Abstract
Introduction
While chronic degenerative arthropathy is the main morbidity of hemophilia, a very high prevalance of low bone density is also seen in men and boys with hemophilia. The current study investigates bone degradation in the knee joint of hemophilic mice resulting from hemarthrosis and the efficacy of aggressive treatment with factor VIII in the period surrounding injury to prevent bone pathology.
Methods
Skeletally mature factor VIII knock-out mice were subjected to knee joint hemorrhage induced by puncture of the left knee joint capsule. Mice received either intravenous Factor VIII treatment or placebo immediately prior to injury and at hours 4, 24, 48, 72 and 96 after hemorrhage. Mice were euthanized two-weeks after injury and the joint morphology and loss of bone in the proximal tibia was assessed using microCT imaging.
Results
Quantitative microCT imaging of the knee joint found acute bone loss at the proximal tibia following injury including loss of trabecular bone volumetric density and bone mineral density, as well as trabecular connectivity density, number, and thickness. Unexpectedly, joint injury also resulted in calcification of the joint soft tissues including the tendons, ligaments, menisci, and cartilage. Treatment with factor VIII prevented this bone and soft tissue degeneration.
Conclusion
Knee joint hemorrhage resulted in acute changes of adjacent bone including loss of bone density and mineralization of joint soft tissues. The rapid calcification and loss of bone has implications for the initiation and progression of osteoarthritic degradation following joint bleeding.
Keywords: Factor VIII, Hemarthrosis, bone density, Joint degradation, Mineralization, MicroCT
Introduction
The most common complication of severe hemophilia is recurrent hemarthrosis, which may lead to destruction of joints. In addition to joint damage, low bone density has been observed in adults [1–4] and children [5] with hemophilia. In children, early institution of regular prophylactic clotting factor infusions to prevent hemarthrosis helps preserve joint function and is the standard of care in resource-rich countries [6]. While indirect evidence suggests that primary prophylaxis preserves bone mineral density [7, 8], there is little information available on acute and subacute bone response to joint bleeding.
The potential significance is great because acute changes in the bone can impact whole joint function. There is evidence that chronic exposure of the joints to blood in hemophilia leads to destruction of both cartilage [9, 10] and bone [11] resulting in osteoarthritis as well as consequent deficits in postural balance [12] and gait abnormalities [13]. In addition, alterations to the joint tissues may lead to joint instability [14–16], which is one of the primary factors for the onset and progression of osteoarthritis in patients with ligament injuries [17]. This initial damage could contribute to recurrent joint bleeding, further accelerating degradation of the joint. In addition, low bone density in the subchondral bone has been related to osteoarthritic joint degradation [18]
This study uses microCT imaging to investigate the acute bone loss in the periarticular bone following induced joint bleeding in factor VIII gene knockout (FVIII−/−) mice and the efficacy of aggressive treatment with recombinant factor VIII(FVIII) following joint injury.
Materials and Methods
All investigations were approved by the University of North Carolina-Chapel Hill Institutional Animal Care and Use Committee. FVIII−/−mice generated by gene targeting (E16 FVIII B6;129S4-F8tm1kaz) were originally supplied by Dr. H. H. Kazazian Jr. (University of Pennsylvania, PA, USA) [19, 20] and bred in house. Twenty-two week old (skeletally mature) FVIII−/− male mice were subjected to knee joint hemorrhage induced by puncture of the knee joint capsule with a 30.5 gauge needle, followed by instillation of 5 microliters of saline into the left knee joint space [21] ; the contralateral right knee joint served as the uninjured control. One group of injured mice received intravenous (I.V.) FVIII treatment (n=9), and the placebo group of injured mice was injected with I.V. saline (n=9). Thirty minutes prior to the joint hemorrhage mice received an I.V. dose of human recombinant FVIII 250 IU kg−1 (AdvateTM; Baxter Bioscience, Deerfield, IL) by tail vein injection. At hours 4, 24, 48, 72 and 96 after the induction of bleeding, 250 IU kg−1 doses of FVIII were repeated. The FVIII dose was selected based on our preliminary studies and those of other investigators [22] demonstrating that plasma from FVIII−/− mice that receive this dose has the same clotting time in seconds as does plasma from hemostatically normal wild type mice, as measured by the activated partial thromboplastin time assay.
Two weeks after hemarthrosis, the mice were euthanized and the hind limbs were collected and fixed in formalin. Hind limbs were scanned at 10µm resolution using microCT (µct80; Scanco Medical AG, Brüttisellen, Switzerland). MicroCT analysis was performed on the trabecular bone at the proximal tibia, inferior to the growth plate, which is a common skeletal site for analysis. Histomorphometric analysis was performed on the trabecular bone using the Scanco analysis software to calculate parameters including trabecular bone volumetric density (BV/TV), trabecular connectivity density, trabecular number and thickness, bone tissue mineral density, and volumetric bone mineral density (vBMD). The BV/TV metric indicates how much trabecular bone is present for a given volume or size of bone. The trabecular connectivity density, number, and thickness are all structural microarchitectural parameters that can correlate with bone strength. The bone mineral density is the average density of the bone present, while the vBMD measures the average mineral density over the entire trabecular compartment, including the space between the bone tissue, which is similar to that of a clinical DEXA bone density scan.
Upon observing gross calcifications on the microCT scans, a more detailed observation of each specimen was performed using three-dimensional renderings using OsiriX Medical Imaging Viewer (www.osirix-viewer.com) to look for abnormal morphologies and calcifications resulting from injury. In addition, Mimics Innovation Suite (Materialise, Leuven Belgium) was used to segment calcified regions from the bone based on thresholding and image processing of the microCT data.
So as to be certain that the injury did result in exposure of the joint to blood, separate groups of FVIII−/− mice (n = 6 mice), not included in the evaluation of bone health, received either I.V. recombinant FVIII 250 IU kg−1 or I.V. saline thirty minutes prior to undergoing an induced hemarthrosis so as to reproduce identical conditions to those used in the bone health study. All of the mice were sacrificed at 4 hours after the injury and the injured legs were dissected free. Half of the injured joints were opened for direct visual examination for gross blood in the intra-articular space. The direct visual examination destroys the architecture of the intact joint, so the other half of the joints were left intact and processed for microscopic examination for blood by histology at 4 hours after the injury.
The statistical significance of the drug treatment and injury were determined using a two-way ANOVA with Tukey Test. (SigmaPlot). Significance was also determined using a two-sided t-test to assess changes resulting from joint injury between the placebo and drug treated mice using the contra-lateral uninjured limb as an internal control.
Results
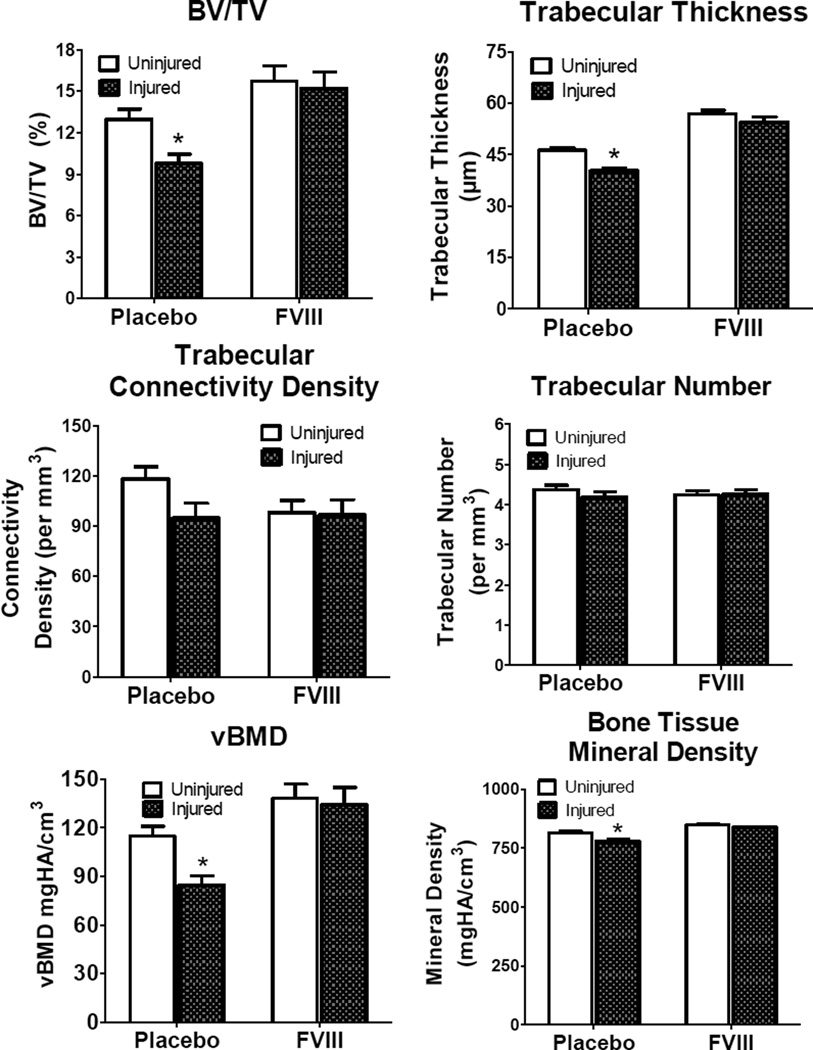
The study quantifies the changes of the bone at the proximal tibia that result following hemarthrosis in the adjacent joint in FVIII−/−mice. This joint injury resulted in an acute reduction of proximal tibial BV/TV, trabecular connectivity density and thickness, bone tissue mineral density, and volumetric bone mineral density (vBMD) (Fig.1 and Fig. 2C, inset). This damage was mitigated by aggressive FVIII replacement (Fig. 1 and Fig 2D, inset)..
Figure 1.
Properties of bone measured by microCT in the proximal tibia adjacent to the joint at two weeks following unilateral induced joint hemorrhage. Separate groups of FVIII−/− mice were treated with either Placebo (physiologic saline) or with recombinant FVIII. Trabecular bone volumetric density (BV/TV), Trabecular Thickness, Trabecular Connectivity Density, and Trabecular Number, Volumetric bone mineral density (vBMD) and Bone Tissue Mineral Density (mean±SEM) for injured/uninjured limbs of placebo-treated compared to FVIII-treated mice (* denotes statistical significance p<0.05 for the difference between injured and uninjured within each treatment group).
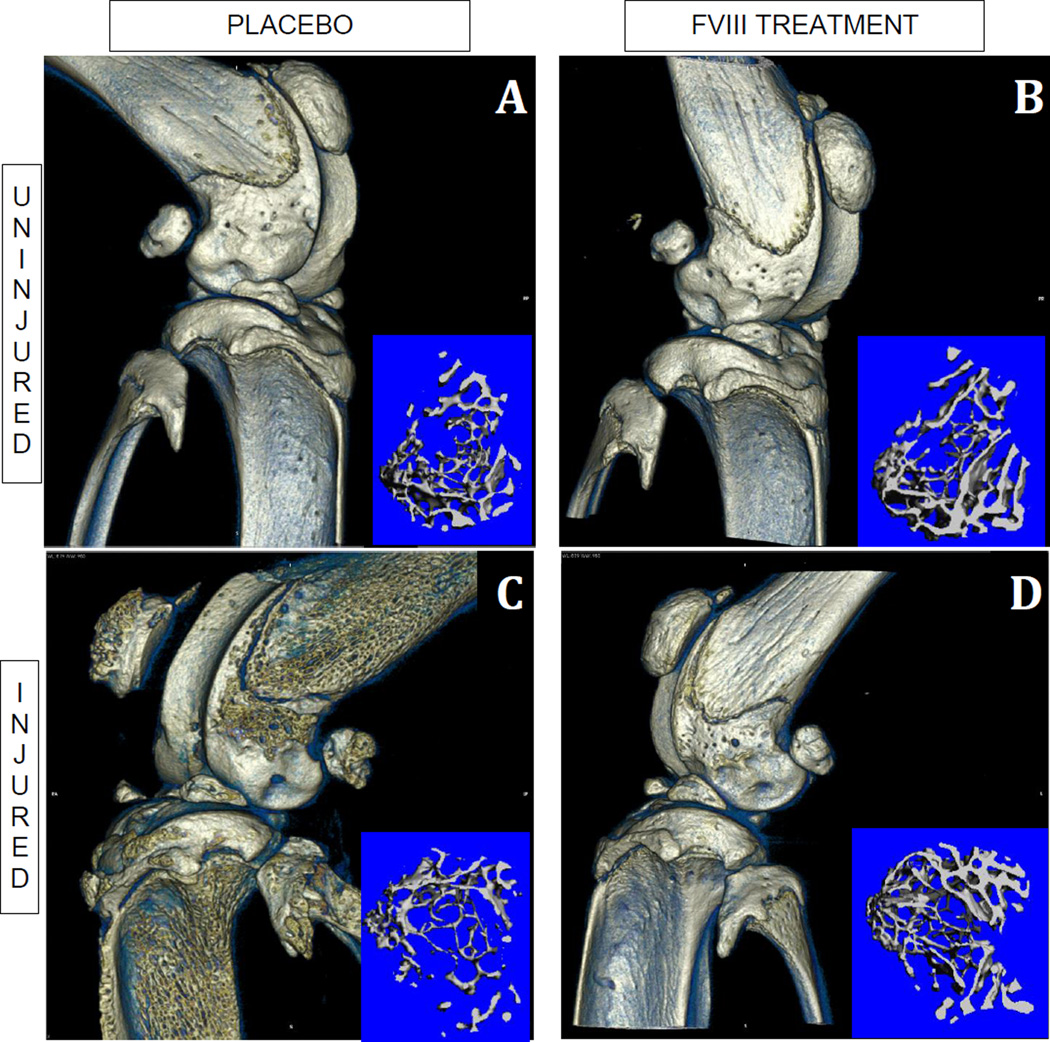
Figure 2.
Three-dimensional rendering of the joint from microCT imaging for the uninjured (A & B) and injured (C & D) knee from the placebo (A & C) and FVIII treated (B & D) mice. The blue inset for each condition is an image of the trabecular bone region of analysis of the proximal tibia, demonstrating less bone in the injured, placebo-treated mouse.
In the absence of FVIII replacement joint injury was associated with a 25% decrease in bone volumetric density (BV/TV) (p<0.05), 13% decrease in trabecular thickness (p<0.05), 5% decrease in tissue mineral density (p<0.05), and 27% decrease in vBMD (p<0.05) compared to the contralateral uninjured limb (Fig. 1). Mice that received the extended FVIII treatment showed no significant changes in any of these bone metrics (Fig. 1).
The difference between the injured and the uninjured contralateral limb of the individual mice in each treatment group (placebo or FVIII-treated) was calculated. The mean % change of the individual animals was derived for each treatment approach. Compared to the changes in the placebo-treated FVIII−/− mice, FVIII replacement significantly prevented bone degradation when measured by BV/TV (p<0.05), trabecular thickness, (p<0.05),tissue mineral density (p<0.05), and vBMD (p<0.05).Also, there was a strong trend for reduction in trabecular connectivity density(p=0.055) and trabecular number (p=0.055) (Table 1).
Table 1.
Morphometric Parameters from microCT of proximal tibia adjacent to the joint at two weeks following hemarthrosis, using contralateral limb as uninjured control (mean ± SD).
| BV/TV (%) |
Trabecular Connectivity Density (per mm3) |
Trabecular Number (per mm3) |
Trabecular Thickness (µm) |
Bone Tissue Mineral Density (mgHA/cm3) |
vBMD (mgHA) |
|
|---|---|---|---|---|---|---|
| Placebo: Control vs Injured Limb-% Change | −25.4 ± 9.4%# | −20.3 ± 15.9% | −4.3 ± 4.0% | −13.0 ± 7.2%# | −4.6 ± 3.0%# | −26.9 ± 8.7 %# |
| FVIII: Control vs Injured Limb-% Change | −3.2 ± 13.6%* | −2.6 ± 20.3% | 0.3 ± 5.5% | −4.7 ± 5.8%* | −1.3 ± 3.0%* | −2.7 ± 14.5%* |
Significant difference resulting from injury compared to contralateral uninjured limb denoted by #(p<0.05).
Significant difference between Factor VIII treatment and Placebo denoted by *(p<0.05).
Direct visual examination of joints collected at four hours after this induced joint hemorrhage demonstrated gross bleeding within the joint capsule and in the adjacent soft tissue of the injured untreated mice and in the mice that had received a single FVIII infusion prior to wounding. Additional joints from this time point that were left intact and processed for histologic staining also showed that blood was present at four hours after the joint capsule puncture in the mice from both groups. Scattered areas of red blood cells layering along the synovial lining of the joint were present in the FVIII-treated mice, whereas in the placebo-treated mice a larger volume of blood partially filled the intra-articular space
In the unprotected (normal saline placebo treated) group, joint injury resulted in acute morphological changes to the cortical bone in the surrounding joint, with the periosteal wall becoming rough (Fig.2C). These damaged regions were observed on the articular surface of the distal femur and proximal tibia, as well as the patella and fibula (Fig.2C and Fig.3 Left). In addition, the patella of the injured and degraded joint was displaced, consistent with hemorrhage and edema. Joints that were injured in mice treated with FVIII did not show these morphological changes (Fig. 2D and Fig.3 Center).
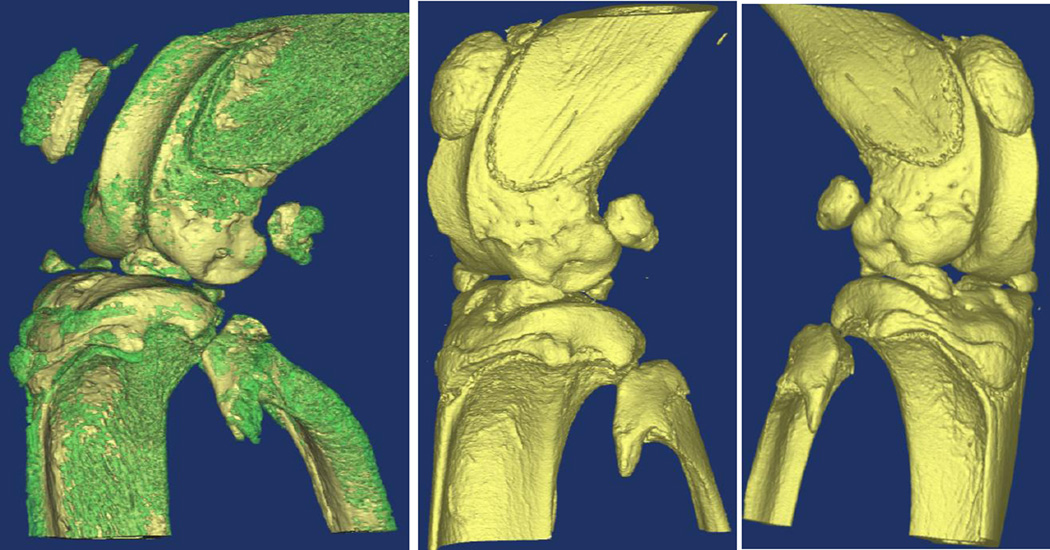
Figure 3.
Left: Injured knee joint from placebo-treated FVIII−/− mouse showing calcification of the patella, patellar tendon, menisci, ligaments, and cartilage. Calcifications rendered in green. Center: Injured knee from FVIII-treated mouse does not have this mineralization present. Right: Uninjured knee from placebo-treated mouse does not have mineralization present
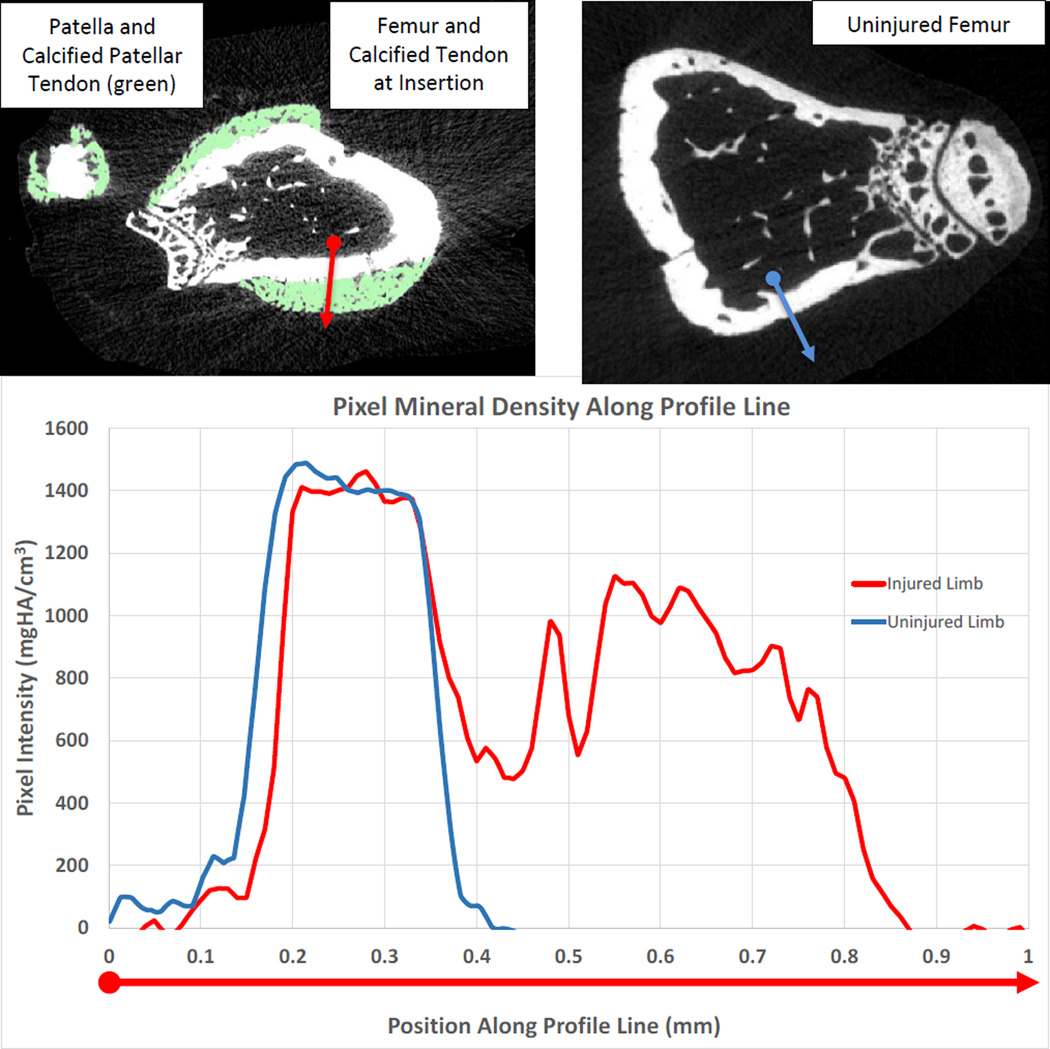
Gross mineralization was found on the femur, tibia, and fibula tendon insertion points, as well as the patella, patellar tendon, menisci, ligaments and cartilage (Fig.3 Left) of injured joints from the placebo group of mice. A more detailed analysis of the calcifications in the distal femur using the Mimics image processing software found that the calcified segment had a lower mineral density (1086 mgHA/cm3) compared to the adjacent cortical bone (1449mgHA/cm3) (Fig. 4), so that a volume of the calcified region of 0.014 mm3 could be delineated. Mineral density is given in units of milligrams of Hydroxyapatite per cm3(mgHA/cm3).The FVIII treatment prevented this acute mineralization as these calcifications were not observed in injured limbs of FVIII treated mice nor non-injured limbs (Fig. 2A, 2B, and Fig.3 Center and Right, Fig. 4).
Figure 4.
Top: Axial, cross-sectional image of the distal femur from injured (left) and uninjured (right) limb from a placebo-treated mice. Calcifications of the patellar tendon and distal femur tendon insertion rendered in green. Profile analysis line spans from the endosteal surface (circle) to the edge of the calcification (arrow). Bottom: Corresponding intensity values along the red (injured limb)and blue (uninjured limb) profile lines shown in above images (location of the circle is at x=0 on the graph). Areas of calcification had lower density (1086 mgHA/cc) than the cortical bone (1449mgHA/cc).
Discussion and Conclusion
The high prevalence of low bone mineral density in men and boys with hemophilia is likely multifactorial. Low bone density has been attributed to the chronic effect of decreased physical activity and disuse atrophy following the development of hemarthropathy, as well as to the chronic effects of HIV and hepatitis infection and their therapies [2]. This study shows that induced joint hemorrhage in hemophilic mice results in an acute loss of trabecular bone in the injured joint as early as 2 weeks after injury. Aggressive replacement of FVIII prevented the deterioration of these morphologic parameters of bone as well as the rapid mineralization in the joint tissues following joint bleeding. Our findings support the hypothesis that reduced bone density in the setting of hemophilia may, at least in part, be secondary to acute hemorrhage and the cumulative effect of acute bleeds.
While measures were taken to have similar weights for both groups of mice, the drug treated group had a slightly larger average weight (26.5 ± 1.9 vs 25.0 ± 2.4 grams), even though this difference is not statistically significant (p=0.16). Any contribution from weight difference was normalized by directly comparing the % change between the injured and non-injured limb within each animal (Table 1).
Intriguingly, one group of investigators has recently reported that in the absence of observed hemorrhage, hemophilia A mice have statistically significant diminution of bone density when compared to their hemostatically normal littermates [23, 24]. These authors concluded that low bone density is an inherent phenotype that is independently associated with congenital coagulation FVIII deficiency and is the subject of further mechanistic investigation [25]. All mice in both our study cohorts were FVIII deficient throughout skeletal maturation and the observed impact of joint hemorrhage, occurring after skeletal maturation, upon bone degeneration is independent of any phenotypic osteopenia that might be a characteristic of congenital FVIII deficiency, per se. Hemarthrosis caused a 25% decrease in trabecular bone BV/TV with some individual mice losing over 30%. This rapid loss of bone density has implications as relates to the onset of osteoarthritis [26]. Changes in the subchondral bone could affect the cartilage and other soft tissues of the joint contributing to osteoarthritis [14, 27].
Uncontrolled bleeding in this experimental model of hemarthrosis is not only associated with acute intra-articular inflammation [28–30] and loss of bone density in the adjacent tibia, but is additionally associated in our study with rapid calcification of the joint soft tissues. Acute mineralization of the joint following joint bleeding and associated inflammation could have long term consequences. Calcification in the joint poses a risk for arthritic joint degeneration and has been associated clinically with osteoarthritis in both the hip and the knee [31–33]. In addition, tendon calcification has been correlated with structural joint disease [34]. Calcification alters the material behavior of these tissues, leading to altered joint biomechanics, which could further degrade the joint and joint tissues. Tendon calcification has been found to lead to degradation of the tissue [35]. In the knee joint, calcifications following injury have been observed clinically at 6-months post-injury [36], which has implications for long term degeneration. Future work could use this mouse joint hemorrhage model to investigate the progression of joint degradation and the onset of osteoarthritis associated with these gross soft tissue calcifications following joint injury and inflammation.
The mechanisms by which exposure of the joint to blood causes these acute morphologic changes warrant further investigation. Increased expression of receptor activator of nuclear factor-κB (RANK) and RANK ligand and decreased expression of osteoprotegerin have been demonstrated in synovial tissue of hemophilic joints, suggesting increased unopposed osteoclastic activity, however these studies were performed on joints with end-stage pathology, resulting from very chronic bleeding [37]. Instead, the current study uses a small needle injury in hemophilic mice to initiate joint bleeding and inflammation and examines acute pathologic changes. It is unknown whether the inflammatory response that occurs following exposure of the joint to blood involves identical mechanisms in hemophilic individuals and in non-hemophilic individuals who experience hemarthrosis. All animals in the current study were congenitally FVIII deficient and our ongoing work compares the response to intra-articular blood in hemostatically normal and hemophilic animals. Future work is needed to investigate the similarities in the pathology of rapid calcifications observed in this study compared to those that have been described in individuals without hemophilia receiving traumatic injury to the joint that results in hematoma and inflammation [36] .
In conclusion, these data support the role of acute hemarthrosis in the absence of FVIII in hemophilia-related bone loss. Whether joint injury is the primary cause of bone loss or a contributing factor deserves further study. Hemophilic joint bleeding tends to recur, establishing a vicious cycle of hemorrhage in affected “target” joints [38]. Our ongoing studies examine the extent and time course of resolution of bone loss following a single or repetitive hemarthroses, and whether full resolution does occur between bleeding episodes. For the studies reported here we chose an aggressive treatment with FVIII replacement throughout wound healing. This treatment schedule essentially reversed the bleeding phenotype of FVIII−/− mice. Our ongoing studies examine whether more abbreviated approaches to FVIII replacement (as are typically used in the clinical treatment of hemophilic bleeding) can achieve bone preservation in this model. Additionally, the rapid mineralization of the joint soft tissues warrants further study to investigate how these calcifications affect arthritic joint health and the progression to osteoarthritis. Overall, this study contributes to an understanding of the underlying cause of low bone density in the setting of FVIII deficiency and identifies calcification in the joint that could be an initiator of osteoarthritic degenerative joint disease.
Acknowledgements
Role of the Funding Sources:
Baxter Health Care Corporation: Provided Investigator-Initiated Study support to Paul E. Monahan and Ted A. Bateman.
NIH: P01 HL112761 - Paul E. Monahan
NIH: 5R01AR059221-02 -Ted A. Bateman
National Space Biomedical Research Institute: PF03003 through NASA NCC 9-58 –Anthony G Lau
Center for the Advancement of Science in Space (CASIS) - Ted A. Bateman
None of these study sponsors had a role in the study design, collection, analysis and interpretation of data, or in the preparation of and decision to submit the manuscript.
Footnotes
Author Contributions
Anthony G. Lau: Imaging and computational analysis of mouse joints; Primary Contributor to the manuscript; final approval of submitted version.
Junjiang Sun: Contributed to the experimental design and experimentation, performed joint injury and fVIII administration; final approval of submitted version.
William B. Hanna: Bred hemophilic mouse strain and contributed to the data acquisition and manuscript; final approval of submitted version.
Eric W. Livingston: Contributed to the data acquisition and manuscript; final approval of submitted version.
Dominique Heymann: Experimental design and contributor to the manuscript; final approval of submitted version.
Ted A. Bateman: Co-PI, experimental design and contributor to the manuscript; final approval of submitted version.
Paul E. Monahan: Co-PI, experimental design and contributor to the manuscript; final approval of submitted version; responsible for the work from inception to finished submission.
Conflict of Interest:
Paul E. Monahan has received remuneration for consulting and speaker work for Baxter Health Care Corporation.
Ted A. Bateman has received remuneration for consulting and speaker work for Baxter Health Care Corporation.
References
- 1.Wallny TA, Scholz DT, Oldenburg J, Nicolay C, Ezziddin S, Pennekamp PH, et al. Osteoporosis in haemophilia – an underestimated comorbidity? Haemophilia. 2007;13:79–84. doi: 10.1111/j.1365-2516.2006.01405.x. [DOI] [PubMed] [Google Scholar]
- 2.Gerstner G, Damiano ML, Tom A, Worman C, Schultz W, Recht M, et al. Prevalence and risk factors associated with decreased bone mineral density in patients with haemophilia. Haemophilia. 2009;15:559–565. doi: 10.1111/j.1365-2516.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostis P, Vakalopoulou S, Slavakis A, Charizopoulou M, Kazantzidou E, Chrysopoulou T, et al. Reduced bone mineral density in patients with haemophilia A and B in Northern Greece. Thromb Haemost. 2012;107:545–551. doi: 10.1160/TH11-08-05563. [DOI] [PubMed] [Google Scholar]
- 4.Kempton CL, Antun A, Antoniucci DM, Carpenter W, Ribeiro M, Stein S, et al. Bone density in haemophilia: a single institutional cross-sectional study. Haemophilia. 2014;20:121–128. doi: 10.1111/hae.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes C, Wong P, Egan B, Speller T, Cameron F, Jones G, et al. Reduced bone density among children with severe hemophilia. Pediatrics. 2004;114:e177–e181. doi: 10.1542/peds.114.2.e177. [DOI] [PubMed] [Google Scholar]
- 6.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus Episodic Treatment to Prevent Joint Disease in Boys with Severe Hemophilia. N Engl J Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 7.Khawaji M, Akesson K, Berntorp E. Long-term prophylaxis in severe haemophilia seems to preserve bone mineral density. Haemophilia. 2009;15:261–266. doi: 10.1111/j.1365-2516.2008.01912.x. [DOI] [PubMed] [Google Scholar]
- 8.Khawaji M, Astermark J, ÅKesson K, Berntorp E. Physical activity for prevention of osteoporosis in patients with severe haemophilia on long-term prophylaxis. Haemophilia. 2010;16:495–501. doi: 10.1111/j.1365-2516.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- 9.Roosendaal G, Van RAC, Vianen M, Van Den Berg H, Lafeber F, Bijlsma J. Haemophilic arthropathy resembles degenerative rather than inflammatory joint disease. Histopathology. 1999;34:144–153. doi: 10.1046/j.1365-2559.1999.00608.x. [DOI] [PubMed] [Google Scholar]
- 10.Hooiveld MJJ, Roosendaal G, Jacobs KMG, Vianen ME, van den Berg HM, Bijlsma JWJ, et al. Initiation of degenerative joint damage by experimental bleeding combined with loading of the joint: A possible mechanism of hemophilic arthropathy. Arthritis Rheum. 2004;50:2024–2031. doi: 10.1002/art.20284. [DOI] [PubMed] [Google Scholar]
- 11.Sokoloff L. Biomchemical and Physiological Aspects of Degenerative Joint Diseases with Special Reference to Hemophilic Arthropathy. Ann N Y Acad Sci. 1975;240:285–290. doi: 10.1111/j.1749-6632.1975.tb53362.x. [DOI] [PubMed] [Google Scholar]
- 12.Souza FMB, McLaughlin P, Pereira RP, Minuque NP, Mello MHM, Siqueira C, et al. The effects of repetitive haemarthrosis on postural balance in children with haemophilia. Haemophilia. 2013;19:e212–e217. doi: 10.1111/hae.12106. [DOI] [PubMed] [Google Scholar]
- 13.Lobet S, Hermans C, Bastien GJ, Massaad F, Detrembleur C. Impact of ankle osteoarthritis on the energetics and mechanics of gait: The case of hemophilic arthropathy. Clin Biomech. 2012;27:625–631. doi: 10.1016/j.clinbiomech.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Yokota H, Leong D, Sun H. Mechanical Loading: Bone Remodeling and Cartilage Maintenance. Curr Osteoporos Rep. 2011;9:237–242. doi: 10.1007/s11914-011-0067-y. [DOI] [PubMed] [Google Scholar]
- 15.Williams GN, Chmielewski T, Rudolph K, Buchanan TS, Snyder-Mackler L. Dynamic knee stability: current theory and implications for clinicians and scientists. J OrthopSports PhysTher. 2001;31:546–566. doi: 10.2519/jospt.2001.31.10.546. [DOI] [PubMed] [Google Scholar]
- 16.Jorge Filho D, Battistella LR, LourenÇO C. Computerized pedobarography in the characterization of ankle–foot instabilities of haemophilic patients. Haemophilia. 2006;12:140–146. doi: 10.1111/j.1365-2516.2006.01187.x. [DOI] [PubMed] [Google Scholar]
- 17.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament Injury, Reconstruction and Osteoarthritis. CurrOpinOrthop. 2005;16:354–362. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doré D, Quinn S, Ding C, Winzenberg T, Cicuttini F, Jones G. Subchondral bone and cartilage damage: A prospective study in older adults. Arthritis Rheum. 2010;62:1967–1973. doi: 10.1002/art.27467. [DOI] [PubMed] [Google Scholar]
- 19.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 20.Bi L, Sarkar R, Naas T, Lawler AM, Pain J, Shumaker SL, et al. Further characterization of factor VIII-deficient mice created by gene targeting: RNA and protein studies. Blood. 1996;88:3446–3450. [PubMed] [Google Scholar]
- 21.Sun J, Hakobyan N, Valentino LA, Feldman BL, Samulski RJ, Monahan PE. Intraarticular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX. Blood. 2008;112:4532–4541. doi: 10.1182/blood-2008-01-131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valentino LA, Hakobyan N, Kazarian T, Sorensen BB, Tranholm M. Prevention of haemarthrosis in a murine model of acute joint bleeding. Haemophilia. 2009;15:314–319. doi: 10.1111/j.1365-2516.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 23.Liel MS, Greenberg DL, Recht M, Vanek C, Klein RF, Taylor JA. Decreased bone density and bone strength in a mouse model of severe factor VIII deficiency. Br J Haematol. 2012;158:140–143. doi: 10.1111/j.1365-2141.2012.09101.x. [DOI] [PubMed] [Google Scholar]
- 24.Recht M, Liel MS, Turner RT, Klein RF, Taylor JA. The bone disease associated with factor VIII deficiency in mice is secondary to increased bone resorption. Haemophilia. 2013 doi: 10.1111/hae.12195. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 25.Baud'huin M, Duplomb L, Teletchea S, Charrier C, Maillasson M, Fouassier M, et al. Factor VIII-von Willebrand factor complex inhibits osteoclastogenesis and controls cell survival. J Biol Chem. 2009;284:31704–31713. doi: 10.1074/jbc.M109.030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bultink IM, Lems W. Osteoarthritis and Osteoporosis: What Is the Overlap? Curr Rheumatol Rep. 2013;15:1–8. doi: 10.1007/s11926-013-0328-0. [DOI] [PubMed] [Google Scholar]
- 27.Funck-Brentano T, Cohen-Solal M. Crosstalk between cartilage and bone: When bone cytokines matter. Cytokine Growth Factor Rev. 2011;22:91–97. doi: 10.1016/j.cytogfr.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Hakobyan N, Enockson C, Cole AA, Sumner DR, Valentino LA. Experimental haemophilic arthropathy in a mouse model of a massive haemarthrosis: gross, radiological and histological changes. Haemophilia. 2008;14:804–809. doi: 10.1111/j.1365-2516.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- 29.Sun J, Monahan PE. Extra-vascular clotting factor VIII protein protects against development of haemophilic synovitis in the presence of pre-existing anti-factor VIII inhibitor. Haemophilia. 2009;15 634-. [Google Scholar]
- 30.Narkbunnam N, Sun J, Hu G, Lin FC, Bateman TA, Mihara M, et al. IL-6 receptor antagonist as adjunctive therapy with clotting factor replacement to protect against bleeding-induced arthropathy in hemophilia. J Thromb Haemost. 2013;11:881–893. doi: 10.1111/jth.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Mauerhan D, Honeycutt P, Kneisl J, Norton HJ, Zinchenko N, et al. Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis Res Ther. 2010;12:R56. doi: 10.1186/ar2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuerst M, Niggemeyer O, Lammers L, Schafer F, Lohmann C, Ruther W. Articular cartilage mineralization in osteoarthritis of the hip. BMC Musculoskelet Disord. 2009;10:166. doi: 10.1186/1471-2474-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abreu M, Johnson K, Chung CB, De LJE, Jr, Trudell D, Terkeltaub R, et al. Calcification in calcium pyrophosphate dihydrate (CPPD) crystalline deposits in the knee: anatomic, radiographic, MR imaging, and histologic study in cadavers. Skeletal Radiol. 2004;33:392–398. doi: 10.1007/s00256-004-0767-9. [DOI] [PubMed] [Google Scholar]
- 34.Kanterewicz E, Sanmarti R, Panella D, Brugues J. Tendon calcifications of the hip adductors in chondrocalcinosis: a radiological study of 75 patients. Br J Rheumatol. 1993;32:790–793. doi: 10.1093/rheumatology/32.9.790. [DOI] [PubMed] [Google Scholar]
- 35.Riley GP, Harrall RL, Constant CR, Cawston TE, Hazleman BL. Prevalence and possible pathological significance of calcium phosphate salt accumulation in tendon matrix degeneration. Ann Rheum Dis. 1996;55:109–115. doi: 10.1136/ard.55.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen WE. Post Traumatic Para Articular Calcification of the Knee Joint. J Natl Med Assoc. 1945;37:164–167. [PMC free article] [PubMed] [Google Scholar]
- 37.Melchiorre D, Milia AF, Linari S, Romano E, Benelli G, Manetti M, et al. RANK-RANKL-OPG in Hemophilic Arthropathy: From Clinical and Imaging Diagnosis to Histopathology. J Rheumatol. 2012;39:1678–1686. doi: 10.3899/jrheum.120370. [DOI] [PubMed] [Google Scholar]
- 38.Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977;59:287–305. [PubMed] [Google Scholar]