Abstract
TRPV4 (Transient Receptor Potentials, vanilloid family, type 4) is widely expressed in vertebrate tissues and is activated by several stimuli, including by mechanical forces. Certain TRPV4 mutations cause complex hereditary bone or neuronal pathologies in human. Wild-type or mutant TRPV4 transgenes are commonly expressed in cultured mammalian cells and examined by Fura-2 fluorometry and by electrodes. In terms of the mechanism of mechanosensitivity and the molecular bases of the diseases, the current literature is confusing and controversial. To complement existing methods, we describe two additional methods to examine the molecular properties of TRPV4. (1) Rat TRPV4 and an aequorin transgene are transformed into budding yeast. A hypo-osmtic shock of the transformant population yields a luminometric signal due to the combination of aequorin with Ca2+, released through the TRPV4 channel. Here TRPV4 is isolated from its usual mammalian partner proteins and reveals its own mechanosensitivity. (2) cRNA of TRPV4 is injected into Xenopus oocytes. After a suitable period of incubation, the macroscopic TRPV4 current is examined with a two-electrode voltage clamp. The current rise upon removal of inert osmoticum from the oocyte bath is indicative of mechanosensitivity. The microAmpere (10-6 to 10-4 A) currents from oocytes are much larger than the subnano- to nanoAmpere (10-10 to 10-9 A) currents from cultured cells, yielding clearer quantifications and more confident assessments. Microscopic currents reflecting the activities of individual channel proteins can also be directly registered under a patch clamp, in on-cell or excised mode. The same oocyte provides multiple patch samples, allowing better data replication. Suctions applied to the patches can activate TRPV4 to directly assess mechanosensitivity. These methods should also be useful in the study of other types of TRP channels.
Keywords: Basic Protocol, Issue 82, Eukaryota, Archaea, Bacteria, Life Sciences (General), Mechanosensation, Ion channels, Lipids, patch clamp, Xenopus Oocytes, yeast, luminometry, force sensing, voltage clamp, TRPV4, electrophysiology
Introduction
TRPs (transient receptor potentials) comprise seven subfamilies of cation channels that serve sensory functions1,2. In mammals, TRPV, the vanilloid subfamily of TRPs, has six varieties. TRPV4 (type 4)3,4 responds to heat, certain chemicals, osmotic swelling, or shear stress. The TRPV4 gene was repeatedly isolated by candidate-gene and/or expression cloning5-8. The latter method followed the gene product's response to hypo-osmolarity. TRPV4 is expressed in nearly all organs and functions in the development, physiology, or pathology of many disparate cell types3,4.
Striking are the >50 human autosomal dominant TRPV4 mutations, causing peripheral neuropathies and/or skeletal dysplasias (abnormalities in skeletal development)9-11. The skeletal dysplasias range from mild brachyomia type 3 (type-3 dwarfism), spondylometaphyseal dysplasia Kozlowski type, to severe dysplasias, some causing neonatal or embryonic death. Though all manners of mechanisms seem possible, none explains the diversity, complexity, variability, and occasional overlaps of these diseases4.
Like other TRP channels1, TRPV4 is a tetramer. In rat or human TRPV4, each subunit is consisted of 871 residues. Its central element is the six transmembrane a helices (S1-S6), which are likely arranged in a manner similar to voltage-gated K+ channels. There, S1 to S4 form a peripheral domain and the S5 and S6 form the permeation pore domain. Between S5 and S6 of TRPV4 is a short pore helix followed by the sequence LDLFKLTIGMGDL, four of which presumably converge to form the cation filter. The 470-residue N-terminal cytoplasmic segment contains 6 ankyrin repeats, known to bind proteins or small ligands. The C-terminal 152-residue cytoplasmic segment includes a calmodulin-binding sequence among other possible sites that bind other elements3.
TRPV4 is a cation channel that essentially excludes anions1. While its physiological function is to transduce stimuli to Ca2+ influx, it is also permeable to other cations with an Eisenman IV permeability sequence, favoring divalent at a PCa : PNa ~7 12. Single-channel conductance rectifies at ~90 pS outward and ~40 pS inward6,13,14. Heterologously expressed current (below) can be activated by hypo-osmotic swelling, shear stress, or warmth15. It is also activated by polyunsaturated fatty acids16,17 and the synthetic phorbal ester 4αPDD 18. At present, the most potent agonist is GSK1016790A 19 and antagonist is GSK2193874, effective in 10-9 to 10-8 M 20, both discovered by high-throughput, small-molecular screen.
Two key areas of TRPV4 research remain confusing: (1) Even as TRPV4 is largely studied for its mechanosensitivity, its molecular basis is controversial. One model describes hypo-osmolarity somehow activates phospholipase A2 (PLA2) to produce the polyunsaturated fatty acid (PUFA) arachidonic acid (AA), which is converted to epoxyeicosatrienoic acid (EET) by an epoxygenase, and the binding of EET activates TRPV4 16,17. Yet, TRPV4 itself has been shown to directly respond to membrane stretch14 (below), providing a simpler explanation. (2) The TRPV4 mutant pathologies are bewildering. At the foundation, one needs to know whether the diseases are due to the loss, the reduction, or the increase of channel activities. Even here, the literature is far from clear. While multiple skeletal-dysplasia alleles were reported to have higher activities, (gain-of-function, GOF)4,21, several were reported to have reduced activities (loss-of-function, LOF)10,22. A systematic study of 14 alleles found them to all be GOF mutations (below)23. The claim that some are LOFs seems to contradict the phenotype of trpV4-/- knockout mouse, which are viable or fertile, with only minor defects, despite a complete loss of TRPV4 function.
A part of these controversies has methodological origin. Laboratories use different methods, or variants of one method, and employ different judging standards. Most commonly, TRPV4 is transiently expressed in cultured mammalian cells (HEK, CHO, HeLa) and the rise of internal [Ca2+] upon agonist or hypotonic stimulation is registered with the Ca2+-sensitive fluorescent dye, Fura-2. The over-reliance on this fluorometric assay has been criticized1. The expression level in different populations, the distribution therein, as well as the effective dye concentration, need to be controlled and documented. More reliable is the direct electrophysiological examinations. Even this, as commonly practiced, is also not without problems. Because the expression levels in individual cultured cells are difficult to control, whole-cell currents have large variations. Further, because the currents are small, reliable statistics will have to rely on large sample sizes, often not practical. Patch-clamp examinations have rarely been performed. Some such recordings show clusters of activity bursts that make statistical evaluation challenging16,17.
To better understand the molecular mechanosensitivity, we have developed two additional systems to examine TRPV4. (1) To isolate TRPV4 away from its usual mammalian partners, we have expressed rat TRPV4 in budding yeast24. Functional expression of TRPV4 in this evolutionarily distant context showed that it could still respond to osmotic force without its usual partners. Because yeast makes no PUFAs such as AA or EET, and its genome has no PLA2 or epoxygenase genes, this expression also shows that they are not required for TRPV4 to sense force. Having TRPV4 in the molecular biologically most amenable eukaryotes also allows efficient forward- or reverse-genetic manipulations25. (2) For in-depth biophysical analyses of TRPV4, we expressed TRPV4 in Xenopus oocytes. Unlike cultured cells, which yield sub-nA to nA (10-10 to 10-9 A) currents, an oocyte expresses currents in µA's (10-6 to 10-4 A). Much larger signal over noise allows better quantification and more confident comparison. TRPV4, so expressed, can also be examined as individual molecules using a patch clamp. A single oocyte allows repeated patch sampling, again making quantification more reliable. Such studies showed that TRPV4 channel itself can directly be activated by membrane stretch force14. Analyses also showed that 14 representative skeletal-dysplasia alleles are all gain-of-function mutations. Further, the degree of this constitutive Ca2+ leakage parallels the severity of the skeletal diseases23.
Because of their novelty and usefulness, the detailed procedures of these two methods are assembled here to allow replications in future research on TRPV4 or similar channels.
Protocol
1. Yeast Luminometry Methods
Use strain BYYT of Saccharomyces cerevisiae. It is a yvc1- tok1- derivative of BY4741 (MATa, ura3D0, his3D1, leu2D0, lys2D0, yvc1::HIS3, tok1::kanMX4). Transform cells with the leu-selectable aequorin-expressing plasmid pEVP1/Aeq and the ura-selectable rat-TRPV4-expressing plasmid p416GPDV4, as described in Batiza et al.26 and Loukin et al.24 Use standard methods of plasmid construction and transformation27. Yeast strain and plasmids are available upon request.
Culture 2 ml of yeast cells overnight to post-logarithmic phase in a 30 °C shaker in leu- ura- "DCD" dropout medium28 (a sulfate-depleted variant of the CMD-leu-ura medium29), supplemented with 1M sorbitol. Inoculate 200 µl of these cultures into 1.8 ml of fresh medium supplemented with 2 µM of the luciferin coelenterazine. Grow in the dark at room temperature for another 24 hr without shaking.
Measure the osmolarity of the culture (typically at 1,400 mOsM) and other solutions (below) using a vapor pressure osmometer.
Aliquot 20 µl of fresh culture into a 12 mm luminometer tube for a single tube luminometer.
For the hypotonic shock, add 200 µl of a solution, containing 25 mM NaEGTA (pH 7.2) or NaMES (pH 7.2) and 500-100 mM NaCl, (total osmolarity of 1,200-400 mOsM) depending on the degree of shock desired. Continuously monitor the luminescence before and at least 120 sec after the osmotic downshock, interrupted only by the brief dilution operation. Register luminometer output on a desktop computer as relative luminescence units (RUL).
Though not for the study of transgenic TRPV4, we have used a variant of the above protocol in an automated system to examine the responses of a large number of yeast strains. Study of the responses to hypo-30 or hyperosmotic31 stimuli of the yeast deletome (the collection of 4,906 yeast single-gene deletants) using a microplate luminometer and analyzed with corresponding software has been successful.
2. and 3. Oocyte Electrophysiology Methods
Electrophysiology uses basic methods32. PCR amplify the open-reading frame of wild-type or mutant TRPV4 using high-fidelity PfuUtra polymerase and integrated into pGH19 33. This entails a precise insertion of the ORF between the 5' and 3' UTRs of the Xenopus β-globin gene and downstream of a T7 RNA-polymerase promoter. Linearize the construct downstream of the 3' UTR and used as templates in an in vitro T7 RNA polymerase reaction. Use standard molecular-biological techniques34.
Use stage V-VI oocytes from X. laevis. Animal husbandry, partial ovariectomy, defolliculation, and vitelline envelope removal follow standard procedures32,33,35. Inject 2-40 ng of cRNA solution per oocyte using a Drummond Nanoinject II automatic microinjector. Inject ~30 oocytes for each set of experiments. After TRPV4-cRNA injection, incubate the oocytes in the ND96 medium with gentamicin (100 µg/ml) and 1 µM ruthenium red14.
2. Two Electrode Voltage Clamp
Pull borosilicate glass recording pipettes from precision disposable 100 µl micropipettes individually with a pipette puller.
Pull both voltage-measuring and current-injecting pipette electrodes are pulled to have a tip aperture of ~1 µm diameter.
Backfill electrodes with 2 M KCl resulting in 0.1-0.2 mΩ resistance. Mount them on HS2A headstages, which together with a VG-2Ax100 virtual-grounded bath clamp, are connected to a GeneClamp 500 amplifier interface through a Digidata 1440 digitizer. Acquire data using pClamp10 software.
Place the oocyte to be tested in a 1 ml bath in a fabricated plastic chamber mounted on the stage of a dissecting microscope with 20X magnification. The bath solution is virtual-grounded by a 3 M KCl agar bridge and a chlorinated silver wire placed close to the oocyte and connected to a VG-2A virtual ground stage.
Construct the chamber for continuous perfusion with plastic tubings as solution inlet and outlet. Connect the inlet tubing is connected to a fabricated perfusion system, which is a manifold of six 50 ml syringe shells, each filled with a different solution. Gravity feed rates of any particular solution is controlled to ~2-3 ml/min using "Keck" ramp clamps on the tubings.
Mount both electrodes with their headstages on micromanipulators. Perform electrode penetrations of the oocyte by micromanipulation.
3. Patch Clamp Examination of Direct Mechanosensitivity
The basic methods of patch clamp including pipette preparation, GΩ-seal formation, and formation of on-cell or excised modes are those in Hamill et al.36 and Conn32.
Fill borosilicate glass pipettes with an ~1 µm diameter opening at the tip (bubble number 5-6) with 98 mM KCl, 1 mM MgCl2, and 10 mM K+-HEPES, pH 7.2.
Attach the patch clamp pipette through a plastic tubing to a 5 ml syringe and through a T-joint, also to a manometer. Use a computer to handle both the electrode and the manometer outputs. Acquire data at 10 kHz, and then play back through an eight-pole Bessel filter at 1 kHz for analysis.
Different patches can be excised from the same oocyte repeatedly. This practice makes the examination of TRPV4's molecular activity more efficient and reliable.
Representative Results
In a typical luminometry experiment, a range of 400-1,200 mOsM hypotonic down shocks is achieved by adding 200 μl of shock solutions of different low osmolarity to 20 μl of culture at 1,400 mOsM. The TRPV4 activity is evident when the RLU of the experimental is compared to that of two negative controls: yeast cells transformed with empty p416GPDV4 plasmid or one that contains the TRPV4 gene with a mutation at the ion filter (Figure 1)24.
In a typical two-electrode voltage-clamp experiment, the oocytes are initially bathed in a flowing isotonic bath solution containing (in mM) 66 KMeSO4, 1.8 Ba(MeSO4)2, 5 K+-HEPES, (pH 7.2) and 100 sorbitol. Peak currents are assessed at the end of 100 msec test pulses to +20 mV from a holding potential of -20 mV every 10 sec. To elicit hypotonic responses flow is changed to a similar solution lacking sorbitol. This hypotonic response is reversible upon return of sorbitol as well as blockable by the TRP-channel blocker ruthenium red (Figure 2A).
In a typical patch-clamp experiment, symmetric solution is used, i.e. the solution bathing the patch and the pipette-filling solution are the same (98 mM KCl, 1 mM MgCl2, and 10 mM K+-HEPES, pH 7.2). Here, the inside-out patch excised from a rat-TRPV4 expression oocyte is held at +50 mV. Pulses of suction are generated from the syringe. Brief suctions, hundreds of msec in duration, applied at tens of mm Hg, elicit the ~40 pS unitary conductance native to the oocyte membrane37 and the ~90 pS unitary conductance of the heterologously expressed TRPV4 (Figure 2B)14.
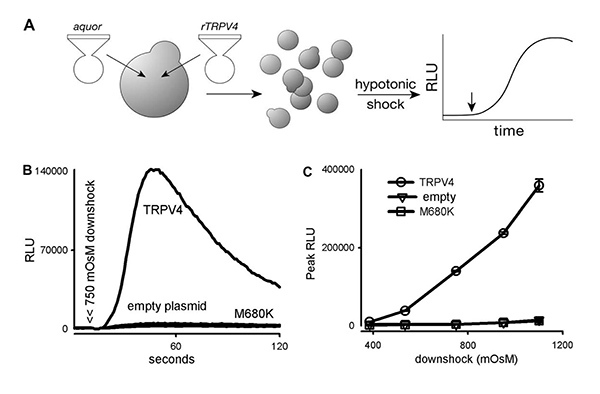 Figure 1. Rat TRPV4 expressed in yeast respond to hypotonic shock. (A) A diagram showing the experimental methods. (B) A 750 mOsM hypotonic shock (arrow heads) triggers a large luminescence increase (in relative luminescence units, RLU) in TRPV4 transformants, but not in transformants of an empty plasmid, or plasmid bearing a TRPV4 with a mutation in its ion filter (M680K). (C) A dose-response relation between hypotonic shock and the peak response (mean + S.D., n = 3). Measurements from 2.4 x 106 cells each24. Click here to view larger image.
Figure 1. Rat TRPV4 expressed in yeast respond to hypotonic shock. (A) A diagram showing the experimental methods. (B) A 750 mOsM hypotonic shock (arrow heads) triggers a large luminescence increase (in relative luminescence units, RLU) in TRPV4 transformants, but not in transformants of an empty plasmid, or plasmid bearing a TRPV4 with a mutation in its ion filter (M680K). (C) A dose-response relation between hypotonic shock and the peak response (mean + S.D., n = 3). Measurements from 2.4 x 106 cells each24. Click here to view larger image.
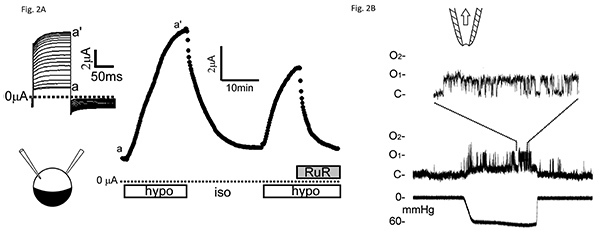 Figure 2. Electrophysiological examinations of TRPV4 activity heterologously expressed Xenopus oocytes.(A) Whole-oocyte macroscopic-current responses to hypotonic stimuli examined with a two-electrode voltage clamp. Peak currents from an oocyte expressing very high levels of wild-type TRPV4 (5 days after injection of 40 ng of cRNA) upon 100 msec voltage steps (from -20 to +20 mV every 10 sec) (inset) in response to the removal of 100 mM sorbitol from the 250 mOsM bath solution (open bars) and the addition of 3 μM ruthenium red (RuR; filled bar). Evident are the repeated peak-current increases upon hypotonic stimuli and its decreases upon the return to isotonic solution or the addition of the channel blocker (RuR). (B) Direct activation of wild-type TRPV4 by membrane stretch seen under a patch clamp. A sample of raw traces of average quality from a patch excised from a TRPV4-expreesssing oocyte, showing activation by 60 mmHg suction (lower trace) applied to an excised inside-out patch held at +50 mV. The uppermost trace is displayed at a faster time base to show the unitary current transition between closed (C) and two open levels (O1 and O2)14. Click here to view larger image.
Figure 2. Electrophysiological examinations of TRPV4 activity heterologously expressed Xenopus oocytes.(A) Whole-oocyte macroscopic-current responses to hypotonic stimuli examined with a two-electrode voltage clamp. Peak currents from an oocyte expressing very high levels of wild-type TRPV4 (5 days after injection of 40 ng of cRNA) upon 100 msec voltage steps (from -20 to +20 mV every 10 sec) (inset) in response to the removal of 100 mM sorbitol from the 250 mOsM bath solution (open bars) and the addition of 3 μM ruthenium red (RuR; filled bar). Evident are the repeated peak-current increases upon hypotonic stimuli and its decreases upon the return to isotonic solution or the addition of the channel blocker (RuR). (B) Direct activation of wild-type TRPV4 by membrane stretch seen under a patch clamp. A sample of raw traces of average quality from a patch excised from a TRPV4-expreesssing oocyte, showing activation by 60 mmHg suction (lower trace) applied to an excised inside-out patch held at +50 mV. The uppermost trace is displayed at a faster time base to show the unitary current transition between closed (C) and two open levels (O1 and O2)14. Click here to view larger image.
Discussion
As stated in the Introduction, the methods commonly used to study TRPV4 functions sometimes resulted in inconsistencies and controversies. The two sets of methods described here offer some advantages and can complement the existing methods. While we describe only the studies on TRPV4, the methods may be extended to study other ion channels as well.
1. Yeast Luminometry Methods
Budding yeast has a native Ca2+-influx response to hypo-osmotic shock that is sensitized by mutations that affect lipid composition30. This signal can be erased with external EGTA. This chelator, however, does not erase the signal from the heterologously expressed TRPV4 24, indicating that the channel is expressed in an internal membrane, most likely that of the endoplasmic reticulum, from which the Ca2+ is released into the cytoplasm upon the osmotic shock. Traffic of heterologously expressed channels has not been studied in yeast. Should this method be extended to the study of other TRP channels or other putative mechanosensitive channels, the chelation test needs to be carried out and the presence of the native system be borne in mind.
2A. Two-electrode Voltage Clamp
Unlike the patch-clamp experiment, the two-electrode voltage-clamp experiment is carried out on intact oocyte, before the removal of the vitelline envelope. Both microscopic examination and capacity values indicate that the plasma membrane is not uniformly spherical but is high invaginated beneath the relatively inelastic sphere of the vitelline envelope. Thus, the mechanical stress caused by hyponicity is not simply an isotropic inflationary tension of an expanded spherical plasma membrane. The stress likely results from the pressing of the membrane against the constraining vitelline envelope and /or away from the established cytoplasmic attachment in the face of increased osmotic pressure.
Expression of TRPV4, and likely certain other channels, is toxic to the oocyte, presumably due to Ca2+ leakage. It is therefore important to continuously incubate the oocyte after TRPV4-cRNA injection in the presence of the channel blocker, ruthenium red. The degree of TRPV4 expression shows variability, which is not completely under experimental control. "Gain-of-function" mutant TRPV4 channels, those that show significant spontaneous opening, are systematically expressed earlier after injection than their wild-type counterpart23.
2B. Patch Clamp
Xenopus oocyte expresses a native mechanosensitive channel, which rectifies in the inward direction (~50 pS inward, ~10 pS outward). One study suggests that it is the expression of TRPC1 37. This constantly expressed native channel provides a calibration for TRPV4, the heterologous expression of which may not always be successfully observed in patches.
The probability of encountering TRPV4 tends to be higher in oocytes after a longer period of incubation, typically 3-4 days after cRNA injection. An efficient way to proceed is to first perform the two-electrode-voltage-clamp experiment (above), making sure that the oocyte is expressing the spontaneous TRPV4 macroscopic current, and then proceed to remove the vitelline envelope before taking sample patches from the same oocyte. The probability of capturing TRPV4 activities can also be enhanced using "macropatches", mounted on pipets with larger bores (bubble number 6-7). Fire polishing these pipettes32 seems to be helpful in achieving the GΩ seal. After the seal formation, the excised patch may show a very high resistance, very little noise usually associated with biological membranes, and no native-channel activity. This most likely indicates a double-membrane formation. A brief air exposure, by transiently lifting the pipette above the meniscus by micromanipulation can usually break the unwanted additional layer. Alternatively, the outer layer can be removed by gently touching the pipette bore against a thread of hardened silicone seal suspended in the bath.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We would like to thank Andrea Kremsreiter for excellent technical assistance. Work in our laboratory is supported by NIH GM096088 and the Vilas Trust of the University of Wisconsin - Madison.
References
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu. Rev. Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12 doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts W, Nilius B, Owsianik G. The vallinoid transient receptor potential channel Trpv4: From structure to disease. Prog. Biophys. Mol. Biol. 2009. [DOI] [PubMed]
- Nilius B, Voets T. The puzzle of TRPV4 channelopathies. EMBO Rep. 2013. [DOI] [PMC free article] [PubMed]
- Liedtke W, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Bodding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett. 2000;485:127–134. doi: 10.1016/s0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- Delany NS, et al. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol. Genomics. 2001;4:165–174. doi: 10.1152/physiolgenomics.2001.4.3.165. [DOI] [PubMed] [Google Scholar]
- Rock MJ, et al. Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat. Genet. 2008;40:999–1003. doi: 10.1038/ng.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow D, et al. Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am. J. Hum. Genet. 2009;84:307–315. doi: 10.1016/j.ajhg.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho N, et al. Dominant TRPV4 mutations in nonlethal and lethal metatropic dysplasia. Am. J. Med. Genet. A. 2010;152:1169–1177. doi: 10.1002/ajmg.a.33392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, et al. Molecular determinants of permeation through the cation channel TRPV4. J. Biol. Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, et al. Modulation of TRPV4 gating by intra- and extracellular Ca2. Cell Calcium. 2003;33:489–495. doi: 10.1016/s0143-4160(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J. Biol. Chem. 2010;285:27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J. Biol. Chem. 2003;278:27129–27137. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Vriens J, et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. U.S.A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F, et al. Identification and characterization of novel TRPV4 modulators. Biochem. Biophys. Res. Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Willette RN, et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J. Pharmacol. Exp. Ther. 2008;326:443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- Kang SS, Shin SH, Auh CK, Chun J. Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp. Mol. Med. 2012;44:707–722. doi: 10.3858/emm.2012.44.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamande SR, et al. Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat. Genet. 2011;43:1142–1146. doi: 10.1038/ng.945. [DOI] [PubMed] [Google Scholar]
- Loukin S, Su Z, Kung C. Increased Basal Activity Is a Key Determinant in the Severity of Human Skeletal Dysplasia Caused by TRPV4 Mutations. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin SH, Su Z, Kung C. Hypotonic shocks activate rat TRPV4 in yeast in the absence of polyunsaturated fatty acids. FEBS Lett. 2009;583:754–758. doi: 10.1016/j.febslet.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin S, Su Z, Zhou X, Kung C. Forward genetic analysis reveals multiple gating mechanisms of TRPV4. J. Biol. Chem. 2010;285:19884–19890. doi: 10.1074/jbc.M110.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiza AF, Schulz T, Masson PH. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 1996;271:23357–23362. doi: 10.1074/jbc.271.38.23357. [DOI] [PubMed] [Google Scholar]
- Amberg DC, Burke D, Strathern JN. Methods in Yeast Genetics: a Cold Spring Harbor Laboratory Course Manual. New York: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- Loukin S, Zhou X, Kung C, Saimi Y. A genome-wide survey suggests an osmoprotective role for vacuolar Ca2+ release in cell wall-compromised yeast. FASEB J. 2008;22:2405–2415. doi: 10.1096/fj.07-101410. [DOI] [PubMed] [Google Scholar]
- Denis V, Cyert MS. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell. Biol. 2002;156:29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin SH, Kung C, Saimi Y. Lipid perturbations sensitize osmotic down-shock activated Ca2+ influx, a yeast "deletome" analysis. FASEB J. 2007;21:1813–1820. doi: 10.1096/fj.06-7898com. [DOI] [PubMed] [Google Scholar]
- Loukin SH, Zhou X-L, Kung C, Saimi Y. A genome-wide survey suggests an osmo-protective role for vacuolar Ca2+ release in cell-wall-compromized yeast. FASEB J. 2008;22:2405–2415. doi: 10.1096/fj.07-101410. [DOI] [PubMed] [Google Scholar]
- Conn PM. Methods in Enzymology. Vol. 294. Academic Press; 1999. [Google Scholar]
- Goldin AL, Sumikawa K. Iverson Rudy LE. Methods in Enzymology. Vol. 207. Harcourt Brace Jovanovich; 1992. pp. 279–298. [DOI] [PubMed] [Google Scholar]
- Loukin SH, et al. Random mutagenesis reveals a region important for gating of the yeast K+ channel Ykc1. EMBO J. 1997;16:4817–4825. doi: 10.1093/emboj/16.16.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AL, Johnson BE, Goodman MB. Patch clamp recording of ion channels expressed in Xenopus oocytes. J. Vis. Exp. 2008. [DOI] [PMC free article] [PubMed]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Maroto R, et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]


