 integrins. This study therefore examined the adhesive, migration, and proliferative characteristics of decorin knockout (Dcn−/−) murine embryonic fibroblasts compared to wild-type controls on collagen-coated, fibronectin-coated, and uncoated tissue culture plates. The Dcn−/− cells showed significantly greater proliferation than wild-type controls on all substrates. The Dcn−/− cells also showed significantly greater adhesion to both collagen and fibronectin; both cell types showed greater adhesion to collagen. The addition of exogenous decorin had a differential effect on adhesion to collagen between cell types, but not on fibronectin. For collagen, blocking either α2 or β1 integrin subunits significantly reduced adhesion for Dcn−/− cells; whereas for fibronectin, blocking either the α5 or β1 integrin subunits reduced adhesion for both cell types. Decorin and the α5β1 integrin may have lesser roles in adhesion to fibronectin than previously presumed. Finally, compared to wild-type cells, Dcn−/− cells showed greater migration on both uncoated and collagen substrates. This study demonstrates that decorin affects the biology of various integrins that participate in cell proliferation, adhesion, and migration on various substrates.
Keywords: decorin, collagen, fibronectin, adhesion, migration, beta 1 integrin
INTRODUCTION
Decorin, a member of the small leucine rich proteoglycan family, is an important regulator of collagen fibrillogenesis,1 collagen degradation,2 and matrix organization.3 Given this close association with collagen, decorin is therefore involved in foreign body reactions to implanted biomaterials4 as well as the remodeling of native and engineered tissues. Decorin also modulates collagenase gene expression and the availability of growth factors in the extracellular matrix by binding of the decorin core protein to fibronectin,5,6 collagen,7 and transforming growth factor beta (TGF-β).8,9 Increased decorin expression has also been reported in healing wounds,10 fibrotic tissues within infarcted myocardium,11,12 tissues undergoing angiogenesis,13 tumor stroma,14 and arthritic joints.15 The involvement of decorin in the above tissue remodeling and pathogenesis can be attributed in part to the inhibitory effect of decorin on cell adhesion16,17 as well as the ability of decorin to bind to TGF-β9 and epidermal growth factor receptors.18–21 Due to both of these effects, the addition of decorin has been shown to influence the proliferation of many different cell types.19,22,23
Cell adhesion to extracellular matrix substrates, predominantly fibronectin, collagen, vitronectin, fibrinogen, and laminin, regulates several aspects of normal cell behavior, such as proliferation, migration, gene expression, and protein synthesis.5,24 Cell adhesion to these matrix molecules occurs mainly through β1 integrins and triggers a variety of signaling pathways.24 Interestingly, the negative influence of decorin on cell adhesion to these substrates has also been shown to be mediated via the β1 subunit of integrin.17,25,26 However, it is still unclear whether this influence is primarily mediated by the β1 subunit binding to the glycosaminoglycan (GAG) chain26 or the core protein of decorin, or the proteoglycan itself binding to the substrate.5,6,27
The influence of decorin on cell adhesion to matrix components likely also affects engineered tissue material behavior, particularly through the regulation of collagen fibrillogenesis. Previously, it was thought that decorin deficiency consistently causes irregular collagen fibril density and morphology.11,28,29 However, it was also shown that tendons from decorin deficient mice actually exhibited greater strength than corresponding tissues from wild-type controls.30,31 Furthermore, prolapsed mitral valves, which exhibit reduced mechanical strength,32 were recently shown to contain a greater abundance of decorin than in normal valves.12 These conflicting reports suggest that the influence of decorin may depend on substrate, environment, and cell type. Therefore, there is a need for further investigation into the role of decorin, particularly with respect to cell-integrin-matrix interactions, in the development, maturation, and mechanical behavior of engineered tissues.
We previously investigated a novel means of elucidating the myriad contributions of decorin to engineered tissue development using decorin knockout (Dcn−/−) murine embryonic fibroblasts (MEFs).33,34 Interestingly, we found that that 3-D collagen gels seeded with Dcn−/− MEFs showed greater matrix organization, cell proliferation, ultimate tensile strength, and elastic modulus than those seeded with wild-type control cells. The properties demonstrated by these collagen gels seeded with Dcn−/− cells were partly influenced by TGF-β and the mechanical environment within the ECM. Specifically, the presence of TGF-β improved gel contraction, matrix organization, and tensile strength of collagen gels seeded with Dcn−/− cells.33 In addition, cyclically straining these collagen gels helped to increase collagen fibril density, proteoglycan density, GAG chain length, and elastic modulus.34 We also found that the Dcn−/− cells expressed greater amounts of α2β1 integrin than wild-type cells. If MEF adhesion to collagen is substantially governed by α2α1 integrins, then the greater expression of α2β1 integrins by the Dcn−/− cells would result in improved cellular adhesion and consequently superior matrix organization. These observed characteristics of the developing 3-D collagen gels could also be attributed to decorin-dependent differences in adhesion and migration behavior between the Dcn−/− and wild-type cells. Although cell adhesion and migration are highly relevant to tissue engineering, these behaviors have not been previously investigated for Dcn−/− cells, yet understanding such behavior could explain the greater contraction of collagen gels seeded with these cells.
In this study, we assessed the influence of decorin on the proliferative, adhesive, and migration behavior of Dcn−/− and wild-type MEFs grown on uncoated, collagen-coated, or fibronectin-coated tissue culture plastic, using the rationale that the surface-dependent cell behavior from these 2-D experiments would be consistent with observations from the 3-D tissue model. Since Dcn−/− cells were previously shown to express a greater amount of α2β1 integrins, we also investigated the contribution of α2 and β1 integrin subunits to cellular adhesion to collagen. In addition, we investigated the influence of decorin on fibronectin adhesion via the α5β1 integrin,35 commonly known as the fibronectin receptor, to investigate the substrate-dependent effects of decorin.
EXPERIMENTAL PROCEDURES
Cell culture
Embryonic fibroblasts were isolated from euthanized Dcn−/− or wild-type mouse embryos (12.5 to 13.5 gestational days old) from Balbc background, as described in previous studies.33 Embryonic fibroblasts were selected for use in this study and in previous studies33 because these less differentiated cells were believed to behave in a manner that could be generalized to multiple, more differentiated cell types. The cells were maintained in an incubator (37°C, 5% CO2, 95% humidity) and supplemented with culture medium containing high glucose Dulbecco's Modified Eagle Medium (DMEM, Mediatech, Inc., Herndon, VA), 10% fetal bovine serum (Hyclone, Logan, UT), 1% antibiotic/antimycotic/antifungal solution (Mediatech, Inc.), and 1% L-glutamine (Mediatech, Inc.). The medium was changed every 2 days and the cells were passaged upon confluence. Cells from passage numbers P5–P8 were used in this study.
Proliferation assays
The growth studies for the different types of cells were performed on uncoated, collagen, and fibronectin-coated tissue culture plastic. For the study using uncoated tissue culture plates, cells were grown in 6-well plates at an initial seeding density of 1×105 cells/well (10.5×3 cells/cm2) and the medium was changed every alternate day. The cells were harvested from triplicate wells every 2 days up to a total of 10 days. Cell numbers were measured using a hemacytometer (trypan blue exclusion).
For the studies using collagen-coated and fibronectin-coated plates, the cells were grown in 12-well plates coated with type I collagen or human fibronectin (both from BD Biosciences, Franklin Lakes, NJ). Cells were seeded at an initial density of 1.5×105 cells/well (3.9×104 cells/cm2) and the medium changed every alternate day. Since the cells were grown on collagen and fibronectin, instead of trypsinizing the cells, an MTT assay (Sigma, St. Louis, MO) was performed according to the manufacturer’s instructions on the cells from triplicate wells every 2 days up to a total of 10 days. Although MTT assays directly measure cell metabolic activity, they are also widely used to assess cell abundance.36 The higher cell density used in this study was required to achieve suitable absorbance values for the MTT assay. To perform the MTT assay, the culture media was first aspirated to remove any unadhered cells, then 1 ml of new media along with 100 µl of MTT reagent (5 mg/ml in sterile PBS, Sigma) was added to each well and the adherent cells were incubated at 37°C for 4 hours. After the incubation period, 1 ml of MTT solvent (0.1 N HCl in anhydrous isopropanol) was added to each well and mixed by trituration until the formazan blue crystals dissolved. The absorbance of the resulting formazan blue product was measured in triplicate at 570 and 690 nm using a spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA). The number of adhered cells per day was calculated by comparing the net absorbance (A570–A690) with net absorbance for a standard curve derived from known cell numbers (measured using a hemacytometer and trypan blue exclusion). The standard curves were created by measuring net absorbance from triplicate wells seeded at 0.1, 0.5, 1.0, 2.0, and 4.0×105 cells/well. Since different cell lines might metabolize MTT differently, standard curves for both cell types were created to verify any difference in absorbance for the same cell number.
Adhesion assays
Cell adhesion to collagen and fibronectin coated surfaces was analyzed for both cell types. To evaluate time dependent adhesion to collagen, cells were plated in triplicate in 12-well tissue culture plates coated with type I collagen at an initial seeding density of 1.5×105 cells/well (3.9×104 cells/cm2). After 0, 5, 10, 30, 60, 90, and 120 min, the culture media was aspirated to remove any unadherent cells, and 1 ml of media was added to the specific wells. The number of the adherent cells at each timepoint was measured using the MTT assay and normalized to the number of cells adhered at the final timepoint.
To evaluate the effect of cell concentration on the adhesion to collagen, cells were plated in triplicate wells at 0.5, 1.0, 2.0, and 4.0×105 cells/well (1.3, 2.6, 5.3, and 10.5×104 cells/cm2). After 1.5 h, the MTT assay was used to determine the number of adhered cells as previously described.
The above studies (time and concentration dependence) were also performed using fibronectin coated plates in order to determine if the role of decorin in cell adhesion was substrate-dependent. Although identical seeding densities were used, the experimental times were reduced to a final time of 60 min for the time dependence study and 30 min for the varying cell concentration study since both cell types adhered to fibronectin more rapidly than to type I collagen. Fibronectin substrate studies were also performed in triplicate wells.
Another study tested the ability of exogenously added decorin (from bovine articular cartilage, Sigma) to restore the Dcn−/− cells to wild-type behavior. The cells were plated in triplicate in a 12-well tissue culture plate coated with type I collagen at an initial seeding density of 2.0×105 cells/well. Immediately after seeding the cells, exogenous decorin (0, 1, 5, 10, or 20 µg/ml) was added to each well. After 1 h, the MTT assay was used to determine the number of adhered cells. The number of adhered cells for each concentration of decorin was normalized to the data from the control wells, to which no exogenous decorin was added.
Effect of blocking β1 integrins on cell adhesion
To determine the influence of β1 integrins on MEF adhesion to different matrix substrates, antibodies against the different integrin subunits were used in a competitive binding study. For adhesion to 12 well collagen-coated plates, antibodies against α2 and β1 were used (Rabbit integrin α2 and β1 from the Integrin β1 Antibody Kit, Chemicon International, Temecula, CA). Because adhesion to fibronectin is heavily mediated by the α5β1 integrin, studies on 12 well fibronectin-coated plates used antibodies against the α5 and β1 (Rabbit integrin α5 and β1 from the Integrin β1 Antibody Kit, Chemicon International). The optimal antibody concentration required to cause an effect on cellular adhesion for both cell types on each substrate was determined to be 1:500. To perform this study, 1 ml of media containing 1.5×105 Dcn−/− or wild-type cells (3.9×104 cells/cm2) and the antibodies against either α2, α5 or β1 integrin subunits was added to triplicate wells of 12-well plates coated with either type I collagen or fibronectin. Control wells were treated with rabbit IgG (Vector Laboratories Inc., Burlingame, CA) as an isotype control antibody to test for non-specific binding. After 90 min (collagen) or 30 min (fibronectin), the number of adhered cells was measured using the MTT assay as previously described. Each study was performed in triplicate wells and repeated three times. The numbers of adhered cells were normalized to the number of adhered cells in the control wells.
Migration study
Cell migration on collagen-coated, fibronectin-coated, and uncoated tissue culture plates was determined for both cell types. The design of this study was based on methods employed in several previously published migration studies.37–39 One cloning cylinder (10 mm diameter × 10 mm high, Fisher Scientific, Pittsburgh, PA) was placed at the center of each well of 6-well tissue culture plates, either uncoated or coated with type I collagen (BD Biosciences), or 12-well tissue culture plates coated with human fibronectin. Cells were seeded inside the cloning ring at 45,000 cells/well (in a volume of 50 µl to avoid leakage from the cloning rings) and incubated at 37°C. After four hours, the cloning ring was removed and the wells were carefully washed twice with PBS to remove unadhered cells. Three ml of fresh media containing mitomyocin C (0.5 µg/ml, Sigma) was then added to each well. Each well was viewed with an inverted microscope to verify that the cells were adherent and confined within the circular region previously created by the cloning cylinder. After incubating the cells for 24 hours, the number of cells that migrated out of the original circular region (based on comparison with an equivalent size circle drawn on a transparency) was counted. The study was performed in triplicate wells for both cell types on collagen-coated and uncoated surfaces.
Statistical analysis
Replicate analyses were averaged to obtain means and standard deviations. Statistical evaluations were performed using SigmaStat software (SPSS, Chicago, IL) for single factor and multiple factor ANOVAs. When a significant difference was observed, post-hoc testing was performed as suggested by the software for pair-wise comparisons. A value of p<0.05 was considered significant.
RESULTS
Greater proliferation for Dcn−/− cells
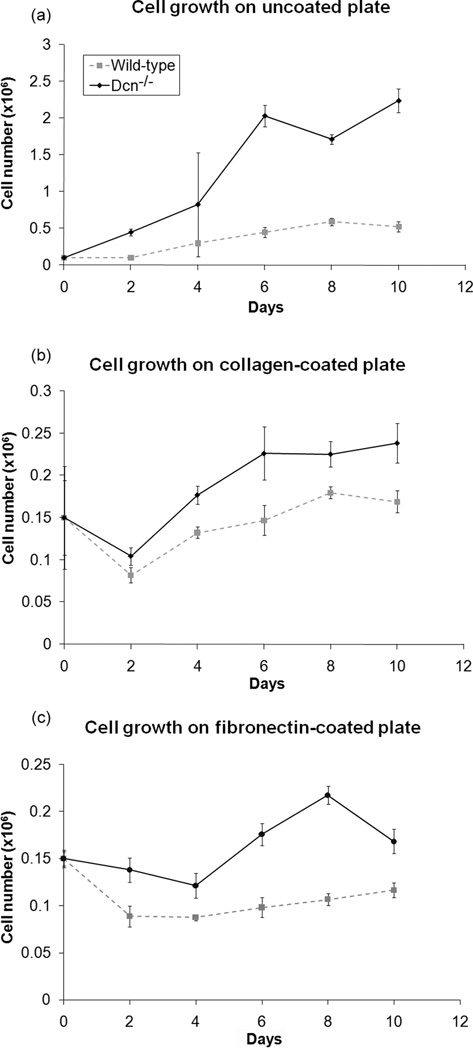
Since decorin inhibits cell growth for a variety of cell types,19,22,23 the cell growth on uncoated, collagen-coated, and fibronectin-coated tissue culture plates were determined for both cell types. The 2-D growth studies performed on all surfaces confirmed the general observation that the Dcn−/− fibroblasts had higher growth rate than the wild-type cells (p<0.001, Fig. 1a–c).
Figure 1.
In 2-D culture, decorin knockout (Dcn−/−) cells grew faster than wild-type cells. The cells were grown on uncoated or collagen coated surfaces. The Dcn−/− cells demonstrated significantly higher proliferation than the wild-type cells (p<0.001) in all three 2-D culture studies. Data represents average values (n=3 for uncoated & collagen-coated, n=6 for fibronectin-coated) at each time point and error bar represents standard deviation.
Greater adhesion for Dcn−/− cells
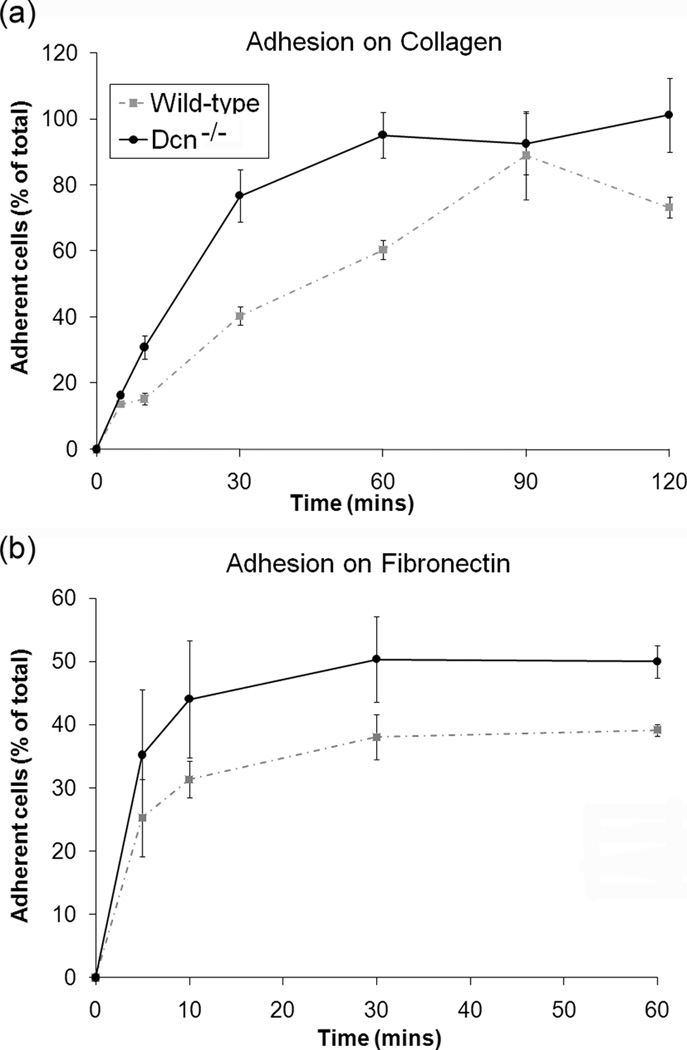
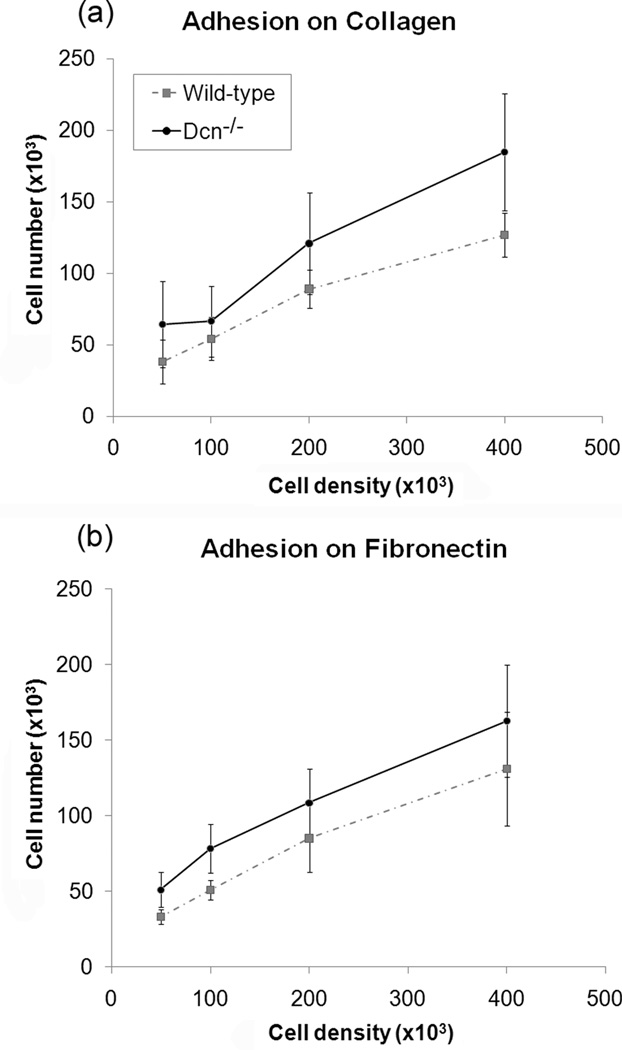
Since cell division is reportedly dominated by cell adhesion,16,17 the adhesion studies investigated which cell line demonstrated a faster adhesion rate and greater overall adherence to collagen and fibronectin. For the varying time experiment, there were differences in adhesion between the cell types after 10 minutes for both collagen and fibronectin substrates, with the maximum differences in adhesion at the 30 and 60 minute timepoints (Fig. 2). Regardless of the substrate, the Dcn−/− cells had a faster adhesion rate and greater overall adhesion than wild-type cells (p<0.001 for cell type and for both substrates). Both types of MEFs demonstrated greater adhesion to collagen than to fibronectin, although their initial rates of adhesion were faster on fibronectin. The varying concentration experiment determined if cellular adhesion was dependent on cell density. For the varying concentration experiment, adhesion was observed to be dependent on the number of seeded cells and the cell type on both substrates (Fig. 3, p<0.001 for cell concentration and cell type on both substrates). At most cell concentrations tested, the Dcn−/− cells showed greater adhesion to both substrates than did the wild-type cells (p<0.001).
Figure 2.
Decorin knockout (Dcn−/−) cells demonstrated greater cellular adhesion on (a) collagen-coated and (b) fibronectin-coated 12-well plates. Cell adhesion at various time point is normalized to 150×103 cells (i.e. total cells). Data represents average values (n=9) at each time point and error bar represents standard deviation. There was a statistically significant difference between the cell-types (p<0.001) for both collagen and fibronectin substrate.
Figure 3.
Cellular adhesion to (a) collagen-coated and (b) fibronectin-coated 12-well plates were dependent on cell concentration for both cell-types. Data represents average values (n=21) for each cell density and error bar represents standard deviation. A significant increase in cellular adhesion was observed for cell concentration for both cell-types (p<0.001) for both collagen and fibronectin substrates. Decorin knockout (Dcn−/−) cells also adhered at a significantly greater amount than wild-type cells on both substrates at all concentrations, except at the concentration of 1×105 on collagen. (p<0.001).
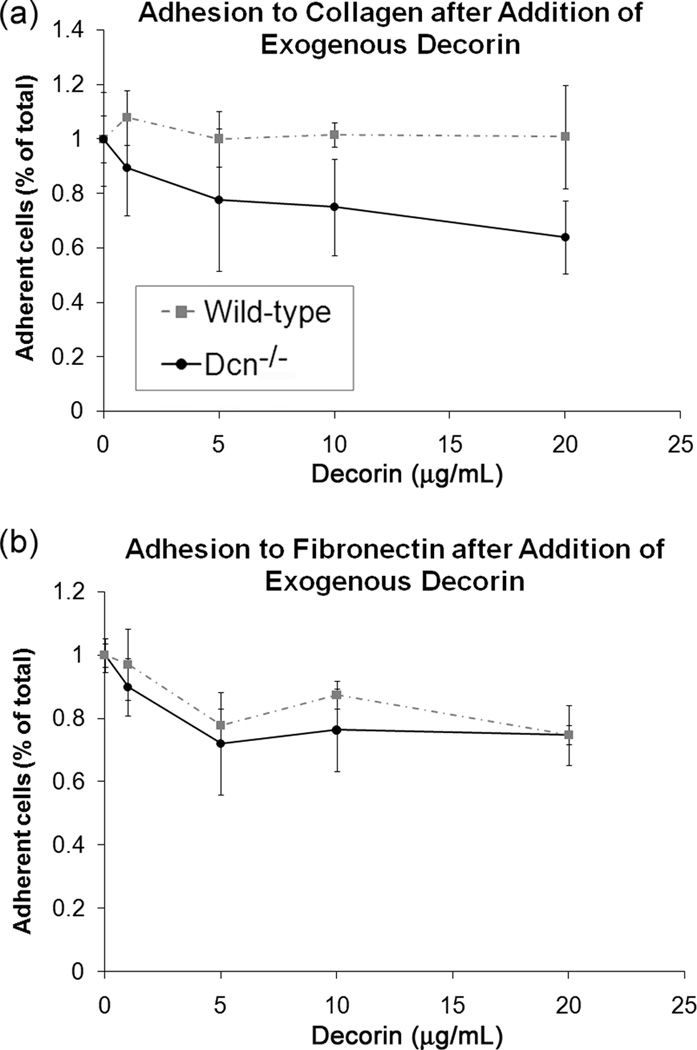
The exogenous decorin study verified the direct effect of decorin on cellular adhesion to collagen and fibronectin substrates. For this study, the adhesion of Dcn−/− and wild-type MEF to either substrate was measured after 60 min. The specific concentration of exogenous decorin added was found to have a significant effect on cell adhesion to both substrates (Fig. 4, p<0.005 for both collagen and fibronectin). With increasing concentrations of exogenous decorin, the Dcn−/− cells demonstrated decreased adherence to collagen (p<0.005 for 10 and 20 µg/ml) and fibronectin (p<0.005 for 5 and 20 µg/ml) compared to untreated controls. At 20 µg/ml, the exogenous decorin had a greater inhibitory effect on Dcn−/− cell adhesion to collagen than on adhesion to fibronectin (p<0.05 for substrate effect). In the presence of exogenous decorin, wild-type cell adhesion did not change significantly on collagen, but was significantly reduced on fibronectin (p<0.001 for 5, 10, and 20 µg/ml vs. control). In other words, in the presence of exogenous decorin, Dcn−/− showed less cell adhesion to collagen than did wild-type cells, but both cell types showed a comparable reduction in adhesion to fibronectin.
Figure 4.
Reduced cellular adhesion by decorin knockout (Dcn−/−) and wild-type cells after addition of exogenous decorin on (a) collagen-coated and (b) fibronectin-coated 12-well plates. Cell adhesion for various concentration of decorin is normalized to 2×105 cells (no added decorin). Sample size (n) was 6–9 for each concentration on each substrate. Data represents average values for each concentration of decorin and error bar represents standard deviation. There was a significant decrease in adhesion for Dcn−/−cells with increasing concentration of exogenously added decorin on both substrates (p<0.005 for control vs. 10 and 20 µg/ml on collagen, p<0.005 for control vs. 5 and 20 µg/ml), and a significant decrease in concentration-dependent adhesion for wild-type cells on fibronectin (p<0.005 for control vs. 5, 10, and 20 µg/ml). There was also a significant effect of cell type on cellular adhesion on both fibronectin and collagen (p<0.001 for cell type on both substrates).
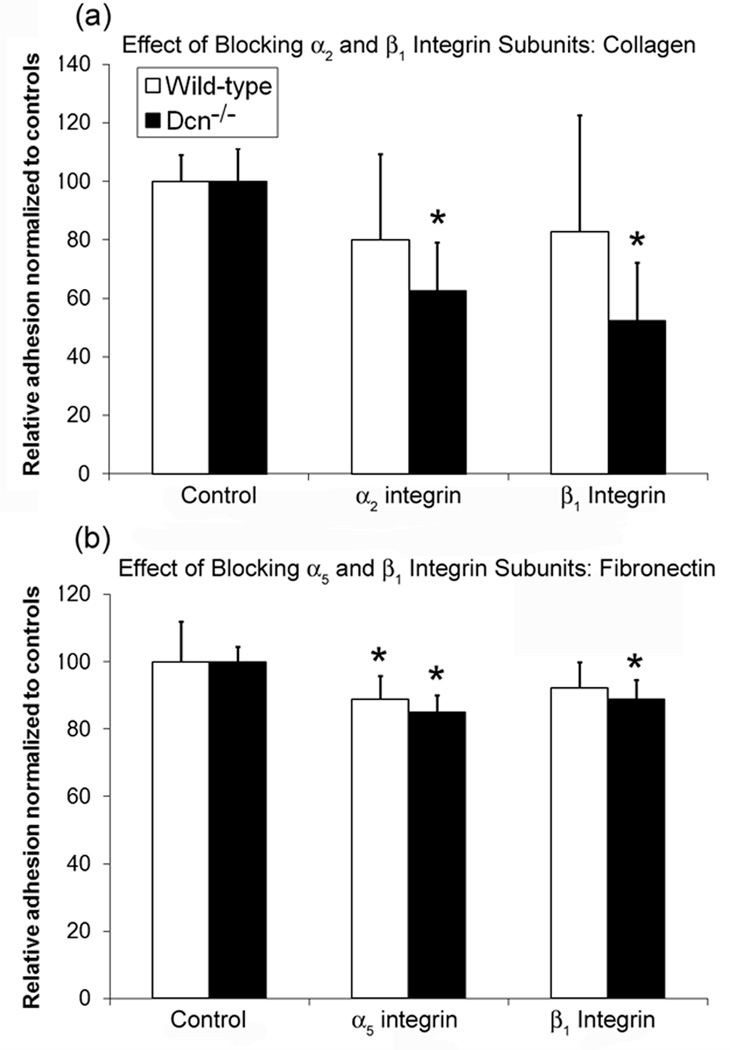
Blocking β1 integrins limited adhesion to collagen and fibronectin
Since β1 integrins are reported to contribute to fibroblast adhesion to collagen type I and fibronectin,40,41 cell adhesion after blocking with antibodies against the β1, α2, or α5 integrin subunits was assessed using the MTT assay. On collagen-coated plates, blocking the β2or β1 subunits caused a significant reduction in adhesion of Dcn−/− cells when compared to wells treated with isotype control antibodies (Fig. 5a, p<0.05 for both antibodies). There was no significant change in wild-type cell adhesion when blocking either the α2 or β1 subunits.
Figure 5.
Effect of (a) blocking α2 and β1 integrin subunits on cellular adhesion by decorin knockout (Dcn−/−) and wild-type cells on collagen-coated 12-well plates and (b) blocking α5 and β1 integrin subunits on cellular adhesion by Dcn−/− and wild-type cells on fibronectin-coated 12-well plates. Cell adhesion after blocking α2 and β1 integrin were normalized to control (rabbit IgG) for each cell-type. Data represents average values (n=6) for each condition and error bar represents standard deviation. For collagen, blocking α2 and β1 integrin subunits resulted in a significant difference in adhesion in Dcn−/− when compared to control (p<0.005), but not wild-type adhesion but not for wild-type cells For fibronectin, there was a significant difference in cell adhesion with respect to controls of both cell types when blocking α5 (p<0.005 for Dcn−/−, p<0.05 for wild-type), but only a significant decrease in Dcn−/− adhesion when blocking β1. (p<0.05) *: p<0.05 with respect to controls.
Using antibodies against the α5 or β1 subunits to block adhesion to fibronectin-coated plates produced similar results to the study on collagen-coated plates. Blocking either the α5 or β1 subunits significantly reduced Dcn−/− cell adhesion to fibronectin when compared to control wells (Fig. 5b, p<0.05 for both antibodies). Wild-type adhesion to fibronectin was significantly decreased when the α5 subunit was blocked (p<0.05), and showed a slight reduction when the β1 subunit was blocked (p=0.085, trend). There was no significant difference between wild-type and Dcn−/− cell adhesion to fibronectin as a result of blocking either the α5 or β1 subunits.
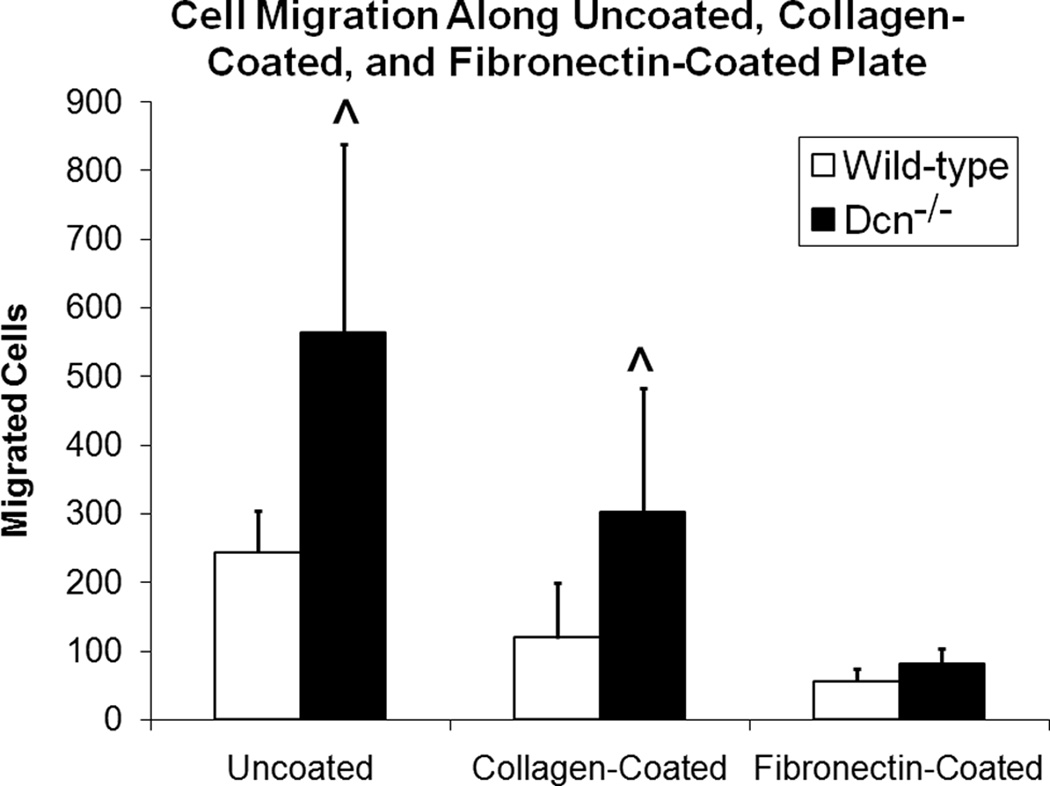
Greater cell migration for Dcn−/− cells
Cell migration on uncoated, collagen-coated, and fibronectin-coated plates was determined by counting the number of cells migrating outwards from the edge of a circular area (previously confined by a cloning cylinder) in wells of a 6-well tissue culture plate either uncoated or collagen-coated, or a 12-well tissue culture plate coated with fibronectin. Dcn−/− cells demonstrated greater migration compared to wild-type cells (Fig. 6) on both uncoated (p<0.01) and collagen-coated plates (p<0.05), but not on fibronectin (p=0.726). Furthermore, there was a significant difference in the number of cells migrating on the different tissue culture substrates (p<0.001). On the collagen and fibronectin-coated plates, cell migration did not advance much further than the outline of the cloning cylinder, whereas in the uncoated plate, cells migrated a slightly greater distance away from the edge of the cloning cylinder.
Figure 6.
Greater cell migration by decorin knockout (Dcn−/−) cells as compared to wild-types on uncoated and collagen-coated tissue culture plastic. Data represents average values (n=3 for uncoated and collagen-coated, n=6 for fibronectin) for each cell type for both substrate and error bar represents standard deviation. There was a significant difference in cell migration for cell-type and substrate (p<0.05). ^ indicates significant difference (p<0.05) between the cell types.
DISCUSSION
The goal of this study was to probe the substrate-dependent influence of decorin on cell proliferation, adhesion, and migration. To our knowledge, this is the first investigation of these characteristics that has employed the unique biological advantages of Dcn−/− cells. The main findings of this study were that Dcn−/− cells demonstrated significantly greater cell adhesion, migration, and proliferation than wild-type cells. In addition, both of these types of MEFs demonstrated different affinities for adhesion to collagen and fibronectin substrates. More specifically, adhesion of these cells to collagen appears to be modulated by a decorin-α2β1 integrin complex. The results of this study help to augment the findings of our previous reports that Dcn−/− cells expressed more α2β1 integrins and caused greater contraction, matrix organization, and cell proliferation within 3D collagen gels than did wild-type cells.33,34 Collagen gel contraction assays are useful in comparing the behavior of different cell types, but can also be used to study specific interactions between cells and collagen because the cells seeded within collagen gels will migrate and align themselves in the direction of tension via α2β1 and other integrins.42–44 These results also help to clarify the substrate-dependent influence of decorin on the material behavior and matrix organization of engineered tissues.
Decorin can inhibit cell growth for a variety of cell types19,22,23 by binding to and sequestering TGF-β9 as well as by binding to the epidermal growth factor receptor.18–21 Correspondingly, we previously showed that Dcn−/− cell-seeded collagen gels had greater cell densities than control wild-type cell-seeded gels. Here, the proliferation studies demonstrated that Dcn−/− cells grow faster on uncoated, collagen-coated, and fibronectin-coated tissue culture plastic than wild-type cells. While the results of this 2-D study might have been influenced by the presence of TGF-β, or lack thereof,9,33 reports that decorin influences cell adhesion to a variety of matrix proteins5–7 lead us to speculate that differential adhesion by the two cell types might have also contributed to the differences in cell growth rate observed in 2D and 3D studies.16,17
Because the influence of decorin on cell adhesion and migration in vivo is relevant to numerous cells types,7,26,27 we investigated the adhesion of the Dcn−/− and wild-type cells to collagen. Our results suggest that Dcn−/− cells exhibit greater adhesion to collagen than wild-type cells through greater expression33 and availability of α2β1 integrins. Indeed, cells expressing α2β1 integrins preferentially bind to type I collagen.40 The reduction of collagen binding by blocking the α2 and β1 integrin subunits on Dcn−/− cells demonstrates that adhesion is strongly mediated by this integrin family. The addition of exogenous decorin resulted in a concentration-dependent reduction in adhesion of Dcn−/− cells to collagen, presumably by the interactions between decorin and α2β1 integrins,25 which were more abundant in the Dcn−/− cells. This result is consistent with previous reports that the external addition of decorin inhibited human skin fibroblast adhesion to collagen.45 Taken together, these results support the findings that Dcn−/− cells caused greater contraction of 3-D collagen type I gels than did wild-type cells.
Although MEF adhesion to collagen was of primary interest, fibroblasts also demonstrate high affinity to fibronectin,46 and reportedly bind via β1 integrins, specifically the α5β1 integrin.40,41 Therefore, fibronectin was also investigated to determine if the different cell types showed substrate-dependent differential adhesion. Dcn−/− cells again exhibited greater adhesion to fibronectin than wild-type cells in the time and concentration-dependent studies. While both types of cells adhered more rapidly to fibronectin than to collagen in the first few minutes, their cumulative adhesion to fibronectin was approximately half that of collagen, suggesting that these cells have a higher affinity for collagen binding than fibronectin binding. Interestingly, our results showed a reduction in both Dcn−/− and wild-type adhesion to fibronectin with the addition of exogenous decorin. Blocking either the α5 or β1 integrin subunit reduced both Dcn−/− and wild-type adhesion as well. That Dcn−/− and wild-type cells adhered similarly to fibronectin in both studies suggests that decorin does not inhibit fibronectin-dependent adhesion through binding to the α5β1 integrin as was previously presumed,40,41 but may act through other integrins known to bind to fibronectin, such as αVβ1,35,47 αVβ3,48 αVβ6,49 and α4β1.50,51 This interpretation is supported by the lesser inhibitory effects of decorin on adhesion to fibronectin. In the exogenous decorin assay, Dcn−/− adhesion to fibronectin, in the presence of 20 µg/ml of decorin, decreased approximately 20% as opposed to a reduction of 40% on collagen. In addition, blocking α5β1 reduced Dcn−/− adhesion to fibronectin only 10%, whereas blocking α2β1 reduced Dcn−/− adhesion to collagen by 40%. Another possibility on the inhibitory mechanism of decorin is that decorin became bound to the fibronectin itself before either cell type could adhere. Decorin has been shown previously to bind to the RGD sequence on fibronectin directly,5,27 although it is not clear whether decorin binds to fibronectin via its core protein or GAG chain. Considered together, our results on adhesion to fibronectin provide insight into the high affinity of fibroblasts for this substrate.
With respect to cell migration, greater numbers of Dcn−/− cells migrated across both type I collagen-coated and uncoated tissue culture plates than did wild-type cells. This finding supports the work of Merle et al., who showed that the addition of decorin to migrating osteosarcoma cells slowed their migration on type I collagen and fibronectin.26 The reduced migration of both cell types on fibronectin also supports the work of Fuja et al., who showed that vocal fold stellate cells exhibit greater affinity to fibronectin.46 Overall, these 2-D studies suggest that the faster migration by the Dcn−/− cells, in combination with their increased adhesion to matrix molecules, would enable them to initiate remodeling of 3-D collagen matrices than would be possible by the wild-type cells.
There were a number of limitations to this study. With respect to the study motivation, we acknowledge that the observed patterns in cell adhesion, proliferation, and migration found in this 2-D study may differ from these same processes within 3-D matrices and in vivo tissues. The cell migration investigated in this study represents random migration by the cells and does not reflect migration dictated by any specific chemoattractant. This study also did not investigate the role of the different structural components of the decorin molecule (i.e., either the core protein or GAG chain) on these outcomes. Further investigation regarding the matrix binding structural components of decorin will continue to shed light on the unique characteristics of these Dcn−/− cells and their study in 2-D and 3-D cultures. Investigation of other integrins, such as α1β1 and α11β1 that are involved in cell adhesion to collagen, and αVβ1, αVβ3, αVβ6, and α4β1 for fibronectin, would continue to reveal the multifaceted role of decorin in cell-matrix adhesion. Finally, our results must be considered in the context of this 2D in vitro study, particularly in light of mixed findings from various cell and tissue systems. For example, we previously reported that collagenous engineered tissues containing Dcn−/− MEFs demonstrated improved matrix organization and strength,33,34 but scar tissues in mature Dcn−/− mice were found to contain loosely packed collagen fibrils with a wide distribution of fibril diameters.11 In another study, the addition of decorin to collagen-coated titanium implants actually increased osteoblast proliferation and accelerated focal adhesion formation.52,53 The differential influences of decorin that have been observed between various tissues and cell types may be a consequence of differential modulation of TGF-β between cell types, as has been shown previously.54,55 Nonetheless, these reports highlight the profound influence of this proteoglycan on the remodeling and healing of native tissues, engineered tissues, and implantable biomaterials, and motivate further research in this area.
CONCLUSION
Although the role of decorin in cell adhesion to fibronectin has been widely investigated, only a few studies have examined its influence in cell adhesion to collagen. Furthermore, no previous study has conclusively demonstrated substrate-specific, decorin-mediated cell behavior. Here, we report that decorin deficient murine embryonic fibroblasts show greater proliferation, adhesion, and migration than wild-type cells. In addition, we observed unique substrate-specific effects, potentially due to interactions between decorin and either the adhesion molecules or substrate, and demonstrated that the adhesion of these cells to fibronectin was only slightly inhibited by blocking β1 integrins. These unique decorin-dependent interactions suggest that decorin actively modulates the cell-mediated matrix organization of engineered and native tissues.
Acknowledgements
The authors thank Dr. Magnus Höök (Center for Extracellular Matrix Biology, Texas A&M University System Health Science Center) for help with the experimental design and discussions of the data presented in this manuscript. We also thank Wei Zhou, Emanuel Smeds, and Adriane M. Joo (Center for Extracellular Matrix Biology, Texas A&M University System Health Science Center) for harvesting the Dcn−/− and wild-type mouse embryos. This research was supported by National Institute of Health Grant R03EB005444 and National Science Foundation REU Grant DBI-0649094.
References
- 1.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19(4–5):249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 2.Bhide VM, Laschinger CA, Arora PD, Lee W, Hakkinen L, Larjava H, Sodek J, McCulloch CA. Collagen phagocytosis by fibroblasts is regulated by decorin. J Biol Chem. 2005;280(24):23103–23113. doi: 10.1074/jbc.M410060200. [DOI] [PubMed] [Google Scholar]
- 3.Kresse H, Schönherr E. Proteoglycans of the extracellular matrix and growth control. J Cell Physiol. 2001;189(3):266–274. doi: 10.1002/jcp.10030. [DOI] [PubMed] [Google Scholar]
- 4.Ward W, Li A, Siddiqui Y, Federiuk I, Wang X. Increased expression of Interleukin-13 and connective tissue growth factor, and their potential roles during foreign body encapsulation of subcutaneous implants. J Biomater Sci Polym Ed. 2008;19(8):1065–1072. doi: 10.1163/156856208784909408. [DOI] [PubMed] [Google Scholar]
- 5.Bidanset DJ, LeBaron R, Rosenberg L, Murphy-Ullrich JE, Hook M. Regulation of cell substrate adhesion: effects of small galactosaminoglycan-containing proteoglycans. J Cell Biol. 1992;118(6):1523–1531. doi: 10.1083/jcb.118.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt G, Hausser H, Kresse H. Interaction of the small proteoglycan decorin with fibronectin. Involvement of the sequence NKISK of the core protein. Biochem J. 1991;280(Pt 2):411–414. doi: 10.1042/bj2800411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huttenlocher A, Werb Z, Tremble P, Huhtala P, Rosenberg L, Damsky CH. Decorin regulates collagenase gene expression in fibroblasts adhering to vitronectin. Matrix Biol. 1996;15(4):239–250. doi: 10.1016/s0945-053x(96)90115-8. [DOI] [PubMed] [Google Scholar]
- 8.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346(6281):281–4. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 10.Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, Gallo RL. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem. 1998;273(43):28116–28121. doi: 10.1074/jbc.273.43.28116. [DOI] [PubMed] [Google Scholar]
- 11.Weis SM, Zimmerman SD, Shah M, Covell JW, Omens JH, Ross J, Jr, Dalton N, Jones Y, Reed CC, Iozzo RV, et al. A role for decorin in the remodeling of myocardial infarction. Matrix Biol. 2005;24(4):313–324. doi: 10.1016/j.matbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Radermecker MA, Limet R, Lapiere CM, Nusgens B. Increased mRNA expression of decorin in the prolapsing posterior leaflet of the mitral valve. Interact Cardiovasc Thorac Surg. 2003;2(3):389–394. doi: 10.1016/S1569-9293(03)00107-5. [DOI] [PubMed] [Google Scholar]
- 13.Jarvelainen HT, Iruela-Arispe ML, Kinsella MG, Sandell LJ, Sage EH, Wight TN. Expression of decorin by sprouting bovine aortic endothelial cells exhibiting angiogenesis in vitro. Exp Cell Res. 1992;203(2):395–401. doi: 10.1016/0014-4827(92)90013-x. [DOI] [PubMed] [Google Scholar]
- 14.Iozzo RV, Cohen I. Altered proteoglycan gene expression and the tumor stroma. Experientia. 1993;49(5):447–455. doi: 10.1007/BF01923588. [DOI] [PubMed] [Google Scholar]
- 15.Witsch-Prehm P, Miehlke R, Kresse H. Presence of small proteoglycan fragments in normal and arthritic human cartilage. Arthritis Rheum. 1992;35(9):1042–1052. doi: 10.1002/art.1780350909. [DOI] [PubMed] [Google Scholar]
- 16.Thery M, Bornens M. Cell shape and cell division. Curr Opin Cell Biol. 2006;18(6):648–657. doi: 10.1016/j.ceb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Xaus J, Comalada M, Cardo M, Valledor AF, Celada A. Decorin inhibits macrophage colony-stimulating factor proliferation of macrophages and enhances cell survival through induction of p27(Kip1) and p21(Waf1) Blood. 2001;98(7):2124–2133. doi: 10.1182/blood.v98.7.2124. [DOI] [PubMed] [Google Scholar]
- 18.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281(36):26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 19.Fischer JW, Kinsella MG, Levkau B, Clowes AW, Wight TN. Retroviral overexpression of decorin differentially affects the response of arterial smooth muscle cells to growth factors. Arterioscler Thromb Vasc Biol. 2001;21(5):777–784. doi: 10.1161/01.atv.21.5.777. [DOI] [PubMed] [Google Scholar]
- 20.Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274(8):4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 21.De Luca A, Santra M, Baldi A, Giordano A, Iozzo RV. Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J Biol Chem. 1996;271(31):18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 22.Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14(3):203–234. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- 23.Hakkinen L, Strassburger S, Kahari VM, Scott PG, Eichstetter I, Iozzo RV, Larjava H. A role for decorin in the structural organization of periodontal ligament. Lab Invest. 2000;80(12):1869–1880. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- 24.Lam K, Zhang L, Yamada KM, Lafrenie RM. Adhesion of epithelial cells to fibronectin or collagen I induces alterations in gene expression via a protein kinase C-dependent mechanism. J Cell Physiol. 2001;189(1):79–90. doi: 10.1002/jcp.1142. [DOI] [PubMed] [Google Scholar]
- 25.Zutter MM, Edelson BT. The alpha2beta1 integrin: a novel collectin/C1q receptor. Immunobiology. 2007;212(4–5):343–53. doi: 10.1016/j.imbio.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Merle B, Durussel L, Delmas PD, Clezardin P. Decorin inhibits cell migration through a process requiring its glycosaminoglycan side chain. J Cell Biochem. 1999;75(3):538–546. [PubMed] [Google Scholar]
- 27.Winnemoller M, Schmidt G, Kresse H. Influence of decorin on fibroblast adhesion to fibronectin. Eur J Cell Biol. 1991;54(1):10–17. [PubMed] [Google Scholar]
- 28.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136(3):729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H. Fibrillogenesis of collagen types I, II, III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules. 2006;7(8):2388–2393. doi: 10.1021/bm0603746. [DOI] [PubMed] [Google Scholar]
- 30.Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127(1):181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- 31.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98(6):1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 32.Barber J, Ratliff N, Cosgrove D, 3rd, Griffin B, Vesely I. Myxomatous mitral valve chordae. I: mechanical properties. J Heart Valve Dis. 2001;10(3):320–324. [PubMed] [Google Scholar]
- 33.Ferdous Z, Wei VM, Iozzo R, Hook M, Grande-Allen KJ. Decorin-TGF beta interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282(49):35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 34.Ferdous Z, Lazaro L, Iozzo R, Höök M, Grande-Allen K. Influence of cyclic strain and decorin deficiency on 3D cellularized collagen matrices. Biomaterials. 2008;29(18):2740–2748. doi: 10.1016/j.biomaterials.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogel B, Tarone G, Giancotti F, Gailit J, Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (αVβ1) J Biol Chem. 1990;265(11):5934–5937. [PubMed] [Google Scholar]
- 36.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 37.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26(16):3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Vernon RB, Gooden MD. New technologies in vitro for analysis of cell movement on or within collagen gels. Matrix Biol. 2002;21(8):661–669. doi: 10.1016/s0945-053x(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 39.Ura H, Takeda F, Okochi H. An in vitro outgrowth culture system for normal human keratinocytes. J Dermatol Sci. 2004;35(1):19–28. doi: 10.1016/j.jdermsci.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZG, Bothe I, Hirche F, Zweers M, Gullberg D, Pfitzer G, Krieg T, Eckes B, Aumailley M. Interactions of primary fibroblasts and keratinocytes with extracellular matrix proteins: contribution of alpha2beta1 integrin. J Cell Sci. 2006;119(Pt 9):1886–1895. doi: 10.1242/jcs.02921. [DOI] [PubMed] [Google Scholar]
- 41.Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102(17):5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13(5):264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 43.Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312(1):86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7(2):157–164. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 45.Noyori K, Jasin HE. Inhibition of human fibroblast adhesion by cartilage surface proteoglycans. Arthritis Rheum. 1994;37(11):1656–1663. doi: 10.1002/art.1780371115. [DOI] [PubMed] [Google Scholar]
- 46.Fuja TJ, Ostrem EM, Probst-Fuja MN, Titze IR. Differential cell adhesion to vocal fold extracellular matrix constituents. Matrix Biol. 2006;25(4):240–251. doi: 10.1016/j.matbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Morla A, Vuori K, Bauer J, Juliano R, Ruoslahti E. The alpha v beta 1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol. 1993;122(1):235–242. doi: 10.1083/jcb.122.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charo I, Nannizzi L, Smith J, Cheresh D. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J Cell Biol. 1990;116(6 Pt 1):2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busk M, Pytela R, Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin binding protein. J Biol Chem. 1992;267(9):5790–5796. [PubMed] [Google Scholar]
- 50.Mould A, Wheldon L, Komoriya A, Wavner E, Yamada K, Humphries M. Affinity chromatographic isolation of the melanoma adhesion receptor for the IIICS region of fibronectin and its identification as the integrin alpha 4 beta 1. J Biol Chem. 1990;265(7):4020–4024. [PubMed] [Google Scholar]
- 51.Akiyama S, Olden K, Yamada K. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995;14(3):173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- 52.Bierbaum S, Douglas T, Hanke T, Scharnweber D, Tippelt S, Monsees T, Funk R, Worch H. Collageneous matrix coatings on titanium implants modified with decorin and chondroitin sulfate: characterization and influence on osteoblastic cells. J Biomed Mater Res A. 2006;77(3):551–562. doi: 10.1002/jbm.a.30572. [DOI] [PubMed] [Google Scholar]
- 53.Douglas T, Hempel U, Mietrach C, Viola M, Vigetti D, Heinemann S, Bierbaum S, Scharnweber D, Worch H. Influence of collagen-fibril-based coatings containing decorin and biglycan on osteoblast behavior. J Biomed Mater Res A. 2008;84(3):805–816. doi: 10.1002/jbm.a.31501. [DOI] [PubMed] [Google Scholar]
- 54.Hausser H, Groning A, Hasilik A, Schonherr E, Kresse H. Selective inactivity of TGF-beta/decorin complexes. FEBS Lett. 1994;353(3):243–245. doi: 10.1016/0014-5793(94)01044-7. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi Y, Kodama Y, Matsumoto T. Bone matrix decorin binds transforming growth factor-beta and enhances its bioactivity. J Biol Chem. 1994;269(51):32634–32368. [PubMed] [Google Scholar]