Abstract
Chemoreception in the mouse olfactory system occurs primarily at two chemosensory epithelia in the nasal cavity: the main olfactory epithelium (MOE) and the vomeronasal epithelium. The canonical chemosensory neurons in the MOE, the olfactory sensory neurons (OSNs), express the odorant receptor (OR) gene repertoire, and depend on Adcy3 and Cnga2 for chemosensory signal transduction. The canonical chemosensory neurons in the vomeronasal epithelium, the vomeronasal sensory neurons (VSNs), express two unrelated vomeronasal receptor (VR) gene repertoires, and involve Trpc2 for chemosensory signal transduction. Recently we reported the discovery of two types of neurons in the mouse MOE that express Trcp2 in addition to Cnga2. These cell types can be distinguished at the single-cell level by expression of Adcy3: positive, type A and negative, type B. Some type A cells express OR genes. Thus far there is no specific gene or marker for type B cells, hampering further analyses such as physiological recordings. Here, we show that among MOE cells, type B cells are unique in their expression of the soluble guanylate cyclase Gucy1b2. We came across Gucy1b2 in an explorative approach based on Long Serial Analysis of Gene Expression (LongSAGE) that we applied to single red-fluorescent cells isolated from whole olfactory mucosa and vomeronasal organ of mice of a novel Trcp2-IRES-taumCherry gene-targeted strain. The generation of a novel Gucy1b2-IRES-tauGFP gene-targeted strain enabled us to visualize coalescence of axons of type B cells into glomeruli in the main olfactory bulb. Our molecular and anatomical analyses define Gucy1b2 as a marker for type B cells within the MOE. The Gucy1b2-IRES-tauGFP strain will be useful for physiological, molecular, cellular, and anatomical studies of this newly described chemosensory subsystem.
Keywords: Main olfactory epithelium, Vomeronasal epithelium, Cyclic-nucleotide gated channel, Trp channel, Guanylate cyclase
Highlights
-
•
Trpc2 + cells exist as type A and type B in the mouse main olfactory epithelium.
-
•
We find no evidence for expression of chemosensory GPCR genes in type B cells.
-
•
We identify the soluble guanylate cyclase Gucy1b2 as a marker for type B cells.
-
•
Gucy1b2-IRES-tauGFP knockin mice will be useful for physiological studies.
1. Introduction
Since the discovery of expression of the Trpc2 cation channel in rat vomeronasal organ (VNO) (Liman et al., 1999), Trpc2 expression was thought to be restricted to VSNs in mouse. We recently challenged this belief by immunohistochemistry (IHC) with the Trpc2 antibody that was used in rat (Liman et al., 1999) and by in situ hybridization (ISH) (Omura and Mombaerts, 2014). We also generated a Trpc2-IRES-taulacZ knockin mouse strain (Omura and Mombaerts, 2014). We found that the mouse MOE actually abounds with Trpc2 + cells, from early stages in development throughout adulthood. We identified two types of Trpc2 + MOE cells, which we refer to as type A and type B cells. These cell types can be distinguished at the single-cell level by Adcy3 expression: type A cells express Adcy3, and type B cells do not express Adcy3. The cell bodies of type A cells form a semicontinuous layer throughout the MOE just below the sustentacular cell layer. The cell bodies of type B cells reside within the lateral region of the MOE and are located at all positions along the basal-to-apical dimension of the MOE. One third of MOE cells labeled with Olfr68/69 riboprobes are type A cells. We were unable to find ISH evidence of expression of the known chemosensory G-protein coupled receptor genes or signaling components in type B cells. Type A and type B cells appear to share only Trpc2 expression with VSNs, and are thus not VSNs that are misplaced in the MOE.
Here, we describe a novel gene-targeted knockin mutation in the Trpc2 locus, designed to coexpress Trpc2 with the red-fluorescent axonal marker taumCherry. We picked single taumCherry + MOE cells from the lateral region of the MOE (type B cells) and carried out RT-PCR analyses. We confirm and extend our ISH observations that type B cells do not express OR or VR genes. Next we applied LongSAGE to single taumCherry + cells, and came across the soluble guanylate cyclase Gucy1b2. We show by ISH and by IHC with a custom Gucy1b2 antibody that Gucy1b2 expression is specific to type B cells in the MOE. We counted ~ 16,000 Gucy1b2+ cells in the MOE of C57BL/6 mice at three weeks, and found that 97% of these cells are Trpc2+. In mice of a novel gene-targeted Gucy1b2-IRES-tauGFP knockin strain, GFP + axons coalesce into a few glomeruli ventrally and posteriorly in the main olfactory bulb. Our results thus define Gucy1b2 as a marker for type B cells in the MOE. The Gucy1b2-IRES-tauGFP strain will enable physiological experiments in type B cells to determine in which regard these cells differ functionally from type A cells, canonical OSNs, and VSNs.
2. Results
2.1. The Trpc2-IRES-taumCherry knockin mouse strain
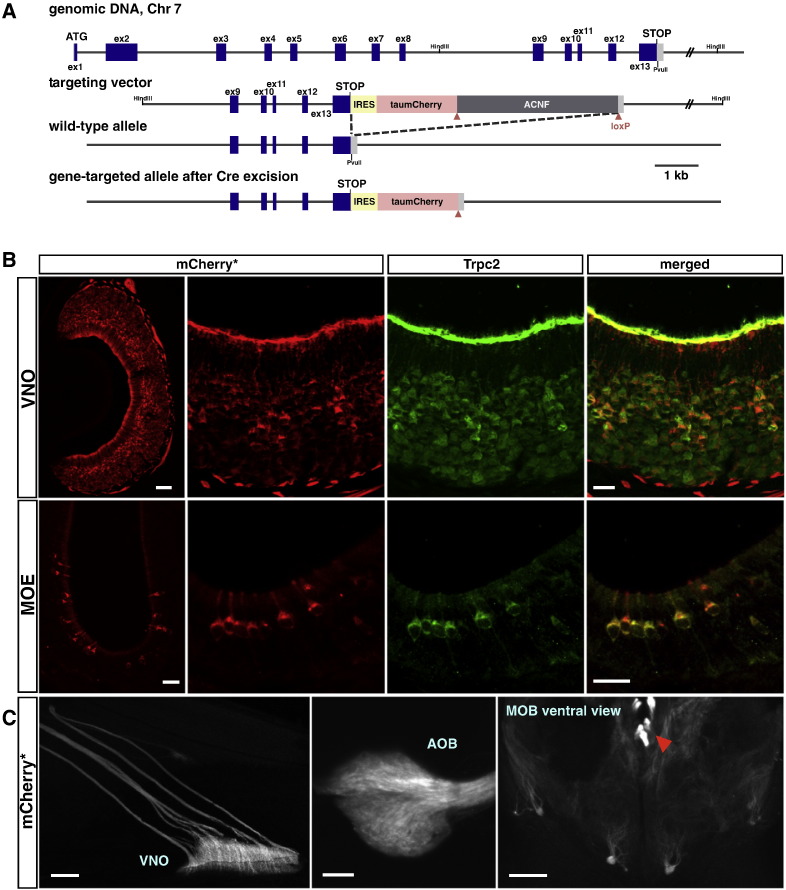
We have previously described a Trpc2-IRES-taulacZ knockin strain, in which Trpc2 + cells coexpress the axonal marker tauβgalactosidase (Omura and Mombaerts, 2014). This mouse strain lends itself well to X-gal histochemistry and IHC with βgalactosidase antibodies, but is less suited to study live, unfixed Trpc2 + cells. We therefore generated another mouse strain by gene targeting using the red fluorescent protein mCherry (Shaner et al., 2004). The genetic design of the Trpc2-IRES-taumCherry targeted mutation (Fig. 1A) mirrors that of Trpc2-IRES-taulacZ. The IRES sequence allows for cotranslation of intact Trpc2 polypeptide with the axonal marker taumCherry, and the endogenous 3′ untranslated sequence of Trpc2 is retained within the transcripts generated from the mutant allele. This mouse strain is publicly available from The Jackson Laboratory.
Fig. 1.
Trpc2-IRES-taumCherry gene-targeted strain. (A) Generation of a Trpc2-IRES-taumCherry knockin mutation in the mouse germline. The IRES-taumCherry-ANCF cassette was inserted after the STOP codon of Trpc2 by homologous recombination with a targeting vector in ES cells. The ACNF cassette is a self-excising neo gene that is removed during the transmission of the targeted allele through the male germ line, leaving a single loxP site (red triangle) behind in the locus. The axonal marker taumCherry is a fusion protein between bovine tau and mCherry and is intrinsically red fluorescent. (B) Intrinsic red fluorescence of taumCherry (mCherry*) of sections of the VNO and MOE of homozygous Trpc2-IRES-taumCherry mice at eight weeks, combined with IHC (green) for Trpc2. The fusion of mCherry to tau promotes subcellular localization at dendritic tips and axons compared to cell bodies. (C) Whole mount view of the olfactory system of a homozygous Trpc2-IRES-taumCherry mouse at four weeks, intrinsic red fluorescence (mCherry*). (Left) Axons project from the VNO across the nasal septum in the form of several vomeronasal fascicles. (Middle) Most axons terminate in the accessory olfactory bulb (AOB). (Right) Some axons coalesce into a few glomeruli ventrally in the main olfactory bulb (MOB). The red arrowhead indicates the vomeronasal nerve. Scale bar: 50 μm in B top left; 20 μm in B other panels; 500 μm in C left; 200 μm in C middle and right.
In a coronal section of the VNO of a homozygous Trpc2-IRES-taumCherry mouse, the intense red fluorescence reflects the broad and high expression of Trpc2 across VSNs (Fig. 1B). A coronal section of the MOE reveals scattered red-fluorescent cells that are also Trpc2 + by IHC (Fig. 1B). There is thus concordance at the cellular level between the intrinsic red fluorescence and the Trpc2 immunoreactive signal, as can be expected from the IRES design. In whole mounts, red-fluorescent cells in the VNO project their axons to the accessory olfactory bulb (AOB) and coalesce into a few glomeruli on the ventral aspect of the main olfactory bulb (Fig. 1C). The Trpc2-IRES-taumCherry strain mimics the expression pattern seen in the Trpc2-IRES-taulacZ strain.
2.2. Single-cell RT-PCR
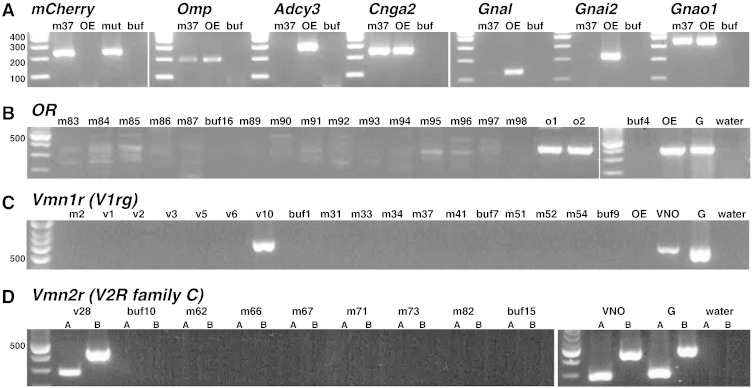
We dissected whole olfactory mucosa (WOM) (Khan et al., 2013) from the lateral region of the MOE of homozygous Trpc2-IRES-taumCherry mice at 8 weeks, and dissociated the tissue samples into single cells. The lateral region of the MOE is enriched in type B cells (Omura and Mombaerts, 2014), but contains also type A cells, which are present throughout the MOE. The expression level of Trpc2 in adult mice is higher in type B cells than in type A cells. We thus picked the brightest mCherry + cells using micromanipulators under a fluorescence microscope. We carried out RT-PCR with mCherry and Omp primers on 59 WOM-derived cells (m-cells), as well as on 31 VNO-derived cells (v-cells). We identified 24 and 18 cells, respectively, from which both transcripts could be amplified; we call these 42 cells the validated cells. An example of the results from a validated WOM cell (m37) is given in Fig. 2A. Cell m37 is positive for mCherry, Omp, Cnga2, and Gnao1, very weakly positive for Gnal, and negative for Adcy3 and Gnai2; this gene expression profile is characteristic of type B cells. Next we subjected validated m-cells to degenerate RT-PCR for OR genes: we could not obtain a distinct RT-PCR product from 0/12 validated m-cells (Fig. 2B). However a strong RT-PCR product was obtained from o1 and o2, two randomly picked single cells from WOM of a heterozygous OMP-YFP gene-targeted mouse; these cells are most likely canonical, OR-expressing OSNs. Indeed, sequencing the PCR products revealed that o1 expressed Olfr1183, and o2 expressed Olfr1607. We did not obtain a band with degenerate primers for Taar genes (Liberles and Buck, 2006) from 8 validated m-cells (data not shown). Next we applied 11 subfamily-specific degenerate primer sets for V1ra to V1rk subfamilies covering all Vmn1r members (Roppolo et al., 2006). Strong PCR products were obtained from 5/6 validated v-cells, but not from 9 validated m-cells with any of these 11 primer sets. Sequencing the PCR products revealed expression of Vmnr68 in v1 and v5, V1re13/Vmn1r71 in v2, V1rf4/Vmn1r236 in v3, and V1rg3/Vmn1r80 in v10; an example is given for the V1rg primer set in Fig. 2C. It is well established that in basal VSNs, one or more genes of the seven-gene family-C Vmn2r are coexpressed with one family-ABD Vmn2r gene (Ishii and Mombaerts, 2008; Ishii and Mombaerts, 2011; Silvotti et al., 2011). Using two sets of degenerate RT-PCR primers for family-C Vmn2r genes, we could amplify a band from 1/2 validated v-cells (v28) with primer set A and 2/2 validated v-cells with primer set B (v28 and v32), but not from 9 validated m-cells (Fig. 2D). We performed RT-PCR with degenerate primers for Fpr genes, the third class of chemosensory G-protein coupled receptor genes expressed in the VNO (Rivière et al., 2009; Liberles et al., 2009). We obtained strong PCR products from v6 and v36, and identified by sequencing expression of Fpr-rs4 expression from v6 and Fpr-rs6 in v36; no PCR products were obtained from 6 validated m-cells (data not shown).
Fig. 2.
Single-cell RT-PCR of red-fluorescent cells from Trpc2-IRES-taumCherry mice. (A) Ethidium-bromide stained agarose gels of RT-PCR products generated from a single red-fluorescent cell (m37) isolated from lateral WOM of a homozygous Trpc2-IRES-taumCherry mouse at eight weeks, in comparison with RT-PCR products of WOM of a WT C57BL/6 mouse at eight weeks (OE), PCR products of genomic DNA (mut) from a homozygous Trpc2-IRES-taumCherry mouse as positive control for mCherry, and buffer (buf) as negative control. (B) Degenerate RT-PCR with primers for OR genes does not reveal OR gene expression in 15 single cells isolated from lateral WOM of a homozygous Trpc2-IRES-taumCherry mouse at eight weeks. We cloned some of the faint bands and found that they did not represent OR genes. A strong RT-PCR product is obtained with o1 and o2, two single cells isolated from WOM of a heterozygous OMP-YFP gene-targeted mouse at three weeks. buf16 is buffer, as negative control. (C) Degenerate RT-PCR for the V1rg family of Vmn1r genes reveals a product in one of six single red-fluorescent cells (v10) isolated from the VNO a homozygous Trpc2-IRES-taumCherry mouse at eight weeks. By contrast, no RT-PCR products are obtained from nine single red-fluorescent cells (such as m2) picked from lateral WOM. Lanes labeled with buf are with buffer, as negative control. (D) RT-PCR for the family-C Vmn2r genes reveals a product in a single red-fluorescent cell (v28) isolated from the VNO a homozygous Trpc2-IRES-taumCherry mouse at eight weeks, but not from six single red-fluorescent cells (such as m62) isolated from lateral WOM of a homozygous Trpc2-IRES-taumCherry mouse at eight weeks. A and B are two distinct primer sets. Lanes labeled with buf are with buffer, as negative control. VNO represents cDNA from whole vomeronasal organ, and Gen represents genomic DNA of a WT C57BL/6 mouse.
Thus, our single-cell RT-PCR analyses on 24 type B cells provide another line of negative evidence that these cells do not express known chemosensory G-protein coupled receptor genes. However some of these genes may not be detectable by the degenerate RT-PCR primers.
2.3. Long Serial Analysis of Gene Expression
The targeted search for expression of known chemosensory G-protein coupled receptor genes and components of signaling pathways by ISH (Omura and Mombaerts, 2014) and here by single-cell RT-PCR has not unveiled any evidence for such expression. In an alternative strategy, we took a non-targeted, discovery-oriented approach by applying a variant of SAGE called LongSAGE (Velculescu et al, 1995; Saha et al., 2002; Saito et al., 2004; Hu et al., 2007) on three red-fluorescent cells from Trpc2-IRES-taumCherry mice: two type B cells and on one VNO cell. The type B cells were validated by RT-PCR for expression of Gapdh, Omp, mCherry, Cnga2, and Gnao and no expression of Adcy3, and the VNO cell was validated for expression of Gapdh, Omp, mCherry, Gnao, and family-C Vmn2r. In LongSAGE, “long” tags representing 17 bp cDNA segments are concatenated, and the concatemers cloned and sequenced. Examples of nucleotide sequences are shown in Fig. 3A, B. From VNO-derived cell v28, we obtained tags for Vmn2r37 or Vm2nr42 (family-ABD genes) and Vmn2r7 (family-C gene); the Omp gene was also represented in the collection of 5998 tags (Fig. 3C). By contrast, in type B cells m85 and m93, there were no tags for OR or VR genes among the 5195 and 5653 tags, respectively; the Omp gene was well represented in both cells (Fig. 3C). We were intrigued by the presence of tags for Gucy1b2 in both m85 and m93. This soluble guanylate cyclase has been poorly studied, and has not been characterized in vertebrate olfactory systems. We decided to evaluate this gene as a marker for type B cells in the MOE.
Fig. 3.
Long Serial Analysis of Gene Expression (LongSAGE) of single red-fluorescent cells from Trpc2-IRES-taumCherry mice. (A) Example of nucleotide sequence of a LongSAGE clone recovered from m93, a red-fluorescent cell isolated from WOM in the lateral region of the MOE of a homozygous Trpc2-IRES-taumCherry mouse at eight weeks. The sequence of the tag representing the gene Omp is highlighted in yellow, and the anchoring NlaIII recognition sites CATG are highlighted in red. (B) Example of nucleotide sequence of another LongSAGE clone recovered from m93. The tag representing the gene Gucy1b2 highlighted. The sequence of the tag representing the gene Gucy1b2 is highlighted in yellow, and the anchoring NlaIII recognition sites CATG are highlighted in red. (C) Summary of tags analyzed in cells m85 and m93 (type B cells) and cell v28 (VNO cell). For Gucy1b2, we identified 8/5195 tags (0.15%) in sample m85, and 9/5653 tags (0.16%) in sample m93, compared to 0/5998 tags in the v28 sample. Sample v28 contains tags for family-ABD Vmn2r and family-C Vmn2r genes: 44 tags (0.73%) for Vmn2r42 or Vmn2r37 (three independent tags were found, however, unfortunately the 17 bp tags are identical in these Vmn2r genes), and 5 tags (0.08%) for Vmn2r7 from family C.
2.4. Gucy1b2 RNA and Gucy1b2 protein expression in the MOE
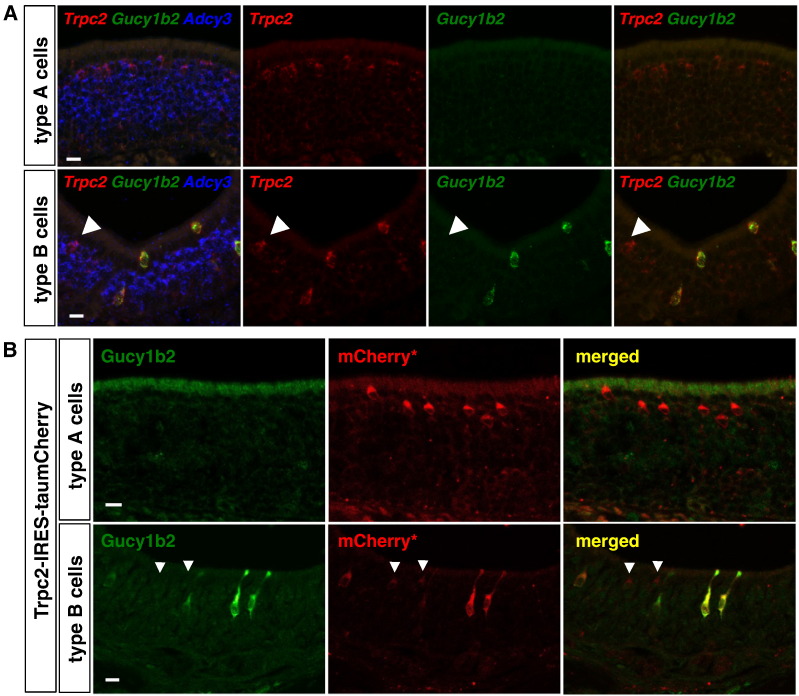
We performed three-color ISH in the MOE of wild-type C57BL/6 mice with riboprobes for Trpc2, Gucy1b2, and Adcy3 (Fig. 4A). Type A cells are Gucy1b2−, and most type B cells are Gucy1b2+, thus providing the first marker that can distinguish type B cells positively from type A cells at the single-cell level. An occasional Trpc2+ cell in the lateral region of the MOE, where type B cells are enriched, appears to be Gucy1b2−. These occasional cells could be type A cells (because this cell type is present throughout the MOE), immature type B cells, or represent another, unrecognized but minor Trpc2 + cell type in the MOE. By two-color ISH in three C57BL/6 mice at three weeks, we counted 16,115 ± 929 (SE) Gucy1b2+ cells in the MOE, of which 15,630 ± 1023 (97.0 ± 1.2%) are Trpc2+.
Fig. 4.
Gucy1b2 RNA and Gucy1b2 protein expression in type B but not type A Trpc2 + MOE cells. (A) ISH with riboprobes for Trpc2, Gucy1b2, and Adcy3 in coronal sections of the MOE of a wild-type C57BL/6 mouse at eight weeks. Type A cells, which are Adcy3+ and have their cell bodies typically just under the sustentacular layer, are Gucy1b2−. By contrast, type B cells, which are Adcy3− and have their cell bodies at all positions along the basal-to-apical dimension of the MOE, are Gucy1b2+. This section through the lateral region of the MOE, where type B cells are concentrated, also contains one Gucy1b2- Trpc2+ Adcy3+ cell (arrowhead); this could be a type A cell, for which there is no unique marker. (B) IHC with antibodies against Gucy1b2 and intrinsic red fluorescence of taumCherry (mCherry*), in coronal sections of the MOE of a wild-type C57BL/6 mouse at two weeks. Type A cells are not immunoreactive for the Gucy1b2 antibody. By contrast, type B cells are Gucy1b2 +. This section through the lateral region of the MOE contains two faintly mCherry* cells that are Gucy1b2 − (arrowheads) and could be type A cells. Scale bar, 10 μm.
Encouraged by the promise of Gucy1b2 as a type B cell marker, we generated a rabbit antiserum against a 22 amino acid sequence near the C-terminus of the Gucy1b2 protein. We performed IHC on coronal sections of the MOE of homozygous Trpc2-IRES-taumCherry mice (Fig. 4B). Consistent with the ISH data, Type A cells are Gucy1b2 −, and most type B cells are Gucy1b2 +.
Thus, our ISH and IHC studies validate Gucy1b2 as a marker for type B cells within the MOE.
2.5. Expression of other signaling molecules
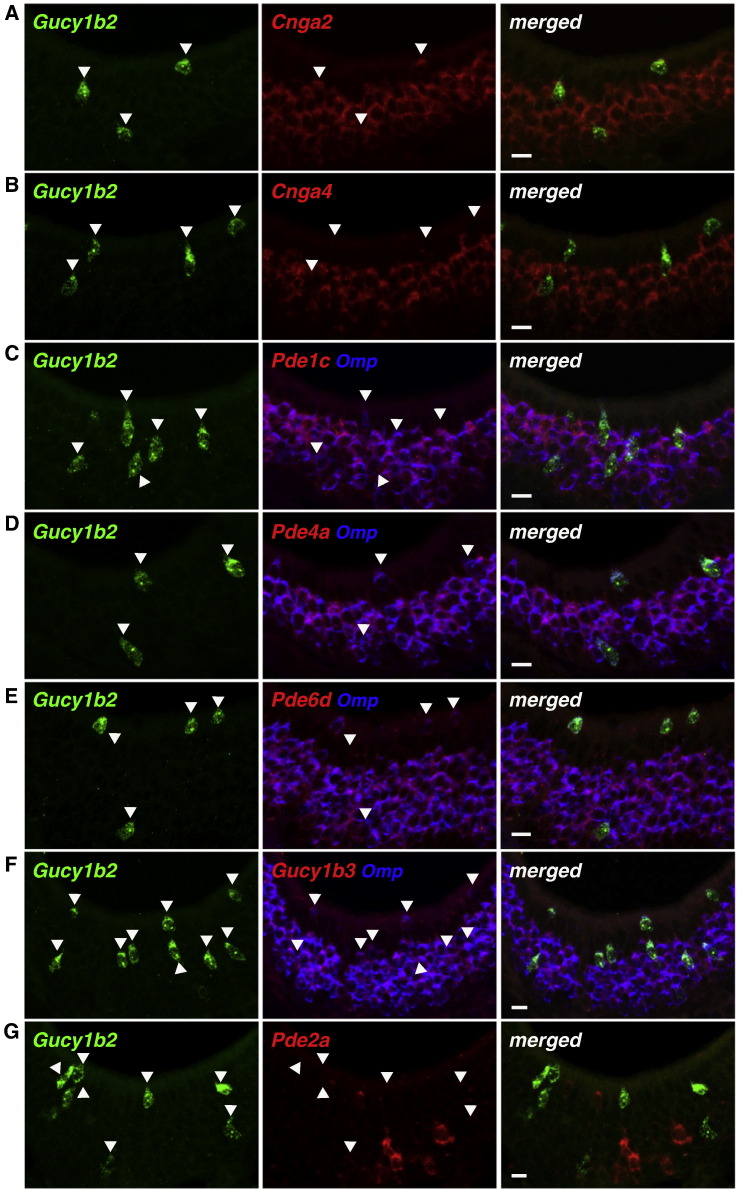
Type B cells do not express Adcy3 but express the cyclic nucleotide-gated channel subunit Cnga2 (Fig. 5A). There must thus be a source of cAMP or cGMP, and Gucy1b2 is an enzyme that catalyzes the production of cGMP. We carried out further ISH analyses for other signaling molecules. Cnga4, another cyclic nucleotide-gated channel subunit (Kelliher et al., 2003), is not expressed in type B cells but is expressed widely across canonical OSNs (Fig. 5B). Among the phosphodiesterases, enzymes that catalyze the degradation of cAMP and/or cGMP, we identify expression of Pde1c, Pde4a, and Pde6d in type B cells (Fig. 5C–E) but not of Pde2a (Fig. 5G), which is expressed in Gucy2d+ cells (Hu et al., 2007; Leinders-Zufall et al., 2007). Interestingly, another soluble guanylate cyclase, Gucy1b3, is widely expressed in the MOE including in type B cells (Fig. 5F).
Fig. 5.
Expression of other signaling molecules in Gucy1b2+ MOE cells. ISH in coronal sections of the MOE of a wild-type C57BL/6 mouse at 8 weeks. Gucy1b2+ cells are also Cnga2+ (A) but Cnga4− (B). These cells express the phosphodiesterases Pde1c (C) and Pde4a (D), as do canonical OSNs. The cGMP-specific phosphodiesterase Pde6d (E) and Gucy1b3 (F) are expressed in Gucy1b2+ cells, as well as in all other Omp+ OSNs. Gucy1b2+ cells are Pde2a− (G). Scale bar, 10 μm.
Thus, the gene expression profile of type B cells differs from canonical OSNs by the absence of expression of Adcy3 and Cnga4.
2.6. The Gucy1b2-IRES-tauGFP knockin mouse strain
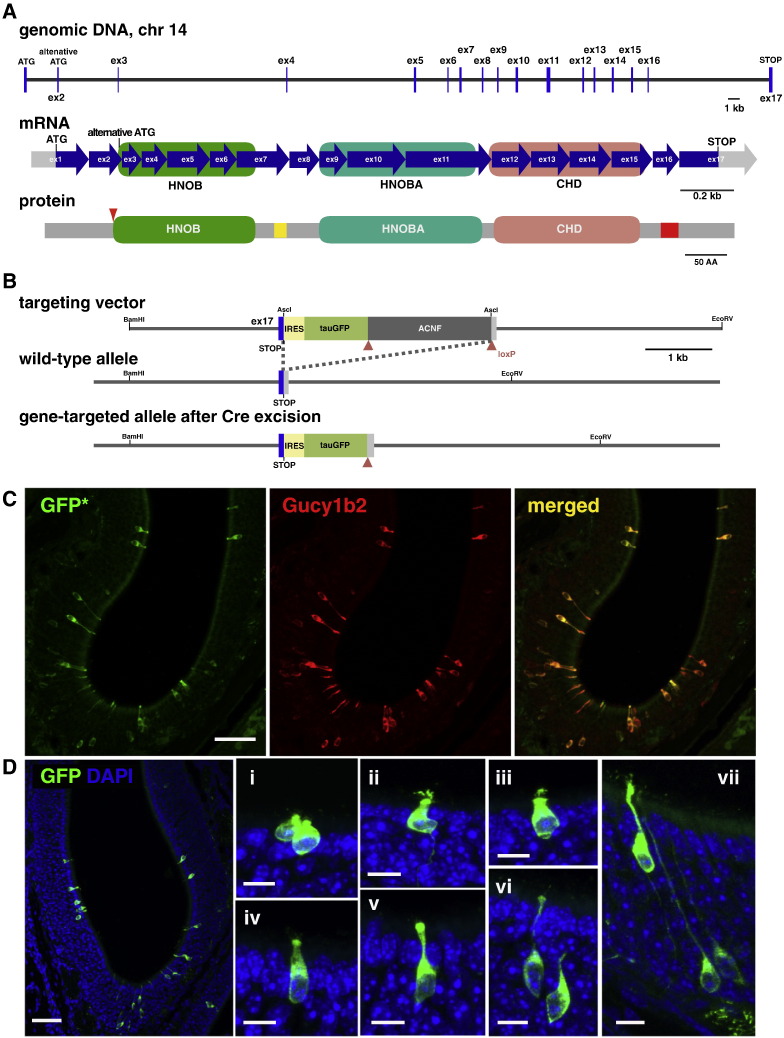
Next we generated an IRES-tauGFP knockin mutation at the Gucy1b2 locus in the mouse germ line by gene targeting in embryonic stem (ES) cells. The Gucy1b2 gene is located on chromosome 14. The RefSeq consists of 17 coding exons with two possible ATG translation initiation sites, encoding a putative polypeptide of 824 amino acids (Fig. 6A). The amino acid sequence of this and other soluble guanylate cyclases features heme NO binding domains HNOB and HNOBA, and a cyclase homology domain CHD. We inserted an IRES-tauGFP cassette immediately after the STOP codon of Gucy1b2 (Fig. 6B), according to the same design as the Trpc2-IRES-taumCherry mutation. This mouse strain is publicly available from The Jackson Laboratory.
Fig. 6.
Gucy1b2-IRES-tauGFP gene-targeted strain: main olfactory epithelium. (A) The mouse Gucy1b2 gene is located on chromosome 14 and consists of 17 coding exons. The various domains of the four known soluble guanylate cyclases are HNOB, HNOBA, and CHD. (B) The Gucy1b2-IRES-tauGFP knockin mutation was generated by gene targeting in ES cells. The IRES-tauGFP-ANCF cassette was inserted after the STOP codon of Gucy1b2 by homologous recombination in ES cells. The ACNF cassette, a self-excising neo gene, was removed during transmission of the targeted allele through the male germ line, leaving a single loxP site (red triangle) behind in the locus. The IRES sequence allows for the cotranslation of intact Gucy1b2 (regardless of which ATG is used for initiation of translation) with the axonal green-fluorescent marker tauGFP. (C) IHC of a coronal section of the MOE of a homozygous Gucy1b2-IRES-tauGFP mouse at 4 weeks shows concordance of the intrinsic fluorescence of GFP (GFP*) with the immunoreactive signal for Gucy1b2. (D) IHC of a coronal section of the MOE of a homozygous Gucy1b2-IRES-tauGFP mouse at three weeks with antibodies against GFP. The immunoreactive GFP + cells i through vii exhibit various morphologies and intra-epithelial locations. Nuclear staining is with DAPI. Scale bar, 50 μm in C; 50 μm in D left; 10 μm in Di–vii.
In coronal MOE sections of a homozygous Gucy1b2-IRES-tauGFP mouse, the intrinsic fluorescence of GFP shows concordance with Gucy1b2 immunoreactivity (Fig. 6C). To reveal the morphology of the GFP + cells more clearly, we performed IHC with antibodies against GFP (Fig. 6D). A variety of cellular morphologies can be discerned among GFP + cells. In some cells fine apical features resembling either short cilia or microvilli can be discerned, and a further characterization of these structures awaits super-resolution or electron microscopy. The Gucy1b2-IRES-tauGFP strain will be useful for molecular, cellular, physiological, and anatomical studies of type B cells.
2.7. Axonal coalescence of type B cell axons into glomeruli
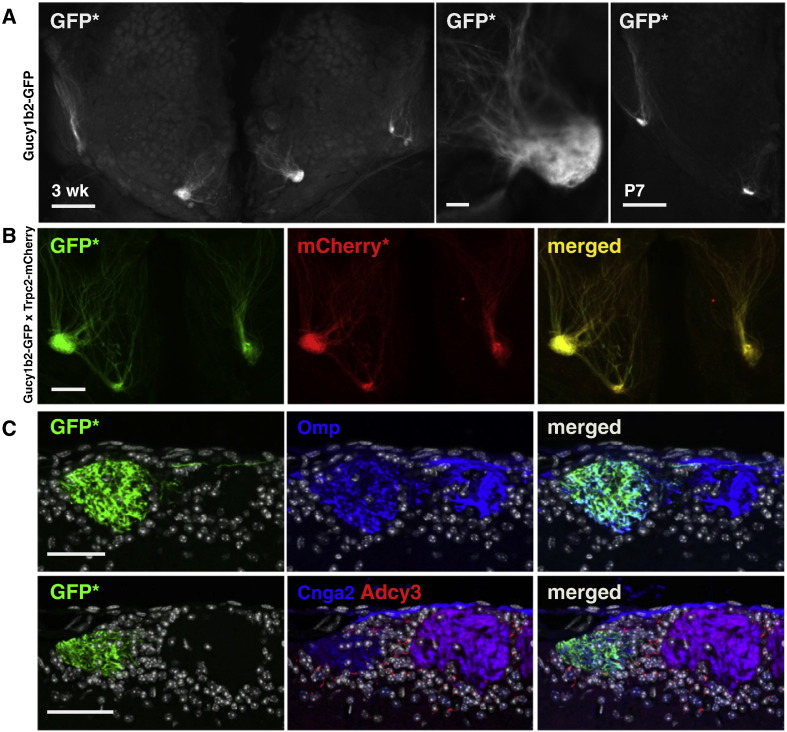
The expression level of the Gucy1b2 locus is sufficiently high for tauGFP translated from the IRES sequence to decorate axonal processes of type B cells all the way to the olfactory bulb. In whole mounts of the main olfactory bulb (n = 14) of homozygous Gucy1b2-IRES-tauGFP mice at three weeks, 3.14 ± 0.21 major green-fluorescent glomeruli are visible on the ventral-posterior aspect (Fig. 7A). At postnatal day 7, the Gucy1b2 glomeruli appear to be fully mature. In a mouse double homozygous for Gucy1b2-IRES-tauGFP and Trpc2-IRES-taumCherry, the fluorescent signals overlap (Fig. 7B), corroborating the concordance of Gucy1b2 and Trpc2 expression in type B cells seen at the level of the MOE. In coronal sections of the main olfactory bulb of a homozygous Gucy1b2-IRES-tauGFP mouse, GFP intrinsic fluorescence overlaps with Omp and Cnga2 immunoreactive signals, and Adcy3 immunoreactivity is absent in the GFP + glomeruli (Fig. 7C). Thus, despite the apparent absence of expression of an OR or VR gene, axons of Gucy1b2 + MOE neurons coalesce into glomeruli that strongly resemble canonical glomeruli, which form in an OR-instructed fashion (Mombaerts et al., 1996; Mombaerts, 2006).
Fig. 7.
Gucy1b2-IRES-tauGFP gene-targeted strain: main olfactory bulb. (A) (Left) Ventral view of a whole mount of a homozygous Gucy1b2-IRES-tauGFP mouse at three weeks, showing intrinsic fluorescence of GFP (GFP*). (Middle) High magnification of a ventromedial glomerulus in the right bulb of the same mouse. (Right) At postnatal day 7, GFP + axons have already coalesced into glomeruli. (B) Whole mount view of a main olfactory bulb of mouse double homozygous for Gucy1b2-IRES-GFP and Trpc2-IRES-taumCherry at four weeks. Fluorescent axons coalesce into a few glomeruli. The signals with green (GFP*) and red (mCherry*) intrinsic fluorescence overlap precisely. (C) IHC of coronal sections of the glomerular layer in the main olfactory bulb of a homozygous Gucy1b2-IRES-tauGFP at eight weeks. Intrinsic GFP fluorescence (GFP*) overlaps with immunoreactivity for Omp and Cnga2 but not for Adcy3, consistent with expression in type B cells. Scale bar, 500 μm in A left and right; 50 μm in A middle; 200 μm in B; 50 μm in C.
3. Discussion
3.1. Gucy1b2 as a marker for type B cells in the MOE
Having established that Trpc2 expression is actually not restricted to VSNs in the mouse (Omura and Mombaerts, 2014), we have here characterized type B cells molecularly and anatomically. This Trpc2 + cell type is confined to the lateral region of the mouse MOE, a region that is similar to that of the most highly expressed OR gene, MOR28/Olfr1507 (Omura and Mombaerts, 2014). Our previous ISH and IHC studies (Omura and Mombaerts, 2014) had not revealed expression of a known chemosensory G-protein coupled receptor gene or component of a signaling pathway other than Cnga2, which is required for odorant-evoked signal transduction in canonical OSNs. The availability of the Trpc2-IRES-taumCherry knockin strain enabled us here to examine live, unfixed cells. Extensive single-cell analyses with degenerate RT-PCR did not reveal expression of the known chemosensory G-protein coupled receptor gene repertoires (OR, Vmn1r, Vmn2r, Taar, Fpr) in putative type B cells. But given the size and complexity of these repertoires, some of these genes may not be detectable by the degenerate RT-PCR primers. A definitive assessment of chemosensory receptor gene expression in type B cells awaits deep RNAseq, ideally of many single cells to exclude that a subset of type B cells may express some of these genes. Our interest in Gucy1b2 was kindled by the unbiased, non-targeted, exploratory approach of LongSAGE, which is conceptually a precursor to RNAseq. ISH and IHC with a custom antibody confirm Gucy1b2 as a marker for type B cells within the MOE. The Gucy1b2-IRES-tauGFP knockin strain has been informative with regard to describing axonal coalescence into glomeruli in the bulb. The initial indication that the few ventral glomeruli in the main olfactory bulb of Trpc2-IRES-taulacZ mice correspond to type B cells (Omura and Mombaerts, 2014) has now been proven in Gucy1b2-IRES-tauGFP mice. Unambiguous tracing of the axonal projections of type A cells to the bulb requires a marker for type A cells that is not expressed in type B cells, canonical OSNs, and VSNs.
3.2. cGMP-mediated signaling
Cnga2 expression in type B cells in the absence of Adcy3 expression raises the question as to which cyclic nucleotide (cAMP or cGMP) is available in these cells to activate this cyclic-nucleotide gated cation channel and what enzyme produces it. The disruption of the glomerular pattern of type B cells in Cnga2 knockout mice indicates that Cnga2 is functionally relevant for these cells (Omura and Mombaerts, 2014). Gucy1b2 is a plausible candidate for the generation of cGMP, possibly as a heterodimer with type-a soluble guanylate cyclases. We find Gucy1b3 to be expressed in type B cells as well as, interestingly, in canonical OSNs. Further progress in the issue of signaling requires the identification of chemosensory stimuli for type B cells that do not act on canonical OSNs and on VSNs. It is tempting to speculate that gases such as nitrogen oxide and/or oxygen (Gray et al., 2004; Morton, 2004; Zimmer et al., 2009; Scott, 2011) stimulate type B cells via Gucy1b2. If that is the case, it remains to be determined if this pathway is the sole or the main chemosensory signaling pathway in these cells. It will be interesting and also challenging to dissect the functional interplay between three distinct signaling pathways in type B cells, involving Cnga2, Trpc2, and Gucy1b2. One or more of these signaling pathways could have a modulating or auxiliary role in chemosensory signal transduction, or may be involved in cellular differentiation or axon guidance rather than be essential for transduction. It is prudent to keep an open mind when designing and interpreting experiments to understand the unprecedented coexistence of these three pathways in the same cells. If Trpc2 affects type B cell function one way or the other, the traditional interpretation of the behavioral phenotypes of Trpc2 knockout mice (Leypold et al., 2002; Stowers et al., 2002; Kimchi et al., 2007) will become problematic.
Thus, for now, Gucy1b2 is merely a marker for type B cells. Functional characterization of Gucy1b2 requires appropriate and specific chemosensory stimuli and Gucy1b2 knockout mice.
4. Conclusion
The unexpected discovery that the mouse MOE abounds with Trpc2 + cells (Omura and Mombaerts, 2014) has raised a series of interesting questions. We have described two types of Trpc2 + MOE cells: type A (Adcy3 +) and type B (Gucy1b2 +). The Gucy1b-IRES-tauGFP mice will be useful for physiological studies of single cells, and for molecular, cellular, and anatomical studies of this newly discovered chemosensory subsystem. The soluble guanylate cyclase Gucy1b2 may be more than a marker for type B cells, and could be relevant for their chemosensory function. In any case, type B cells are not VSNs that are misplaced in the MOE, and their gene expression profile suggests that they are physiologically distinct from VSNs, sharing only Trpc2 expression. Our results serve as further caution against the traditional interpretation of the behavioral phenotypes of Trpc2 knockout mice, as if only VSNs would be impaired in these mice, and no other neurons or no other cells in the mouse's body. Specifically, there is no reason to expect that Trpc2 + type B cells respond to pheromones.
5. Experimental methods
5.1. Generation of mouse strains with gene-targeted knockin mutations
To generate the targeting vector for the Trpc2-IRES-taumCherry mutation, a 4.8 kb HindIII–PvuII fragment containing exons 9–13 of Trpc2 was used for the left homology arm, and a 4.8 kb PvuII–HindIII fragment containing a part of exon 13 for the right homology arm. A PacI–AscI site was generated at the PvuII site located 32 bp after the STOP codon of Trpc2. The cassette IRES-taumCherry-loxP-ACNF was inserted in the newly generated AscI site. The vector was linearized and electroporated into E14 ES cells. Targeted ES cell clones were injected into C57BL/6 blastocysts, and chimeras were bred with wild-type C57BL/6 mice. A strain was established from ES clone A75. To generate the targeting vector for the Gucy1b2-IRES-tauGFP mutation, a 4.5 kb BamHI fragment containing a part of exon 17 and intron between exons 16 and 17 and a 6.7 kb BamHI–EcoRV fragment containing a part of exon 17 and 3′ of Gucy1b2 gene were isolated from bacterial artificial chromosome clone bMQ-312D1 (Source Bioscience). An AscI site was generated immediately downstream of the STOP codon of Gucy1b2. The cassette IRES-tauGFP-loxP-ACNF was inserted in the newly generated AscI site. The vector was linearized and electroporated into E14 cells. Targeted ES clones were injected into C57BL/6 blastocysts, and chimeras were bred with wild-type C57BL/6 mice. A strain was established from ES clone C20. Mice were maintained in specified pathogen-free conditions in individually ventilated cages of the Tecniplast green line. Mouse experiments were carried out in accordance with NIH guidelines and the German Animal Welfare Act, European Communities Council Directive 2010/63/EU, and the institutional ethical and animal welfare guidelines of the Max Planck Institute of Biophysics and the Max Planck Research Unit for Neurogenetics. Approval came from the IACUC of The Rockefeller University and the Veterinäramt of the City of Frankfurt. The following two strains are publicly available from The Jackson Laboratory in a mixed 129 × C57BL/6 background: Trpc2-IRES-taumCherry as official strain name B6;129P2-Trpc2 < tm2Mom >/MomJ and JR#6733, and Gucy1b2-IRES-tauGFP as official strain name B6;129P2-Gucy1b2 < tm3Mom >/MomJ and JR#21063.
5.2. Single-cell RT-PCR
We dissected out WOM (Khan et al., 2013) from the lateral region of the MOE and VNE of Trpc2-IRES-taumCherry homozygous mice at 8 weeks and heterozygous OMP-tauYFP mice at 3 or 8 weeks. Tissue samples were dissociated with dispase (Invitrogen), collagenase (Invitrogen) and DNase I (Roche). We picked mCherry + cells or YFP + cells picked under an inverted fluorescence microscope (TE200-1, Nikon) using micromanipulators (N88NEN2, Nikon Narashige) (Fuss et al., 2013). The isolated cells were washed individually at least three times in separate drops of buffer under mineral oil. The cells were transferred to 0.4 μl of nuclease-free water in each PCR tubes. The samples were immediately snap frozen. Reverse transcription and amplification of cDNA were conducted as described previously (Saito et al., 2004) with modifications.
For single cell RT-PCR, reverse transcription (RT) was performed with anchor-T primer (TATAGAATTCGCGGCCGCTCGCGA[T]24) and Superscript III (Invitrogen) for 90 min at 37 °C and 15 min at 50 °C. Reactions were stopped by heating at 70 °C for 10 min. Tailing reaction was followed with TdT (Roche), dATP (Roche) and RnaseH (Roche) for 20 min at 37 °C, then for 10 min at 65 °C. To validate taumCherry expression in the single cells, RT-tailed products were first amplified with mCherry-F1: GGATAACATGGCCATCATCAAGG and Trpc2 3′UTR-R: CATCTGACTTCCACAGCAGA. Then a second round of PCR was performed with mCherry-F2: CTGTTCCACGATGGTGTAGTCC, and mCherry-R1: CGTAATGCAGAAGAAGACCATGG. For analysis of expression of other genes, RT-tailed products were first amplified by PCR reaction with EX-Taq HS DNA polymerase (Takara) and anchor-T primer by incubating at 95 °C for 2 min, 37 °C for 5 min, 72 °C for 20 min, then 30 cycles of 95 °C for 30 s, 67 °C for 1 min, 72 °C for 6 min plus 6 s extension for each cycle, then 72 °C for 10 min. The second round of PCR was with the following primers: for Omp, Omp-F: GCACAGTTAGCAGGTTCAGCT, Omp-R: GGTTTGCAGTCCTGGCAGC, for Gapdh, Gapdh-F: TTAGCCCCCCTGGCCAAGG, Gapdh-R: CTTACTCCTTGGAGGCCATG, for Cnga2, Cnga2-F: GGAGATCCTGATGAAGGAAGG, Cnga2-R: AACAGCTGGCTCAGGGGTGT, for Adcy3, Adcy3-F: GCATGAACAAAGGAGGGGTTC, Adcy3-R: TCAGGGGTTGTCCACCACTT, for Gnai2, Gnai2-F: GAGCATGAAGCTGTTTGACAGC, Gnai2-R: CTCCTTGGTGTCTTTGCGC, for Gnao1, Gnao-F: CTCCACGAGGACGAAACCAC, Gnao-R: GCCCCGGAGATTGTTGGCA, for Gnal (Roppolo et al., 2006). For OR genes, we used generate RT-PCR primer sets D1–D2 and D4–D6 (Oka et al., 2006), and P26–P27 (Malnic et al., 1999). For Taar genes, we used Taar-F: TIGAGRGMTGCTGGTAYTTYGG and Taar-R: RGTYTTKGCWGCYTTYCTITC. For Vmn1r genes, we used the 11 primer sets (V1ra-V1rk) as previously described (Roppolo et al., 2006). For family-C Vmn2r genes, we used for set A, Family-C-F1: AAGCCATGCAACTGGTCCTG and Family-C-R1: GCATTCAAAGATGATCTTTACA, and for set B, Family-C-F1 and Family-C-R2: GACAAAAGAGATCCAGACAATG. For Fpr genes, we used for set A, Fpr-F1: TTCTTTGTCTGTTGGTTCCC and Fpr-R1: ACATAGAGTATTGGGTTGAG, and for set B, Fpr-F2: TCTTGACTACAGTGAGAGATG and Fpr-R2: GAGGACACGTAAAGGACG. Primers for Fpr-rs1 were: Fpr-rs1-F, ATCCTGGGGCAACTCTGTTGAG and Fpr-rs1-R: CACAGCCCCCTCCTCATATT.
5.3. LongSAGE
All steps until the first amplification of cDNA were done as described for single-cell RT-PCR except for the RT reactions: these were done with biotin anchor-T primer (biotin-TATAGAATTCGCGGCCGCTCGCGA[T]24) and Superscript III (Invitrogen) for 10 min at 37 °C and 10 min at 50 °C then for 10 min at 65 °C. A biotin-anchor-T primer was used also first amplification step. Amplified PCR products were screened for expression of Gapdh, Omp, mCherry, Adcy3, Cnga2 and Gnao with gene-specific primers. Samples m85 and m93 from cells (Gapdh, Omp, mCherry, Cnga2 and Gnao positive, and Adcy3 negative) and v28 (Gapdh, Omp, mCherry, and Gnao positive) from the VNO were analyzed using LongSAGE (Saha et al., 2002) according to the protocol published at http://www.sagenet.org/protocol/index.htm. Briefly, amplified single-cell PCR products were digested with anchoring enzyme NlaIII (New England BioLabs). Cleaved samples were divided into two fractions and were bound to streptavidin magnetic beads (Dynal), and linkers were ligated. The ligated DNA was digested with tagging enzyme MmeI (NEB). The cleaved tags were ligated to form ditags and amplified by PCR. The PCR product was cleaved with anchoring enzyme NlaIII and the ditags were ligated to form concatemers. The concatemers were cloned into pZero-1 vector (Invitrogen). Clones were picked and sequenced. Tag sequences were analyzed using SAGE2002 software (Johns Hopkins University) and NCBI Blast searches.
5.4. In situ hybridization
ISH was performed as described (Ishii et al., 2004). The following riboprobes have been described previously: Omp (Ishii et al., 2004), Trpc2, Adcy3, and Cnga2 (Omura and Mombaerts, 2014). We used a mix of two riboprobes for Gucy1b2: riboprobe 1, nt 951–2040, and riboprobe 2, nt 1747–2630 from NM_172810.3. We designed riboprobes for Cnga4, nt 125–1263 from NM_001033317.3; for Pde1c, nt 1253–2398 from NM_001025568; for Pde2a, nt 2361–3358 from NM_001008548; for Pde4a, nt 2077–2838 from NM_183408; for Pde6d, nt 153–908 from NM_008801.2; and for Gucy1b3, nt 1901–2835 from NM_017469. Images were collected with a Zeiss LSM 710 confocal microscope.
5.5. Generation of antibodies against mouse Gucy1b2
As antigen for raising antibodies against mouse Gucy1b2 protein, we used the 22 amino-acid peptide RPSALADGKEASTPRNQVKKPR (654–675 in the Gucy1b2 sequence), which is C-terminal to the predicted catalytic domain CHD. Rabbit antiserum (ImmunoGlobe, Himmelstadt, Germany) was raised against KLH-conjugated polypeptide (Schafer-N Copenhagen, Denmark). Antibodies were affinity-purified with using affinity columns conjugated with the synthetic peptide.
5.6. Immunohistochemistry
Mice were anesthetized by injection of ketamine HCl and xylazine (210 mg/kg and 10 mg/kg body weight, respectively) and perfused with ice-cold PBS, followed by 4% PFA in PBS. The mouse heads were dissected, post-fixed in 4% PFA, and decalcified in 0.45 M EDTA in 1 × PBS overnight at 4 °C. Samples were cryoprotected in 15% and 30% sucrose in 1 × PBS at 4 °C, frozen in O.C.T. compound (Tissue-Tek), and sectioned at 12 μm with a Leica CM3500 cryostat. Sections were washed with 1 × PBS. Washed sections were blocked with 10% normal goat serum or normal donkey serum, 0.1% Triton X-100 in 1 × PBS for 1 h at room temperature. After the blocking step, sections were incubated in 3% BSA, 0.1% Triton X-100 in 1 × PBS for overnight at 4 °C with the following primary antibodies: rabbit anti-Gucy1b2 (1:500), rabbit anti-Trpc2 (1:500, Liman et al., 1999), goat anti-Omp (1:1000, Wako Chemicals), rabbit anti-Adcy3 (1:1000, Santa Cruz Biotechnology), goat anti-Cnga2 (1:100, Santa Cruz Biotechnology), and chicken anti-GFP (1:3000, Aves Labs). After incubation with primary antibodies, sections were incubated at 1.5 h at room temperature with secondary antibodies: goat anti-rabbit IgG-Alexa647 (Molecular Probes) for rabbit anti-Trpc2 in Fig. 1B, goat anti-rabbit IgG-Alexa488 (Molecular Probes) for rabbit anti-Gucy1b2 in Figs. 4B, goat anti-rabbit IgG-Alexa546 (Molecular Probes) for rabbit anti-Gucy1b2 in Fig. 6C, goat anti-chicken IgG Alexa488 (Molecular Probes) in Fig. 6D, donkey anti-rabbit IgG Alexa555 (Molecular Probes) for rabbit anti-Adcy3 in Fig. 7C, donkey anti-goat IgG Cy5 (Jackson ImmunoResearch) for goat anti-Omp, and goat anti-Cnga2 in Fig. 7C. For sections of the olfactory bulb, nuclear staining was with DAPI (1:10,000, Molecular Probes) after the washing steps. Sections were analyzed with a Zeiss LSM 710 confocal microscope.
Acknowledgments
We thank Wei Tang and Zhaodai Bai for injecting ES cells into blastocysts; Yan Zhu and Manxi Jiang for picking single red-fluorescent cells from Trpc2-IRES-taumCherry mice; Hiro Matsunami for sharing experimental protocols for single-cell RT-PCR and LongSAGE; Tobias Burbach and Bartos Machert for technical assistance; and Bolek Zapiec and Anna Holl for help with SAGE tag analysis. We are grateful to Emily Liman for a gift of a copious quantity of Trpc2 antibodies. We acknowledge Johns Hopkins University for SAGE2002 software and a detailed experimental protocol. P.M. received grant support from European Research Council Advanced Grant ORGENECHOICE, and generous support from the Max Planck Society.
References
- Fuss S.H., Zhu Y., Mombaerts P. Odorant receptor gene choice and axonal wiring in mice with deletion mutations in the odorant receptor gene SR1. Mol. Cell. Neurosci. 2013;56:212–224. doi: 10.1016/j.mcn.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Gray J.M., Karow D.S., Lu H., Chang A.J., Chang J.S., Ellis R.E., Marletta M.A., Bargmann C.I. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Hu J., Zhong C., Ding C., Chi Q., Walz A., Mombaerts P., Matsunami H., Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Ishii T., Mombaerts P. Expression of nonclassical class I major histocompatibility genes defines a tripartite organization of the mouse vomeronasal system. J. Neurosci. 2008;28:2332–2341. doi: 10.1523/JNEUROSCI.4807-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Mombaerts P. Coordinated coexpression of two vomeronasal receptor V2R genes per neuron in the mouse. Mol. Cell. Neurosci. 2011;46:397–408. doi: 10.1016/j.mcn.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Ishii T., Omura M., Mombaerts P. Protocols for two- and three-color fluorescent RNA in situ hybridization of the main and accessory olfactory epithelia in mouse. J. Neurocytol. 2004;33:657–669. doi: 10.1007/s11068-005-3334-y. [DOI] [PubMed] [Google Scholar]
- Kelliher K.R., Ziesmann J., Munger S.D., Reed R.R., Zufall F. Importance of the CNGA4 channel gene for odor discrimination and adaption in behaving mice. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4299–4304. doi: 10.1073/pnas.0736071100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Vaes E., Mombaerts P. Temporal patterns of odorant receptor gene expression in adult and aged mice. Mol. Cell. Neurosci. 2013;57:120–129. doi: 10.1016/j.mcn.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Kimchi T., Xu J., Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T., Cockerham R.E., Michalakis S., Biel M., Garbers D.L., Reed R.R., Zufall F., Munger S.D. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold B.G., Yu C.R., Leinders-Zufall T., Kim M.M., Zufall F., Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles S.D., Buck L.B. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Liberles S.D., Horowitz L.F., Kuang D., Contos J.J., Wilson K.L., Siltberg-Liberles J., Liberles D.A., Buck L.B. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9842–9847. doi: 10.1073/pnas.0904464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman E.R., Corey D.P., Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B., Hirono J., Sato T., Buck L.B. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the olfactory system. Annu. Rev. Cell Dev. Biol. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Wang F., Dulac C., Chao S.K., Nemes A., Mendelsohn M., Edmondson J., Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Morton D.B. Atypical soluble guanylyl cyclases in Drosophila can function as molecular oxygen sensors. J. Biol. Chem. 2004;279:50651–50653. doi: 10.1074/jbc.C400461200. [DOI] [PubMed] [Google Scholar]
- Oka Y., Katada S., Omura M., Suwa M., Yoshihara Y., Touhara K. Odorant receptor map in the mouse olfactory bulb: in vivo sensitivity and specificity of receptor-defined glomeruli. Neuron. 2006;52:857–869. doi: 10.1016/j.neuron.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Omura M., Mombaerts P. Trpc2-expressing sensory neurons in the main olfactory epithelium of the mouse. Cell Rep. 2014;8:583–595. doi: 10.1016/j.celrep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Rivière S., Challet L., Fluegge D., Spehr M., Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- Roppolo D., Ribaud V., Jungo V.P., Lüscher C., Rodriguez I. Projection of the Grüneberg ganglion to the mouse olfactory bulb. Eur. J. Neurosci. 2006;23:2887–2894. doi: 10.1111/j.1460-9568.2006.04818.x. [DOI] [PubMed] [Google Scholar]
- Saha S., Sparks A.B., Rago C., Akmaev V., Wang C.J., Vogelstein B., Kinzler K.W., Velculescu V. Using the transcriptome to annotate the genome. Nat. Biotechnol. 2002;19:508–512. doi: 10.1038/nbt0502-508. [DOI] [PubMed] [Google Scholar]
- Saito H., Kubota H., Roberts R.W., Chi Q., Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–691. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Scott K. Out of thin air: sensory detection of oxygen and carbon dioxide. Neuron. 2011;69:194–202. doi: 10.1016/j.neuron.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N.C., Campbell R.E., Steinbach P.A., Giepmans B.N., Palmer A.E., Tsien R.Y. Improved monomeric red, orange, and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Silvotti L., Cavalca E., Gatti R., Percudani R., Tirindelli R. A recent class of chemosensory neurons developed in mouse and rat. PLoS ONE. 2011;6(9):e24462. doi: 10.1371/journal.pone.0024462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L., Holy T.E., Meister M., Dulac C., Koentges G. Loss of sex discrimination and male–male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Velculescu V.E., Zhang L., Vogelstein B., Kinzler K.W. Serial analysis of gene expression. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- Zimmer M., Gray J.M., Pokala N., Chang A.J., Karow D.S., Marletta M.A., Hudson M.L., Morton D.B., Chronis N., Bargmann C.I. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]