Abstract
Advances in stem cell technology have engendered keen interest in cell-based therapies for neurological disorders. Postnatal engraftments of most neuronal precursors result in little migration, a critical step for transplants to integrate within the host circuitry. This may occur because most neurons migrate along substrates, such as radial glial processes, that are not abundant in adults. However, cortical GABAergic interneurons migrate tangentially from the subcortical forebrain into the cerebral cortex. Accordingly, transplants of cortical interneuron precursors migrate extensively after engraftment into a variety of CNS tissues, where they can become synaptically connected with host circuitry. Here we review how this remarkable ability to integrate post-transplant is being applied to the development of cell-based therapies for a variety of CNS disorders.
Keywords: GABAergic interneurons, transplantation, cell-based therapy, fate determination, neurological disease, cortical development
Tangential (non-radial) migration of cortical GABAergic interneurons, and initial transplantation studies
GABAergic interneurons represent about 20% of the neurons in the cerebral cortex [1]. In most instances, GABA hyperpolarizes target cells largely via postsynaptic chloride-permeable receptors. In addition to an inhibitory effect on cortical activity, a single interneuron can synchronize the firing of projection neuron ensembles, which is crucial to normal cerebral cortical function. Accordingly, cortical interneuron dysfunction is associated with a variety neuropathologies, including epilepsy, schizophrenia, and autism.
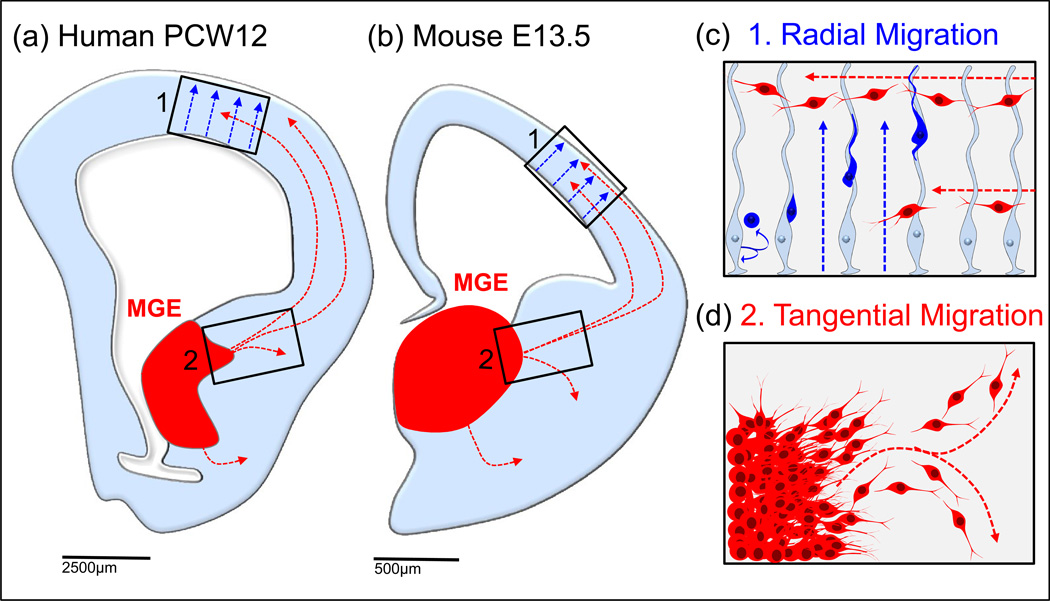
Multiple studies have demonstrated that cortical interneurons in rodents are born in subcortical regions and migrate tangentially (non-radially) over long distances to populate both the neocortex and the hippocampus [2] [3] (Figure 1). Similar migrations were also identified in gyrencephalic mammals, including ferrets and humans [4, 5]. While a cortical source of cortical interneurons has also been suggested to occur in primates [5–7], two recent studies found that the vast majority of GABAergic interneurons in primate cortex arrive there via non-radial migration from subcortical origins [8, 9] (Figure 1).
Figure 1. Subcortical origins and migration of mouse and human neocortical interneurons.
Cortical interneurons in rodents and primates originate embryonically in subcortical structures, then migrate non-radially into the cerebral cortex (MGE in red, a=human post conception week 12 and b=mouse embryonic day 13.5). Unlike radially migrating pyramidal cells born in the cortical ventricular zone (VZ) (box c, blue cells), the movement of interneurons is not restricted to radial glial scaffolds (boxes c and d, red cells). Mouse interneuron migration routes schematized as reviewed in [2], human interneuron migratory routes schematized from data in [8, 9].
The discovery of this remarkable capacity for cortical interneurons to migrate substantial distances, across the radial-glial scaffold, led Alvarez-Buylla and colleagues to test the idea that this capacity would permit their dispersion following engraftment into adult brain. Indeed, transplantation of interneuron progenitors from the medial ganglionic eminence (MGE; Figure 2) into the adult striatum resulted in widespread migration and survival [10] (Figure 2). Neither transplants of progenitors of striatal GABAergic projection neurons (from the lateral ganglionic eminence; LGE), nor transplants of neocortical glutamatergic projection neurons, showed a significant capacity to migrate into host brain tissues. This differential capacity to migrate post transplantation into the adult brain may relate to the general tendency for forebrain projection neuron populations to migrate radially, in contrast to forebrain interneuron populations [11]. These results suggested that MGE-derived interneuron precursors may be especially suited for use in cell-based therapies [10], a notion supported by the finding that MGE transplants into postnatal cortex differentiate into GABAergic interneurons that enhance local synaptic inhibition [12] (Figure 3a).
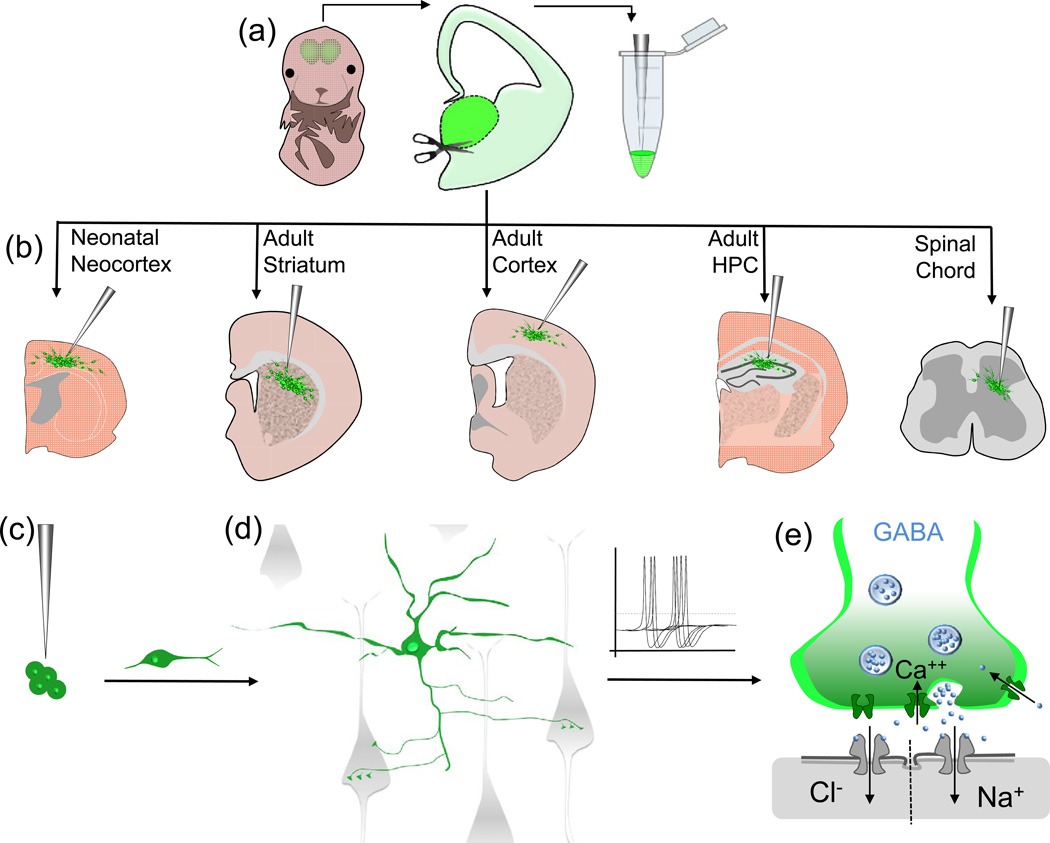
Figure 2. MGE-sourced interneurons survive and synaptically integrate upon transplantation into a variety of CNS tissues.
Interneurons sourced from embryonic mouse MGE are amenable to transplantation. GFP+ MGE tissue is dissected out, and resuspended as single cells (a) then injected into the desired host tissue (b). Various adult and neonatal tissues have been proven to be permissive to support transplanted interneurons derived from GFP+ MGE. These cells migrate away from the transplant core and many are able to integrate within the host circuitry (c and d, MGE derived interneurons = green, host cells = grey). Transplanted interneurons mature into GABAergic cells competent to release GABA upon depolarization along with trophic factors that can influence the downstream activity of host cells via opening GABA selective ion permeable channels (e).
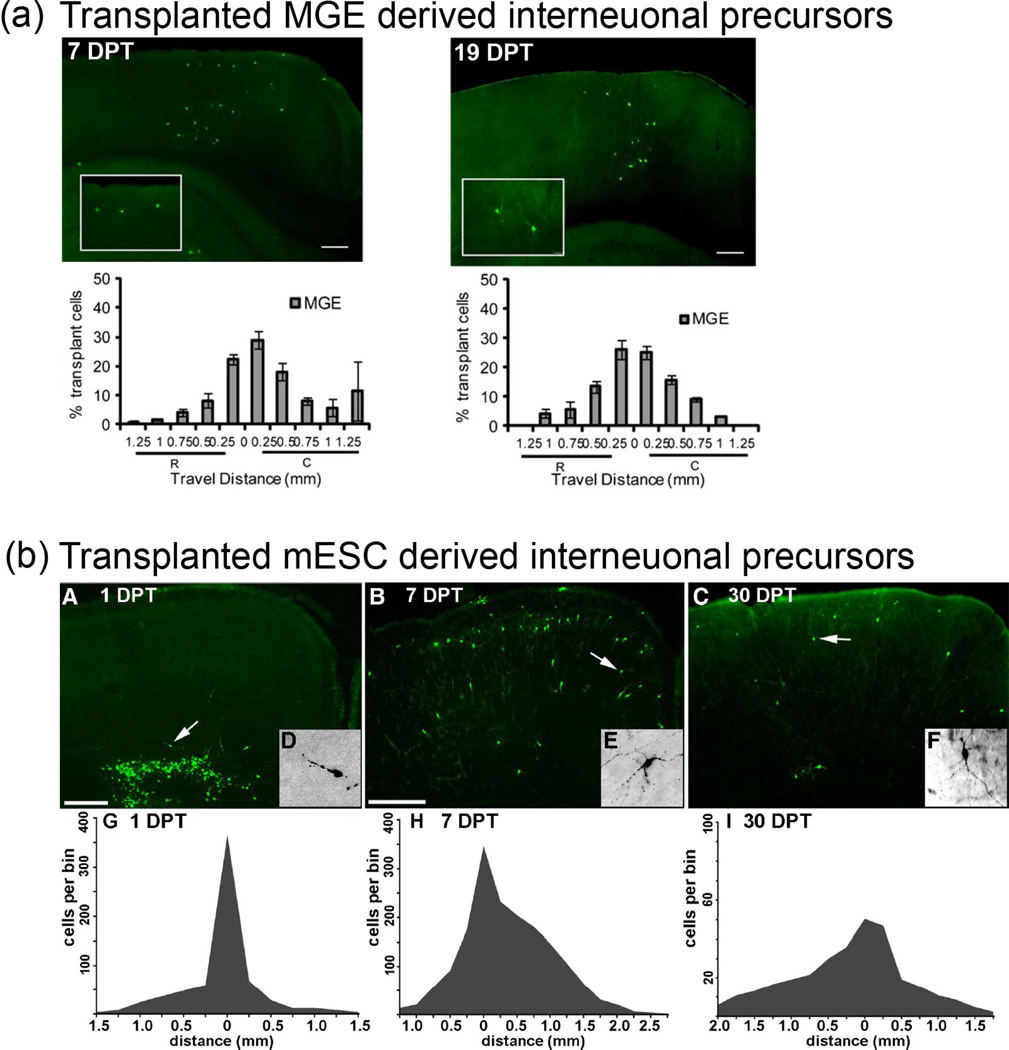
Figure 3. Migration of MGE and mESC-sourced interneuron precursors in neonatal cortical transplantation.
Composite image from ([68] and [40]). Interneurons sourced from either mouse embryonic MGE (a) or mouse embryonic stem cells (b) differentiated towards an Lhx6+, cortical interneuron precursor fate are capable of extensive dispersion from a transplant core in both neonatal (2mm+ ([68]) and adult (1mm+ [40]) brains. Top Panel (a): MGE sourced interneuron progenitors migrating from a transplant core. Left panel = 7 days post transplant (DPT). Right panel = 19 days post transplant (DPT). The X-Axis shows rostral-caudal distance from the injection site. The Y-axis shows the percentage of cells in each 250-µm bin. R=rostral and C=caudal. Bottom Panel (b): A–F, Immunofluorescence labeling of GFP in 50µm coronal sections. Arrows indicate cells shown at higher magnification in the insets At 1 DPT, the GFP+ cells were distributed close to the injection site, and many appear to be migrating into the cortical parenchyma. B, E, At 7 DPT, the GFP+ cells are much more broadly distributed, and many have multipolar morphologies suggestive of postmigratory neurons. C, F, The mediolateral extent of these cells at 30 DPT is 2.5-0.3 mm. G–I, Distributions of transplanted cells 1 (G), 7 (H), or 30 (I) days after transplantation. The x-axes show rostral–caudal distance from the injection site. The y-axes show cell profile number per 250µm bin made conservatively by multiplying the number of GFP+ cell profiles in the most distal section of that bin by 5. Note that in I, the y-axis scale is larger. G, After 1 DPT, most transplanted cells are within 300µm of the injection site. H, By 7 DPT, some of the cells have dispersed as far as 2 mm from the injection site. I, Although the survival or detectability of GFP+ cells had significantly decreased by 30 DPT, the rostral– caudal distribution is similar to that seen at 7 DPT. Scale bars: A, 200µm; B, C, 400µm. Permission has been granted to reproduce the copyrighted elements of this figure.
MGE transplants to study interneuron fate
While analysis of transgenic mice and genetic fate-mapping have made invaluable contributions to understanding cortical interneuron development, the migration and differentiation of MGE-derived cortical interneurons post-transplant has enabled a variety of studies on interneuron migration, fate determination, and function. Embryonic transplantation studies revealed that MGE interneurons will migrate into the overlying cortex, and differentiate into parvalbumin (PV) or somatostatin (SST) expressing interneuron subgroups when transplanted heterotopically into more caudal regions of the basal forebrain [13]. In addition, MGE interneurons lacking reelin signaling, transplanted homotopically into the wild-type MGE in utero, still migrate into the temporally-appropriate layers of cortex [14]. This study and others led to the important conclusion that cortical pyramidal neurons have a major influence on the layer-specific targeting of temporally-defined cohorts of MGE-derived interneurons [14–16].
Transplantations of MGE-derived interneuron progenitors into postnatal cortex have also been used to study interneuron subgroup origins. Neonatal cortical transplantation of different regions of the MGE (dorsal vs. ventral), revealed a strong bias for SST-expressing interneurons to originate early from the dorsal-most region of the MGE, and a weaker bias for PV-expressing interneurons to originate in the ventral two thirds of the MGE later in neurogenesis [17–19]. This approach also revealed a remarkable bias for a subtype of cortical interneuron, the axo-initial segment targeting Chandelier cell, to be generated from the ventral most region of the MGE at the latest stages of cortical neurogenesis [18, 20]. This latter finding is spurring follow-up studies to discover the fate determination of Chandelier cells, a neuron whose dysfunction is implicated in epilepsy and schizophrenia [21–23].
In addition to their utility for studying interneuron origins, MGE transplantation methods, by allowing the examination of chemically or genetically manipulated cells developing within an otherwise wild-type cortex, have also been invaluable for studying interneuron fate. For example, addition of the morphogen sonic hedgehog to slice cultures, followed by transplantation of the MGE region into wild-type cortex, can convert ventral MGE progenitors from the generation of PV to the generation of SST-expressing fates [24]. The approach can also be used to study the transcriptional regulation of interneuron fate. Electroporation of Lhx6, a target of the interneuron-fate determining transcription factor Nkx2.1, into the MGE-like region of slices cultures from Nkx2.1 nulls, can rescue the ability of these cells to express PV or SST after transplantation into neonatal neocortex [25].
In addition to the study of early aspects of interneuron development, MGE transplantation approaches can also be used to study the regulation of interneuron maturation and survival. For example, Dlx1 is required for the dendritic maturation and survival of interneuron subclasses [26]. Remarkably, interneuron survival both in vivo and in transplantation studies appears to be determined by an interneuron-intrinsic mechanism that results in the death of roughly 50% of cells, independently, in the case of transplant studies, of the number of cells transplanted [27].
MGE transplants in the study of interneuron-induced cortical plasticity
A potential role for cortical interneuron maturation in the process of adolescent-age range cortical plasticity was posited based on the tight correlation between PV expression in a subset of cortical interneurons, and dendritic spine density on pyramidal neurons, during prefrontal cortical development in the macaque [28]. Subsequent studies using transgenic mice and visual cortical plasticity in response to monocular deprivation revealed that the maturation of GABAergic interneurons is indeed crucial in determining the temporal window of plasticity [29, 30]. To examine whether the maturation of inhibition induces this type of plasticity based on cell-intrinsic aspects of inhibitory circuitry, MGE progenitors were transplanted into either neonatal (P0–2) or postnatal (P9–11) mouse cortex. The transplants were able to re-open ocular dominance plasticity in recipient cortex according to the age of the engrafted interneurons, specifically when they reached an age equivalent to when this plasticity occurs normally (P26–28) [31]. Of note, the effects of transplanted MGE on cortical plasticity probably cannot be exclusively attributed to increased inhibition, since enhancing inhibition pharmacologically does not similarly modulate plasticity after the end of the critical period [32]. However, this study raises the intriguing possibility that MGE transplants could facilitate aspects of circuitry repair by reopening a developmentally transient window of enhanced plasticity.
MGE transplants in the development of cell-based therapies
Seizure disorders
As noted above, cortical transplants of MGE-progenitors migrate from the site of engraftment and differentiate into interneurons that are synaptically connected to excitatory projection neurons in the recipient cortex [12, 31, 33] (Figure 2b–e). Accordingly, in the past few years a number of groups have established pre-clinical models of interneuron-based therapies to treat neurological disorders where impaired balance of excitation and inhibition results in pathology (Table 1). Epilepsy is one such disorder where a decrease of GABA-mediated inhibition is among the known pathological mechanisms. Drug treatment is ineffective for many epileptic patients, and surgical resection can itself cause neurological defects; these failures necessitate the development of alternative treatments for seizure disorders [34, 35]. In fact, the concept of treating seizures using interneuron-based cell therapy was first enumerated over two decades ago [36, 37], but awaited progress in identifying the sources of cortical interneurons in the subcortical forebrain that are amenable to transplantation.
Table 1.
Rodent models of human disorders treated by interneuron transplantation
| Disorder | Rodent Model | Cell Source | Target Tissue |
Results | Refs |
|---|---|---|---|---|---|
| Seizures | Kv1.1 Mutant Mouse | E13.5 MGE (GFP+) | Neonatal Cortex | Reduced abnormal electrical discharges | [38] |
| Seizures | Kainate Rat Model, TLE | E19 Hippocampal CA1 | Adult Hippocampus | Suppressed aberrant MFS in adult hippocampus, no EEG | [42] |
| Seizures | Kainate Rat Model, TLE | E19 Hippocampal CA1 | Adult Hippocampus | Restored loss of calbindin in adult hippocampus, no EEG | [43] |
| Seizures | Acute cortical 4-aminopyridine (4-AP) | E13.5 MGE (GFP+) | Adult Sensorimotor Cortex | Decreased local field potential power at site of transplantation | [40] |
| Seizures | Adult elimination of hippocampal interneurons SSP-Sap injection | E12.5 MGE (GFP+) | Adult Hippocampus | Decreased induced seizure severity and post seizure mortality | [33, 39] |
| Seizures | Kainate Rat Model, TLE | E14 MGE (CldU Labeled) | Adult Hippocampus | Reduced seizure frequency, duration and severity | [66] |
| Seizures | Pilocarpine, TLE | E13.5 MGE (GFP+) | Adult Hippocampus | Reduced seizures via EEG improved behavioral deficits | [41] |
| Seizures | Pilocarpine, TLE | mESC derived neural precursors | Adult Hippocampus | Cells express GABA but did not modify epileptic phenotypes | [70] |
| Parkinson's Disease | 6-OHDA Rat Model | E14.5 MGE/CGE (GFP+) | Adult Striatum | Improved motor scores on the apomorphine-stimulated rotation test | [49] |
| Neuropathic Pain | Spared nerve injury (SNI) model | E13.5 MGE (GFP+) | Adult Spinal Chord (dorsal horn) | Promoted reversal of mechanical hypersensitivity | [52] |
| Stroke | Transient cerebral artery occlusion | E15.5 MGE (GFP+) | Adult Cortex | Reduced motor deficits in the rotarod and elevated body swing tests | [53] |
| Anxiety | GABAA deficient mice | E14.5 MGE (GFP+) | Neonatal Neocortex | Reduced anxiety like behavior in an elevated plus maze paradigm | [56] |
| Psychosis | MAM Schizophrenia mouse model | E14.5 MGE (GFP+) | Adult Hippocampus | Reversed aberrant dopamine system function and behavior | [64, 65] |
| Psychosis | CD2 −/− mouse | E13.5 MGE (GFP+) | Adult Hippocampus | Reversed aberrant dopamine system function and behavior | Holly Moore, in review |
| Psychosis | PCP mouse model of shizophreniform cognitive deficits | E13.5 VZ and SVZ of MGE (GFP+) | Neonatal mPFC | Prevented cognitive and sensory-motor gating deficits by PCP. Activated projection neurons in the mPFC | [59] |
| Learning and Memory Deficits | Medial septum lesion | Human ESC-derived GABAergic neurons | Adult Hippocampus | Improved learning and memory deficits | [78] |
ESC=embryonic stem cell, GABA=γ-Aminobutyric acid, GFP=green fluorescent protein, MAM=methylazoxymethanol acetate, MGE=medial ganglionic eminence, mPFC=medial prefrontal cortex, MFS=mossy fiber sprouting, PCP=1-(1-phenylcyclohexyl)piperidine), SSP-Sap=saporin conjugated to substance P, SVZ=sub-ventricular zone, TLE=temporal lobe epilepsy, VZ=ventricular zone
The first study of MGE-derived interneuron transplants in a rodent model of epilepsy found that MGE progenitors, transplanted into the neonatal neocortex of mice lacking the potassium channel Kv1.1 resulted in a significant reduction of spontaneous electrographic seizures [38]. Subsequently, a series of papers from multiple groups have together shown that interneuron precursor transplantation into neonatal or adult neocortex or hippocampus can ameliorate both the development of seizure activity and the intensity of seizures already established [33, 39, 40] and even attenuate behavioral phenotypes [41] in rodent seizure models. Additionally, transplantation of fetal CA1 hippocampus also demonstrated potential seizure reducing properties. However, these studies did not confirm seizures by EEG, making it difficult to assess their therapeutic implications [42],[43] (Table 1; for reviews of this topic, see [35, 44, 45]).
The general assumption is that these transplants reduce seizure-related activity via their demonstrated enhancement of synaptic inhibition [12, 31, 33]. However, one study used the acute focal induction of epileptiform activity in adult neocortex to evaluate seizure propagation across a previously established MGE transplant, then compared the density of transplanted interneurons within a given mouse to the seizure propagation across the transplant in that same mouse [40]. Surprisingly, relatively low transplant densities still significantly influenced this propagation. Conceivably, the transplanted cells could have exuberant connectivity with the somata of host projection neurons, thus concentrating their inhibitory effects close to the source of axon-potential generation (Figure 2d). However, an additional possibility is that MGE transplants can affect local activity via a non-synaptic mechanism, such as the enhancement of tonic (extrasynaptic) inhibition, or the regulation of local blood flow.
Parkinson’s Disease
Parkinson’s Disease (PD) is characterized by motor deficiencies in addition to cognitive dysfunction and disturbances of mood. The hallmark motor symptoms arise from loss of dopamine producing (DA) neurons that originate in the substantia nigra pars compacta (SNc) and project to the striatum. Many studies have attempted to replace DA neurons directly in models of Parkinson’s related pathology, with varied levels of success (reviewed in [46]). However, alterations in striatal inhibition also occur in PD, and enhanced striatal inhibition can improve symptoms associated with PD [47, 48]. Accordingly, MGE-progenitors transplanted into rats that had received unilateral SN lesions with 6-OHDA differentiated into interneuron and glial subclasses and partially rescued motor behavior in lesioned animals [49]. While the physiological relationships between the transplants and the motor behaviors are unclear, this study highlights the capability of MGE progenitor transplants to affect complex neuronal circuits and improve deficits in a diseased brain. The survival of some interneurons at least one year after transplantation into lesioned brains also highlights the possibility of using these cells as a vector for the long-term delivery of a therapeutic agent.
Neuropathic Pain & Stroke
Neuropathic pain (NP) is defined as a chronic and debilitating condition characterized by abnormal and unpleasant sensations produced by otherwise innocuous stimuli [50]. Damage to inhibitory interneurons in the spinal chord is thought to significantly contribute to persistent pain caused by nerve injury, and dorsal spinal cord transplants of a neuronal cell line engineered to overexpress GABA reduced measures of pain in a rodent model of NP [51]. Astoundingly, MGE progenitors can migrate, survive, and differentiate into GABAergic interneurons upon transplantation into the spinal chord of adult mice [52] (Figure 2b). Grafted cells display mature interneuronal markers, integrate into spinal chord circuitry and achieve an almost complete reversal of mechanical hypersensitivity in a standard mouse model of nerve injury-induced neuropathic pain [52].
Additionally, interneuron-based therapies may enhance recovery after stroke. MGE progenitors were transplanted into multiple cortical regions in a rat model of stroke via transient middle cerebral artery occlusion [53]. Cells were delivered focally to regions surrounding the infarction two weeks after the occlusion, and grafted animals displayed reduced motor deficits compared to sham and fibroblast-transplanted controls. Curiously, unlike many of the other transplant studies reviewed herein, authors report only 20% of MGE-derived cells differentiated into GABAergic neurons in the occluded animals, suggesting the stroke environment may alter transplant fate. Regardless, transplanted animals displayed axonal sprouting and reformation in concert with neurite outgrowth suggesting that the graft reorganized circuitry in the stroke region.
Anxiety & Psychosis
The GABAergic system is implicated in modulation of overall anxiety levels, and depleted GABA tone may contribute to the development of generalized anxiety disorders [54]. In support of this connection, mice with GABAA deficits display behavioral, cognitive, and neuroendocrine hallmarks of melancholic anxious depression [55]. Interestingly, MGE-derived transplants delivered bilaterally to the neocortex of neonatal mice were also able to reduce anxiety like behavior in an elevated plus maze paradigm [56].
Schizophrenia is also associated with abnormalities of GABA signaling [57]. For example, PCP, arguably the psychotomimetic drug that best produces schizophrenia-like symptoms, may act primarily by blocking NMDA receptors on cortical interneurons [58]. Interestingly, acute PCP administration in mice results in schizophrenia-related cognitive deficits that can be prevented by MGE transplantation into the prefrontal cortex, but not by transplants into other cortical regions [59]. While the mechanisms underlying illness progression in schizophrenia are not well understood, one hypothesis suggests that hyperactive dopamine signaling in the mesolimbic system underlies the “positive” symptoms, hallucinations and delusions [60]. Hippocampal hyperactivation at rest has also been observed in schizophrenic patients [61], and rodent studies have identified a hippocampal-nucleus accumbens-substantia nigra circuit whereby hippocampal overactivation results in hyperactivation of midbrain dopaminergic neurons and their striatal targets [62]. While the mechanism behind hippocampal overactivation in schizophrenia is likely to be complex and heterogenous, reductions of MGE-derived hippocampal interneurons have been reported in post-mortem studies of the hippocampus from schizophrenic patients [63]. Based on this link between hippocampal overactivation and striatal dopamine release, a recent study examined the effects of MGE progenitor transplants into the adult hippocampus of mice treated by the methylazoxymethanol (MAM) model of psychosis-related phenotypes. The MAM model recapitulates some of the behavioral abnormalities and alterations in forebrain networks seen in schizophrenia, through perturbing embryonic brain development [64]. Remarkably, these transplants normalized measures of hippocampal hyperactivation [65]. Additionally, transplanted animals showed a reversal in hyperactive locomotion in response to amphetamine, implying that the hippocampal MGE transplants corrected the enhanced striatal dopamine release (by substantia nigra neurons) that is associated with the model used. While this paper did not explore how the transplants influence hippocampal activity, these results suggest that MGE-transplant based therapy targeting the hippocampus may be a future option for severely affected, treatment-resistant patients who also show hippocampal hyperactivation at rest by functional MRI.
In sum, MGE transplants can affect neuronal activity in remarkably diverse contexts, including neonatal and adult ages, and cortical as well as some non-cortical brain regions. The precise mechanisms by which MGE interneuron transplants affect this activity remain to be determined, and will probably be interneuron-subtype specific. However, it is likely that the mechanisms allowing for their normal process of non-radial migration across developmental domains followed by synaptic integration have endowed them with this extraordinary capacity.
Generation of GABAergic interneurons from stem cells
As demonstrated through the above findings, progenitors of MGE-derived GABAergic cortical interneurons appear to be extraordinarily suitable for a variety of cell-based therapeutic applications. But what cell source is amenable to a therapeutic trial? MGE derived cells from rats can be passaged many times while retaining a small capacity to differentiate into GABAergic interneuron-like cells [66]. These cells can also diminish hippocampal seizures post transplant, although the contribution of the minority of neurons in these cultures to seizure-reducing effects is not clear. However, this work does raise the possibility that a similar approach could generate a large amount of transplantable, MGE-related cells from fetal tissue.
Perhaps a more realistic approach would be application of pluripotent stem cell technology to the generation of cortical interneurons. Mouse embryonic stem cells have been directed into MGE-like cells with the capacity to differentiate into neurons with migratory and neurochemical features of cortical interneurons (Figure 3) [67–72]. These features included expected electrophysiological properties of neurochemically-defined cortical interneuron subgroups one-month following transplantation into neonatal neocortex [68]. In addition, mESCs differentiated towards MGE-like fates could survive and differentiate upon transplantation into the adult hippocampus in a mouse model of temporal lobe epilepsy (TLE) [70]. These heterogeneous mESC-sourced cells cannot be directly compared to MGE cells as upon differentiation few cells (<10%) went on to express markers of mature interneurons, which may explain why no changes in epilepsy were observed [70]. Despite the lack of an effect, it is likely that additional studies will be conducted where particular subgroups of interneurons are enriched in the ESC-derived transplant to maximize the epilepsy-ameliorating effects on host circuitry.
It has also become possible to generate MGE and cortical interneuron-like cells from human stem cells [73–76] using similar protocols to those that have been successful in mouse owing to the similar origins of cortical interneurons in these species [8, 9]. The stem cell-derived human interneuron precursors can undergo non-radial migration from MGE to cortex in an embryonic mouse forebrain slice preparation [75], and also migrate extensively into the surrounding cortical parenchyma following transplantation into neonatal mouse neocortex in vivo [75, 77]. Unfortunately, although the human stem cell-derived interneurons show input and output synaptogenesis and neurochemical interneuron subgroup expression after one month of culture with mouse neocortical cells [75], they show limited evidence of maturation in vivo even 6 months after transplantation into neonatal mouse neocortex [77]. Perhaps it should not be surprising that human interneurons follow their highly protracted, intrinsic developmental program in xenographic transplants. However, since the first human trials of stem cell derived interneuron transplants would likely involve very ill patients, the protracted maturation may be problematic for such trials unless approaches are discovered to accelerate human interneuron differentiation.
One study has transplanted human stem cell derived MGE-like cells into adult mouse hippocampus, resulting in a mixed population of astrocytes, GABAergic neurons, and cholinergic neurons [78], with survival to at least 6 months. Prior to transplant, some mice were subjected to a toxic lesion to the septal area, resulting in cognitive deficits. Remarkably, transplanted mice showed recovery on cognitive deficits relative to sham transplant controls. In sum, exciting progress is underway with human stem cell derived MGE-like transplants, such that we consider it likely that an interneuron-fated or mixed transplant of MGE-like progenitors will be feasible for a human trial in interneuron-related illnesses. Some remaining challenges are listed in Box 1.
Box 1 Challenges to the Implementation of MGE/Interneuron.
Transplantation Therapy
Despite results from animal studies suggesting that MGE/Interneuron transplantation therapy may have a variety of applications in humans, major hurdles remain to bringing this approach to the bedside. What will the source of the cells be? There have been tremendous advances in generating MGE progenitor and also interneuron-like cells from mouse and human stem cells, but the protracted differentiation, particularly of the human cells, after xenographic transplantation raises serious concerns about the feasibility of this approach. If a human stem cell source is used, what method will ensure that no tumor-producing cells are transplanted along with therapeutic ones? The use of reporters of newly born post-mitotic interneurons, such as Lhx6, in mouse ESC studies is encouraging. However, the application of this approach to human cells, and the use of this approach to generate or identify surface antigens that can be used to FACS-purify the fate committed, post-mitotic interneurons from any human source, remains to be explored. In addition, how long will an interneuron transplant function, and what will be the long-term effects on circuitry that is not dysfunctional? For example, would transplant of interneurons into a neocortical seizure focus reduce seizures, but alter local circuitry in a manner that disrupts cortical function? Perhaps most importantly, the interneuron subgroups and subtypes that derive from the MGE have remarkably distinct properties, such as axon targeting, sensitivity to extrinsic factors, and firing rate and pattern. To date, studies of MGE transplantation have barely scratched the surface of the question of how distinct types of interneurons are influencing host circuitry and activity in the transplantation context. As methods are discovered for the selective generation of cells committed to distinct interneuron fates, the potential of interneuron-based transplantation therapy, first proposed more than two decades ago, may finally be realized.
Future directions
As noted above, transplantation studies involving MGE progenitors have been used to study aspects of interneuron development, function, and have been applied to the development of cell-based therapies for seizures, Parkinson’s disease, neuropathic pain, stroke, psychosis, and anxiety (Table 1). A comprehensive discussion of the implications of this work is beyond the scope of this article, but a few areas warrant special mention. First, further elucidation of the intrinsic mechanisms for interneuron non-radial migration could be used to develop genetic tools for inducing the post - transplant migration and integration of projection neuron populations, such as cholinergic neurons for Alzheimer’s disease, medium spiny neurons for Huntington’s disease, or cortical pyramidal neurons for stroke. In addition, from fundamental studies methods can be learned to reliably control interneuron migratory behavior, for example by introducing hyperpolarizing signals [79, 80], in order to optimize their final density within a given distance from the initial graft. On a related topic, optogenetic methods can be combined with the generation of MGE-like progenitors from stem cells to allow the transplantation of photoactivatable interneurons. In addition to further studies on inhibition in plasticity, such a tool could be used in conjunction with patient or EEG-activated LEDs to instantly modulate seizure propagation [81], neuropathic pain, or perhaps even psychotic symptoms. Finally, the possibility of using stem cell derived interneurons as vectors for the delivery of therapeutic agents beyond GABA remains an underexplored possibility for a wide variety of therapeutic interventions.
Highlights.
Cortical GABAergic interneurons migrate tangentially from the subcortical forebrain into the cerebral cortex.
Transplants of cortical interneuron precursors migrate extensively after engraftment into a variety of CNS tissues.
This ability to integrate post-transplant is being applied to the study of aspects of development and excitingly to the development of cell-based therapies for a variety of CNS disorders.
Box 2. Outstanding Questions.
How will the large size of the human brain impact the potential for developing interneuron-transplantation based therapeutics?
Will it be possible to accelerate the maturation of human stem cell derived interneurons?
Do subtypes of interneurons differentially affect host neural activity post transplant? Are there implications of such differential effects for the development interneuron-based therapies?
Since key aspects of interneuron function depend not only on the presence of class-defining markers, but also on specific patterns of inputs, intrinsic activity, and axonal targeting onto other neurons, what assays will allow us to study these features?
How do we direct the differentiation of ESCs into interneuron subtypes, how can we efficiently purify these subtypes from the differentiation culture, and how can we define these subtypes outside of the human brain?
Can we use our knowledge of the regulation of non-radial migration to control the dispersion of interneurons post transplant?
Can we use our knowledge of the regulation of non-radial migration to modify stem cell derived projection neuron populations such that they undergo interneuron-like migration after transplantation into adult brain?
Acknowledgements
We apologize to all of our colleagues whose work we did not discuss due to space limitations. Authors thank Dr. Timothy Petros for editorial suggestions on the manuscript. This work was supported by NIH grants from the National Institutes of Mental Health (R01 MH066912, K02 MH070031; SA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tamamaki N, et al. Green Fluorescent Protein Expression and Colocalization with Calretinin, Parvalbumin, and Somatostatin in the GAD67-GFP Knock-In Mouse. Journal of Comparative Neurology. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 2.Marin O. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. The European journal of neuroscience. 2013;38:2019–2029. doi: 10.1111/ejn.12225. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka DH, Nakajima K. Migratory pathways of GABAergic interneurons when they enter the neocortex. The European journal of neuroscience. 2012;35:1655–1660. doi: 10.1111/j.1460-9568.2012.08111.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson SA, et al. Distinct origins of neocortical projection neurons and interneurons in vivo. Cerebral cortex. 2002;12:702–709. doi: 10.1093/cercor/12.7.702. [DOI] [PubMed] [Google Scholar]
- 5.Letinic K, et al. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 6.Jones EG. The origins of cortical interneurons: mouse versus monkey and human. Cerebral cortex. 2009;19:1953–1956. doi: 10.1093/cercor/bhp088. [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Zecevic N. Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:2413–2420. doi: 10.1523/JNEUROSCI.5249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen DV, et al. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci. 2013 doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma T, et al. Subcortical origins of human and monkey neocortical interneurons. Nature neuroscience. 2013 doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- 10.Wichterle H, et al. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nature neuroscience. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- 11.Marin O, Rubenstein JL. A long, remarkable journey: Tangential migration in the telencephalon. Nature Reviews Neuroscience. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Dolado M, et al. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7380–7389. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wichterle H, et al. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 14.Pla R, et al. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:6924–6934. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hevner RF, et al. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience. 2004;124:605–618. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Lodato S, et al. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69:763–779. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flames N, et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inan M, et al. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cerebral cortex. 2012;22:820–827. doi: 10.1093/cercor/bhr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wonders CP, et al. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Developmental biology. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniguchi H, et al. The spatial and temporal origin of chandelier cells in mouse neocortex. Science. 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeFelipe J. Chandelier cells and epilepsy. Brain : a journal of neurology. 1999;122(Pt 10):1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- 22.Woodruff AR, et al. The enigmatic function of chandelier cells. Front Neurosci. 2010;4:201. doi: 10.3389/fnins.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis DA, et al. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in neurosciences. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Q, et al. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du T, et al. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- 26.Cobos I, et al. The vertebrate ortholog of Aristaless is regulated by Dlx genes in the developing forebrain. The Journal of comparative neurology. 2005;483:292–303. doi: 10.1002/cne.20405. [DOI] [PubMed] [Google Scholar]
- 27.Southwell DG, et al. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491:109–113. doi: 10.1038/nature11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson SA, et al. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- 29.Toyoizumi T, et al. A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron. 2013;80:51–63. doi: 10.1016/j.neuron.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 31.Southwell DG, et al. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagiolini M, et al. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- 33.Zipancic I, et al. Transplant of GABAergic precursors restores hippocampal inhibitory function in a mouse model of seizure susceptibility. Cell transplantation. 2010;19:549–564. doi: 10.3727/096368910X491383. [DOI] [PubMed] [Google Scholar]
- 34.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 35.Richardson RM, et al. Developing cell transplantation for temporal lobe epilepsy. Neurosurgical focus. 2008;24:E17. doi: 10.3171/FOC/2008/24/3-4/E16. [DOI] [PubMed] [Google Scholar]
- 36.Buzsaki G, et al. Suppression and induction of epileptic activity by neuronal grafts. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:9327–9330. doi: 10.1073/pnas.85.23.9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindvall O, Bjorklund A. Intracerebral grafting of inhibitory neurons. A new strategy for seizure suppression in the central nervous system. Advances in neurology. 1992;57:561–569. [PubMed] [Google Scholar]
- 38.Baraban SC, et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calcagnotto ME, et al. Grafting of GABAergic precursors rescues deficits in hippocampal inhibition. Epilepsia. 2010;51(Suppl 3):66–70. doi: 10.1111/j.1528-1167.2010.02613.x. [DOI] [PubMed] [Google Scholar]
- 40.De la Cruz E, et al. Interneuron progenitors attenuate the power of acute focal ictal discharges. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2011;8:763–773. doi: 10.1007/s13311-011-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunt RF, et al. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nature neuroscience. 2013;16:692–697. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shetty AK, et al. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8391–8401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shetty AK, Hattiangady B. Restoration of calbindin after fetal hippocampal CA3 cell grafting into the injured hippocampus in a rat model of temporal lobe epilepsy. Hippocampus. 2007;17:943–956. doi: 10.1002/hipo.20311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shetty AK. Progress in cell grafting therapy for temporal lobe epilepsy. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2011;8:721–735. doi: 10.1007/s13311-011-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naegele JR, et al. Recent advancements in stem cell and gene therapies for neurological disorders and intractable epilepsy. Neuropharmacology. 2010;58:855–864. doi: 10.1016/j.neuropharm.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Munter JP, et al. Cell based therapy in Parkinsonism. Translational neurodegeneration. 2013;2:13. doi: 10.1186/2047-9158-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mallet N, et al. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplitt MG, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Cerdeno V, et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell stem cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mann R, et al. Burden of spinal cord injury-related neuropathic pain in the United States: retrospective chart review and cross-sectional survey. Spinal cord. 2013;51:564–570. doi: 10.1038/sc.2013.34. [DOI] [PubMed] [Google Scholar]
- 51.Eaton MJ, et al. Transplants of neuronal cells bioengineered to synthesize GABA alleviate chronic neuropathic pain. Cell transplantation. 1999;8:87–101. doi: 10.1177/096368979900800102. [DOI] [PubMed] [Google Scholar]
- 52.Braz JM, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daadi MM, et al. Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell transplantation. 2009;18:815–826. doi: 10.3727/096368909X470829. [DOI] [PubMed] [Google Scholar]
- 54.Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 55.Shen Q, et al. GABAergic control of critical developmental periods for anxiety- and depression-related behavior in mice. PloS one. 2012;7:e47441. doi: 10.1371/journal.pone.0047441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valente MF, et al. Postnatal transplantation of interneuronal precursor cells decreases anxiety-like behavior in adult mice. Cell transplantation. 2013;22:1237–1247. doi: 10.3727/096368912X657422. [DOI] [PubMed] [Google Scholar]
- 57.Inan M, et al. Losing your inhibition: Linking cortical GABAergic interneurons to schizophrenia. Neurobiology of disease. 2012 doi: 10.1016/j.nbd.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korotkova T, et al. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka DH, et al. GABAergic precursor transplantation into the prefrontal cortex prevents phencyclidine-induced cognitive deficits. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14116–14125. doi: 10.1523/JNEUROSCI.2786-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2004;7(Suppl 1):S1–S5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- 61.Schobel SA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lisman JE, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in neurosciences. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Konradi C, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophrenia research. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lodge DJ. The MAM rodent model of schizophrenia. Current protocols in neuroscience / editorial board, Jacqueline N. Crawley … [et al.] 2013;Chapter 9(Unit9):43. doi: 10.1002/0471142301.ns0943s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perez SM, Lodge DJ. Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Molecular psychiatry. 2013 doi: 10.1038/mp.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waldau B, et al. Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem cells. 2010;28:1153–1164. doi: 10.1002/stem.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe K, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nature neuroscience. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 68.Maroof AM, et al. Prospective isolation of cortical interneuron precursors from mouse embryonic stem cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:4667–4675. doi: 10.1523/JNEUROSCI.4255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Danjo T, et al. Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1919–1933. doi: 10.1523/JNEUROSCI.5128-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maisano X, et al. Differentiation and functional incorporation of embryonic stem cell-derived GABAergic interneurons in the dentate gyrus of mice with temporal lobe epilepsy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:46–61. doi: 10.1523/JNEUROSCI.2683-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petros TJ, et al. Enhanced derivation of mouse ESC-derived cortical interneurons by expression of Nkx2.1. Stem cell research. 2013;11:647–656. doi: 10.1016/j.scr.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen YJ, et al. Use of "MGE Enhancers" for Labeling and Selection of Embryonic Stem Cell-Derived Medial Ganglionic Eminence (MGE) Progenitors and Neurons. PloS one. 2013;8:e61956. doi: 10.1371/journal.pone.0061956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Germain ND, et al. Derivation and isolation of NKX2.1-positive basal forebrain progenitors from human embryonic stem cells. Stem cells and development. 2013;22:1477–1489. doi: 10.1089/scd.2012.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goulburn AL, et al. Generating GABAergic cerebral cortical interneurons from mouse and human embryonic stem cells. Stem cell research. 2012;8:416–426. doi: 10.1016/j.scr.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Maroof AM, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell stem cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, et al. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nature protocols. 2013;8:1670–1679. doi: 10.1038/nprot.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nicholas CR, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell stem cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, et al. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nature biotechnology. 2013;31:440–447. doi: 10.1038/nbt.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Marco Garcia NV, et al. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature. 2011;472:351–355. doi: 10.1038/nature09865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krook-Magnuson E, et al. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nature communications. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]