Abstract
Impact on health by nanomaterials has become a public concern with the great advances of nanomaterials for various applications. Surface coating agents are an integral part of nanoparticles, but not enough attention has been paid during toxicity tests of nanoparticles. As a result, there are inconsistent toxicity results for certain nanomaterials. In this study, we explore the cytotoxicity of eleven commonly used surface coating agents in two cell lines, human epidermal keratinocyte (HaCaT) and lung fibroblast (CRL-1490) cells, at surface coating agent concentrations of 3, 10, 30, and 100 μM. Two exposure time points, 2 h and 24 h, were employed for the study. Six of the eleven surface coating agents are cytotoxic, especially those surfactants with long aliphatic chains, both cationic (cetyltrimethylammonium bromide, oleylamine, tetraoctylammonium bromide, and hexadecylamine) and anionic (sodium dodecylsulfate). In addition, exposure time and the use of different cell lines also affect the cytotoxicity results. Therefore, factors such as cell lines used and exposure times must be considered when conducting toxicity tests or comparing cytotoxicity results.
Keywords: Surface coating agents, nanoparticle, cytotoxicity, aliphatic amines, SDS
Introduction
Engineered metal nanoparticles (NPs) have been widely used in cosmetics, painting, textiles, water treatment, batteries, and automobiles. Since their size scale is similar to that of biological macromolecules such as DNA and protein, NPs have also found enormous applications in medicine for therapy, imaging, drug delivery, and gene therapy. Due to their increased use in humans and release to the environment, it has become an important branch of nanoscience for the study to understand the impact of NPs on human health (Colvin 2003; Borm 2006; Ray 2009; Hwang 2012; Ray 2013).
Surface coating agents, whether organic, inorganic, or polymeric, are an integral part of the NPs. They are used for size and shape control during synthesis, and for stabilization, protection, recognition, and delivery (Brust 1994; Vogel 1997; Boennemann 1998; Wong 2001; Caruntu 2002; Chen and Hsieh 2002; Cushing 2004; Ray 2010; Bae 2011; Levard 2011). For most liquid phase preparation methods, surface coating agents are present at the initial step. These molecules may interact with the metal ions in solution and affect the reaction equilibrium, particle nucleation and growth rates, and thus the entire course of the reaction, leading to size and shape control (Murray 1995; Sun 1999; Ray 2010). Surface coating agents can bind preferentially to one or several facets of the seed particles, either inhibiting or promoting crystal growth on some facets depending on the nature of the coating agents. Surface coating agents bonded to the surface of NPs can protect NPs from direct interaction with the environment, thus avoiding reactions between them. For example, surface coating agents for silver NPs can prevent oxidation of the surface silver atoms to silver oxides (Lu 2010, McShan 2014), while the coating agents for ZnO NPs help prevent dissociation of ZnO in acidic aqueous solutions (Wong 2001). Charged bulky organic surface coating agents can cause electrostatic repulsion as well as steric hindrance between particles, thus stabilizing the NPs from agglomeration (Caruntu 2002). Specific agents coated on the surface of NPs can help to ease the passing of the NPs through the cell membrane to deliver NPs to the target. Finally, various molecules such as ligands, RNA/DNA aptamers, and antigens strategically placed on the NP surface have been used for special recognition and sensing (Ray 2010).
Organic surface coating agents can be divided into several categories: 1). Covalent bonded, such as thiol-containing compounds for gold and silver NPs; 2). Ionic compounds, such as anionic alkyl sulfate and fatty acids, or cationic alkyl ammonium salts; 3). Neutral molecules; and 4). polymeric molecules. Some of the coating materials are also called “surfactants” because they are ionic molecules with long aliphatic chains, such as alkyl ammonium halides, fatty acids, and alkyl sulfates. Coating agents are mostly absorbed onto the NPs surface, but a certain amount of the free molecules must exist in solution to maintain the equilibrium and thus the stabilization of the NPs. As Wang et al pointed out, gold nanorods are not stable when the concentration of the coating agent is too low after multiple centrifugations and replacement of the supernatant with nanopure water (Wang 2008).
Although coating agents are an integral part of the NPs, they have not received sufficient attention for their potential contribution to the overall toxicity of the NPs. Information of NP’s surface coating agents is often not even available from suppliers, making it even more difficult to judge what roles the surface coating agents might play during the toxicity tests. There are several recent reports that determined certain coating agents’ contribution to the overall toxicity of NPs. They found that some are toxic at certain concentrations (Modrow 2003; Hoshino 2004; Yang and Watts 2005; Wang 2008; Lu 2010; Ying and Hwang 2010), can alter the release of NPs into the aqueous medium (Yang 2012), improve solubility and interaction of the NPs with biological molecules (Levard 2011; Li 2013), or affect the amount of reactive oxygen species (ROS) formation (Chairuangkitti 2012; Li 2013; Zhang 2013). Thus, negligence of the contribution of coating agents may result in wrong conclusions on the toxicity of certain nanomaterials (Ray 2009; Hwang 2012; Yang 2012; Ray 2013). For example, Connor et al (Connor 2005) found that gold NPs are not toxic against K562 leukemia cells during a 3-day exposure test with up to 100 μM concentration (in Au atom) when coated with citrate, cysteine, glucose, or biotin. Conversely, Pernodet et al (Pernodet 2006) reported that human dermal fibroblast cells showed impaired spreading, division, and changes in cell morphology after treatment with gold NPs coated with citrate. Wang et al (Wang 2008) found that gold nanorods, prepared using cetyltrimethylammonium bromide (CTAB) as a growth-directing surfactant, are highly toxic to human skin cells due to the presence of CTAB, not the gold nanorods themselves. Alkilany et al. (Alkilany 2009) reported that over-coating gold nanorods with polymers substantially reduces their cytotoxicity. Similar studies on silver NPs also pointed out these inconsistencies (Yang and Watts 2005; Lu 2010; Suresh 2010; Yang 2012).
Due to the use of surfactants in detergents and cosmetics, the toxicity of some surfactants, including some of those used as surface coating agents for nanoparticles, was studied decades ago and was published in an excellent summary by Gloxhuber (Gloxhuber 1974). Those tests used animals at surfactant concentrations being high (> 1000 μM). They found that most surfactants were not acutely toxic. Although they did find that exposure to high levels of surfactants (millimolar to molar range) could dissolve the cell membrane. It was pointed out that the toxicity of some surfactants is caused by their strong protein-binding ability and the solubilization of cholesterol and phospholipoids.
In this report, we present the cytotoxicity study of eleven commonly used surface coating agents (Table 1) in two different cell lines, human skin keratinocytes (HaCaT) and human lung fibroblast cells (CRL-1490), using MTT/MTS assay with both a long (24 h) and a short (2 h) exposure time. The aims of this study are to explore the difference in toxicity of these coating agents in different cells and exposure times, thus providing a possible guide for researchers dealing with toxicity studies of NPs with certain surface coating agents. Since coating agents are in equilibrium with coating on particles, and since the coatings can be exchanged from the particles, this study can inform nanomaterial researchers of the extent of problems their choice in coatings can have in tissue culture model systems.
Table 1.
List of selected coating agents, molecular formula, and applications in nanoparticles
| Name | Molecular formula | Nanoparticles | Reference |
|---|---|---|---|
| Cationic | |||
| Oleylamine (OA) | CH3(CH2)7CH=CH(CH2)8NH2 | In2O3 | (Seo 2003) |
| Tetraoctylammonium bromide (OcBr) | (CH3(CH2)7)4N+ Br− | Co, Pd, PtRu, Pt3Sn | (Vogel 1997; Boennemann 1998; Bucher 2002; Modrow 2003) |
| Cetyltrimethyl ammonium bromide (CTAB) | CH3(CH2)15N+ (CH3)3 Br− | Au, Ag, Ni, CeO2, CuM2O5(M=Ho, Er), NiO, ZnO, SnO2, BaFe12O19 | (Pillai 1993; Palla 1999; Porta 1999; Chen and Wu 2000; Chen and Hsieh 2002; Wang 2002; Wu 2002) |
| Hexadecylamine (HDA) | CH3(CH2)14CH2NH2 | Ni, Cu2O, CdSe, CdSe@ZnS | (Rockenberger 1999; Talapin 2001; Hou and Gao 2003) |
| Neutral | |||
| p-Aminothiophenol (4-A) | HSPhNH2 | Pt | (Gomez 2001) |
| Triphenylphosphine (TPP) | P(Ph)3 | Cu146Se73(PPh3)30 | (Krautscheid 1993) |
| Dodecanethiol (DDE) | CH3(CH2)10CH2SH | ZnO, Ru, Au | (Brust 1994; Wong 2001; Viau 2003) |
| Tributylphosphine (TBP) | (CH3(CH2)3)3 P | CdS, CdTe@CdS, | (Schreder 2000) |
| Anionic | |||
| Sodium dodecyl sulfate (SDS) | CH3(CH2)6CH2SO4− Na+ | Cu, CoCrFeO4, CoFe2O4 | (Liu 2000; Vestal and Zhang 2002; Gui 2003) |
| Myristic acid (MA) | CH3(CH2)11CH2COOH | MnFe2O4, Fe3O4, CoFe2O4, NiFe2O4, ZnFe2O4 | (Murray 1993; Murray 1995; Sun 1999; Murray 2001; Caruntu 2002) |
| Tetradecylphosphonic acid (TDPA) | CH3(CH2)12CH2PO(OH)2 | CdTe | (Peng and Peng 2001) |
Experimental Section
Materials
Among the selected coating agents, oleylamine (AC12954-1000), dodecanethiol (AC11762-0025), myristic acid (AC15696-1000), and tetradecylphosphonic acid (50-901-10305) were purchased from Fisher Scientific (Houston, TX). Tetraoctylammonium bromide (294136-5G), hexadecylamine (445312-5G), tributylphosphine (247049-100G), p-Aminothiophenol (42296-5G), cetyltrimethylammonium bromide (52365-50G), and triphenylphosphine (93092-250G) were from Sigma-Aldrich (St. Louis, MO). Sodium dodecyl sulfate (161-0302) was purchased from Bio-Rad Laboratories (Hercules, CA). They can be divided into cationic (first four), neutral (next four), and anionic (last three) compounds at physiological pHs (Table 1). Further details of their properties and usage on NPs are listed in Table 1. Stock solutions in DMSO or ethanol (10 mM) were prepared and stored at 4 °C. It was diluted with 1x PBS buffer at pH 7.4 to the desired concentration before use.
HaCaT keratinocytes, a transformed human epidermal cell line, was obtained from Dr. Norbert Fusenig of the Germany Cancer Research Center (Heidelberg, Germany). The lung fibroblast cell line, CRL-1490, was purchased from ATCC (Manassas, VA). Trypsin EDTA solutions were purchased from Cambrex Bio Science (Walkersville, MD). Fetal bovine serum (FBS), Dulbecco’s Minimum Essential Medium (DMEM), penicillin/streptomycin, DMSO, phosphate buffered saline (PBS), and CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) were purchased from Fisher Scientific (Houston, TX). Thiazolyl blue tetrazolium bromide (MTT) was obtained from Sigma-Aldrich (St. Louis, MO). Eagle’s Minimum Essential Medium (EMEM) was purchased from ATCC (Manassas, VA).
MTT/MTS assay
HaCaT cells were grown in complete medium (DMEM, 10% FBS, 1% penicillin/streptomycin) in 25 cm2 cell culture flasks. CRL-1490 cells were grown in complete medium (EMEM, 10% FBS, 1% penicillin/streptomycin) in 75 cm2 culture flasks. Cells were cultured in a humidified incubator with 5% CO2 at 37 °C. After the cells grew to confluence, they were detached by 25% trypsin/EDTA, and diluted to 3×105 cells/mL by their respective complete media as previously reported (Fullove and Yu 2013; McShan and Yu 2014).
A 200 μL cell suspension in complete medium was added to each well of a 96-well plate and incubated under 5% CO2 at 37 °C for 24 h for cell adhesion. After incubation, the supernatant was removed through pipetting and the adherent cells were washed with 1× PBS buffer before exposed to coating agents.
A total of 90 μL of DMEM (for HaCaT) or EMEM (for CRL-1490) and 10 μL of a coating agent solution in PBS with 10% DMSO or ethanol (final DMSO or ethanol concentration 1%) at desired concentrations were added into each well. A total of 5 wells were used for the test at each concentration. After 2 h or 24 h of incubation under 5% CO2 at 37 °C, cell viability was determined by MTT or MTS assays. The control tests were with 1% DMSO or ethanol and they did not result in any significant cell death. Negative controls of cell medium with the proper amount of PBS, DMSO/ethanol, and DMEM/EMEM were used to obtain the background absorbance values at 490 nm for MTS test and at 540 nm for MTT test. These values were subtracted from the final absorbance reading to eliminate background interference.
For MTS test, 20 μL MTS solution was added directly to each well after treatment and incubated for further 2 h. The absorbance was read at 490 nm using a 96-well Multiskan Ascent Plate Reader with Ascent software. For MTT assay, the supernatant was removed from each well and washed twice with 1× PBS buffer. MTT solution (50 μL of 5 mg/mL MTT) and DMEM (200 μL) were added into each well. After 1 h of incubation, MTT solution was separated from the adherent cells and 200 μL DMSO was added to the cells of each well. The plate was left at room temperature for 30 min to completely solubilize the formazan salt. The absorbance was read at 540 nm using Multiskan Ascent Plate Reader with Ascent software as it was done before (Wang 2008; Lu 2010; Fullove and Yu 2013).
Statistical analysis
All statistical analyses were performed with the SAS 9.3 software using the Generalized Linear Model (Anderson 2004) with data from the average of three independent experiments (N = 3). Statistical significance was determined for the cytotoxicity of each coating agent at a certain concentration in comparison to the control without added coating agent. Statistical significance was also determined for cytotoxicity of a coating agent in HaCaT cells in comparison to that in CRL-1490 cells. The significant level was defined as p < 0.05.
Results
Cytotoxicity of coating agents on HaCaT and CRL-1490 cells
Two exposure time points (2 h and 24 h) were used for cell viability tests with either the HaCaT or the CRL-1490 cells. The concentration for all coating agents was at 100 μM. The cell viability is expressed as percent of viable cells versus the control and is shown in Figure 1 and Table 2.
Figure 1.
Viability of HaCaT (A) and CRL-1490 (B) cells treated with the eleven selected surface coating agents at 100 μM for 2 h or 24 h. Error bars are the average of three separate experiments. Statistical significance was determined with SAS 9.3 using the Generalized Linear Model (N = 3) in comparison to the control without added coating agents and those with a p < 0.05 are shown by a *. Statistical significance between the cytotoxicity results of the two cell lines were compared with each other at the same concentration of the coating agent and those with a p < 0.05 are shown by **.
Table 2.
Cell viability after exposure to the coating agents at 100 μM for 2 or 24 hours.
| Coating agents | Cell Viability % (± standard error) | |||
|---|---|---|---|---|
| HaCaT | CRL-1490 | |||
| 2 h | 24 h | 2 h | 24 h | |
| Cationic | ||||
| Oleylamine (OA) | 33±10* | 2±2* | 8±13* | 3±1* |
| Tetraoctylammonium bromide (OcBr) | 14±1* | 6±2* | 37±26* | 4±1* |
| Hexadecylamine (HDA) | 86±13** | 13±7* | 53±12*&** | 3±2* |
| CTAB | 64±19*&** | 1±0* | 0±3*&** | 7±1* |
| Neutral | ||||
| p-Aminothiophenol (4-A) | 116±20 | 71±13 | 80±9** | 61±16* |
| Dodecanethiol (DDE) | 63±19*&** | 80±13 | 111±14 | 100±17 |
| Tributylphosphine (TBP) | 121±14 | 76±19 | 84±6 | 94±9** |
| Triphenylphosphine (TPP) | 102±14 | 81±19 | 93±10 | 93±19 |
| Anionic | ||||
| Tetradecylphosphonic acid (TDPA) | 78±9* | 90±17 | 93±7 | 76±4 |
| SDS | 115±24 | 22±12* | 65±6*&** | 33±4* |
| Myristic acid (MA) | 109±8 | 75±21 | 101±8 | 98±12 |
Statistical significance was determined with SAS 9.3 software using the Generalized Linear Model in comparison to the control without any added coating agents. Data were the average of three independent experiments. Those significant with P < 0.05 against the control are shown by *, and those significant with P < 0.05 between the two cell lines are shown by **.
HaCaT Cells
For the 2 h exposure time, oleylamine, tetraoctylammonium bromide, CTAB and dodecanethiol are toxic, with cell viability of 33%, 14%, 64% and 63%, respectively (Figure 1A and Table 2). Hexadecylamine and tetradecylphosphonic acid are slightly toxic (P<0.05) with cell viability of about 80%. The rest of the coating agents do not show significant toxicity on the P < 0.05 level.
For the 24 h exposure time, HaCaT cell viabilities treated with oleylamine, tetraoctyl ammonium bromide, hexadecylamine, CTAB, and SDS are greatly decreased compared with the respective 2 h exposure results (P < 0.05). It was also decreased for p-aminothiophenol from 116% to 71%. Tetraoctylammonium bromide, oleylamine, and CTAB are all highly toxic with close to 0% cells viability, and SDS and hexadecylamine are also toxic with about 20% cell viability. The remaining surface coating agents have low or no toxicity with less than 25% cell death (Table 2).
CRL-1490 cells
For the 2 h exposure test, three surface coating agents, tetraoctylammonium bromide, oleylamine, and CTAB, which are cytotoxic in HaCaT cells, are also cytotoxic in CRL-1490 cells, resulting in 37, 8, and 0% cell viability, respectively. In addition, hexadecylamine and SDS are also cytotoxic with 53 and 65% cell viabilities, respectively (Table 2). The remaining coating agents do not show significant toxicity on the p < 0.05 level.
For the 24 h exposure test, most of the surface coating agents exhibit cytotoxicity higher than that observed for the 2 h exposure test result. Among them, oleylamine, CTAB, SDS, tetraoctylammonium bromide, and hexadecylamine are highly toxic, leading to almost 100% cell death. p-Aminothiophenol is cytotoxic with 61% cell viability. The remaining coating agents are not cytotoxic, with a minimum of 76% cells viability.
By comparing the cytotoxicity results, cell viabilities for the 24 h exposure time are in general much lower than that for the 2 h exposure time, indicating that a longer exposure time results in more cell death. Although the coating agents that are toxic to one cell line are also toxic to the other, the cell viability values against the two cell lines can be different for the same coating agent. For the 2 h exposure tests, as the statistical analyses indicate, the toxicity of CTAB, SDS, hexadecylamine, p-aminothiophenol, tributylphosphine and dodecanethiol against HaCaT cells are significantly different from that against CRL-1490 cells. CTAB, SDS, hexadecylamine and p-aminothiophenol cause HaCaT cell viabilities to decrease to 64%, 115%, 86% and 116%, but cause CRL-1490 cell viabilities to decrease to 0%, 65%, 53% and 80%, respectively. For dodecanethiol and tributylphosphine, they cause HaCaT cell viability to be at 63% and 121%, respectively, but cause CRL-1490 cell viability to be at 111% and 84%, respectively (Table 2). All these numbers are significantly different.
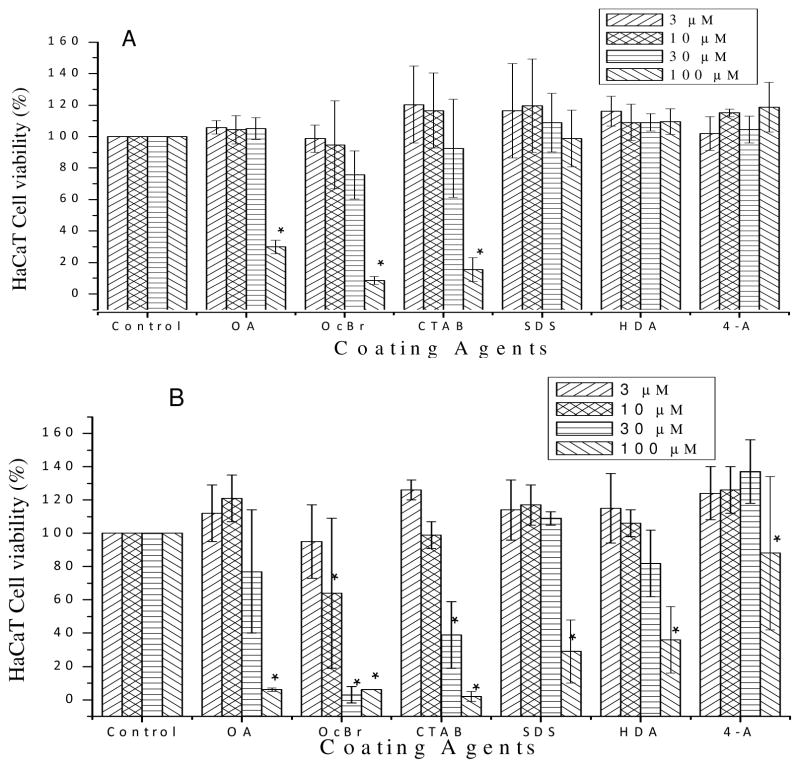
Concentration Dependence
To explore concentration dependence of the cell viabilities exposed to coating agents, HaCaT cells were exposed to 3, 10, 30, and 100 μM of each of the six coating agents that are tested toxic to HaCaT cells at exposure times of 2 h (Figure 2A) or 24 h (Figure 2B), respectively. For the 2 h exposure test, while SDS, hexadecylamine and p-aminothiophenol are not cytotoxic, CTAB, tetraoctylammonium bromide and oleylamine cause increased cytotoxicity with increasing concentration.
Figure 2.
Concentration dependence of HaCaT cell viability exposed to surface coating agents at 3, 10, 30, and 100 μM for 2 h (A) and 24 h (B). Statistical significance was determined by the average of three independent experiments (N = 3) with SAS 9.3 software using the Generalized Linear Model in comparison to the control without added coating agent and those with p < 0.05 are shown by *.
For 24 h exposure results (Figure 2B), toxicity responses shift to lower concentrations compared to the 2 h exposure results. For oleyamine, CTAB, tetraoctylammonium bromide, the lowest concentration at which they show toxicity decreased from 100 μM for the 2 h exposure tests to 30, 30, 10 μM, respectively, for the 24 h exposure tests. SDS, hexadecylamine and p-aminothiophenol are also toxic at 24 h exposure, but only showing significant toxicity at the highest concentration of 100 μM.
The IC50 are calculated by plotting the cell viability versus the concentration of the surface coating agents and listed in Table 3. The IC50 values for the 24 h exposure tests are clearly lower than the 2 h tests.
Table 3.
IC50 (μM) of the six cytotoxic surface coating agents
| Exposure time | Oleylamine | Tetraoctylammonium bromide | CTAB | SDS | Hexadecyl amine | 4-Amino-thiophenol |
|---|---|---|---|---|---|---|
| 2 h | 80 | 56 | 69 | -# | -# | -# |
| 24 h | 61 | 16 | 26 | 80 | 79 | 189* |
Determined by extrapolation;
Not determined due to needs of much higher concentration.
Discussions
The coating agents’ concentrations we used are 3–100 μM in the medium. The concentration of the free coating agents in solutions is generally not tested, and it depends on the nature of the coating agents, the NP, and the media. For wet chemistry synthesis of NPs, it also depends on whether the NP is treated or not to remove excess coating agents before use. Without treatment, the concentration of coating agents could be as high as 10–100 mM. After 3 centrifugations, as people mostly do, removal of supernatants and refill with water, the estimated concentration of the coating agents will decrease a maximum of 1,000 times to 10–100 μM, within the range of our tested concentrations. If NPs aggregate in medium (extra cellular liquid), they will release the absorbed coating agents, and the concentration of the free coating agents could be higher (Wang 2008).
Because surface coating agents are an integral part of all NPs, the test of NP toxicity is a test of a mixture. Therefore, all considerations for the test of a mixture should be considered (Hoshino 2004; Yang and Watts 2005; Yin 2005; Alkilany 2009; Lu 2010; Ying and Hwang 2010; Levard 2011; Yang 2012). In particular, 1) surface coating agents must be included as a control since some of them are toxic as it is shown here; 2) exposure time and use of cell lines must also be considered since these factors affect the toxicity test results, especially when comparing toxicities of NPs; 3) as pointed out by Yang and Watts (Yang and Watts 2005; Yang 2012), the effects of surface coating agents on NPs’ release, transport, and ROS formation must also be considered; 4) consideration that the surface coating agents may modulate (reduce or increase) the toxicity of the NPs depending on the mechanism they induce toxicity.
Six of the eleven tested coating agents are toxic to both human skin keratinocyte and human lung fibroblast cells. Among them, all cationic surface coating agents, ammonium/amino compounds with long aliphatic chains, are toxic to both cell lines. Similar test results were reported by McLaughlin et al. (McLaughlin 2011) that the longer the aliphatic chain, the more toxic the coating agent is. Another surface coating agent, SDS, a commonly used anionic surfactant for many applications, is also toxic at 100 μM for the 2 h exposure and 30 μM for the 24 h exposure. The neutral and anionic coating agents, whether sulfur, phosphorous, or carboxylic compounds, are with low or no toxicity under these testing conditions.
Other factors that can affect the toxicity test result are the use of a particular cell line and exposure time. The use of cell lines, although produces the same toxicity trend, the level of toxicity could be different. This is particularly important when we toxicity test results. Exposure time is another variable for cytotoxicity test results. For the 2 h and 24 h tests, the longer test time (24 h) almost exclusively caused more cell death. This means that test results with different exposure times may not be compared.
Regarding the effect of coating agents on NPs’ toxicity, we hypothesize that long aliphatic chain surface coating agents are toxic due to the fatty acid-effect on the cell membrane. Perturbations of the phospholipid-rich cell membrane of target cells can result in a robust activation of phospholipases (PLs), notably PLA2 and PLC. Activation of PLA2 in human cells by lipid and phospholipid derivatives have been demonstrated to induce a significant activation of PLA2 and phosphoinositide (PI)-specific PLC resulting in the release of free arachidonic acid (AA) and inositol triphosphate (IP3) in human bronchial epithelial cells (Kafoury 1998). The generation of these signaling mediators was associated with increased ROS. We have also demonstrated that activation of PLA2 and PI-PLC can result in the activation of the nuclear transcription factor-κB (NF-κB), a key regulator of pro-inflammatory genes (Kafoury 1999; Kafoury 2007), for example, interleukins 1β, 6, 8 and tumor necrosis factor-α (TNF-α). Decades ago, studies on the toxicity of surfactants used in detergents or cosmetics suggested that the surfactants greatly affect cell membrane (Gloxhuber 1974). However, a detailed mechanism on the cellular level warrants further investigation.
Therefore, surface coating agents can be one of the factors contributing to the toxicity of NPs. Control experiments with pure surface coating agents used for that particular NPs must be included for tests of all NPs. The use of exposure time, concentration, cell type, and test methods must be carefully considered when comparing toxicity results. The impact of surface coating agents on the overall toxicity of NPs, whether through the intrinsic toxicity of the surface coating agents or through the synergetic or antagonistic activity when considered as a unit, must be carefully considered.
Highlights.
Nanoparticle surface coating agents can be cytotoxic
Not enough attention paid to their potential contribution to nanoparticles toxicity
Long aliphatic chain surface coating agents are cytotoxic in general
The cytotoxicity depends on chemical structure, cell line used, and incubation time
Acknowledgments
This research was supported in part by NSF for the Partnership for Research and Education in Materials (DMR-0611539). Core research facilities were supported by grants from the NSF (CHE-0840450) and NIH (NCRR 2G12RR013459-11). This article is not an official U.S. Food and Drug Administration guidance or policy statement. No official support or endorsement by the U.S. FDA is intended or should be inferred.
Abbreviations
- NP
Nanoparticle
- CTAB
Cetyltrimethylammonium bromide
- OA
oleylamine
- OcBr
tetraoctylammonium bromide
- HAD
hexadecylamine
- 4-A
p-aminothiophenol
- TPP
triphenylphosphine
- TBP
tributylphosphine
- SDS
sodium dodecyl sulfate
- MA
myristic acid
- TDPA
tetradecylphosphonic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkilany AM, Nagaria PK, Hexel CR, Shaw TJ, Murphy CJ, Wyatt MD. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small. 2009;5(6):701–708. doi: 10.1002/smll.200801546. [DOI] [PubMed] [Google Scholar]
- Anderson D, Feldblum S, Modlin C, Schirmacher D, Schirmacher E, Thandi N. 2004 Discussion Paper Program - Applying and Evaluating Generalized Linear Models Including Research Papers on the Valuation of P&C Insurance Companies. Vol. 2014. Colorado Springs, CO: Casualty Actuarial Society; 2004. A Practitioner’s Guide to Generalized Linear Models; pp. 1–117. [Google Scholar]
- Bae E-J, Park H-J, Park J-S, Yoon J-Y, Kim Y-H, Choi K-H, Yi J-H. Effect of Chemical Stabilizers in Silver Nanoparticle Suspensions on Nanotoxicity. Bull Korean Chem Soc. 2011;32(2):613–619. [Google Scholar]
- Boennemann H, Britz P, Vogel W. Structure and Chemical Composition of a Surfactant-Stabilized Pt3Sn Alloy Colloid. Langmuir. 1998;14(23):6654–6657. [Google Scholar]
- Borm PJ, Robbins D, Haubold S. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fiber Toxicol. 2006;3:11–35. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatized gold nanoparticles in a two-phase liquid-liquid system. J Chem Soc Chem Comm. 1994;(7):801–2. [Google Scholar]
- Bucher S, Hormes J, Modrow H, Brinkmann R, Waldofner N, Bonnemann H, Beuermann L, Krischok S, Maus-Friedrichs W, Kempter V. Interaction between core and protection shell of N(butyl)4Cl- and N(octyl)4Cl-stabilized Pd colloids. Surface Sci. 2002;497(1–3):321–332. [Google Scholar]
- Caruntu D, Remond Y, Chou NH, Jun M-J, Caruntu G, He J, Goloverda G, O’Connor C, Kolesnichenko V. Reactivity of 3d Transition Metal Cations in Diethylene Glycol Solutions. Synthesis of Transition Metal Ferrites with the Structure of Discrete Nanoparticles Complexed with Long-Chain Carboxylate Anions. Inorg Chem. 2002;41(23):6137–6146. doi: 10.1021/ic025664j. [DOI] [PubMed] [Google Scholar]
- Chairuangkitti P, Lawanprasert S, Roytrakul S, Aueviriyavit S, Phummiratch D, Kulthong K, Chanvorachote P, Maniratanachote R. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol In Vitro. 2012;27(1):330–338. doi: 10.1016/j.tiv.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Chen D-H, Hsieh C-H. Synthesis of nickel nanoparticles in aqueous cationic surfactant solutions. J Mat Chem. 2002;12(8):2412–2415. [Google Scholar]
- Chen D-H, Wu S-H. Synthesis of Nickel Nanoparticles in Water-in-Oil Microemulsions. Chem Mat. 2000;12(5):1354–1360. [Google Scholar]
- Colvin V. The potential environmental impact of engineered nanmoaterials. Nature Biotech. 2003;21:1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- Cushing BL, Kolesnichenko VL, O’Connor CJ. Recent Advances in the Liquid-Phase Syntheses of Inorganic Nanoparticles. Chem Rev. 2004;104:3893–3846. doi: 10.1021/cr030027b. [DOI] [PubMed] [Google Scholar]
- Fullove TP, Yu H. DNA damage and repair of human skin keratinocytes concurrently exposed to pyrene derivatives and UVA light. Toxicol Res. 2013;2(3):193–199. doi: 10.1039/C3TX20085J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloxhuber C. Toxicological properties of surfactants. Arch Toxicol. 1974;32(4):245–270. doi: 10.1007/BF00330108. [DOI] [PubMed] [Google Scholar]
- Gomez S, Erades L, Philippot K, Chaudret B, Colliere V, Balmes O, Bovin J-O. Platinum colloids stabilized by bifunctional ligands: self-organization and connection to gold. Chem Comm. 2001;(16):1474–1475. [Google Scholar]
- Gui Z, Fan R, Mo W, Chen X, Yang L, Hu Y. Synthesis and characterization of reduced transition metal oxides and nanophase metals with hydrazine in aqueous solution. Mat Res Bull. 2003;38(1):169–176. [Google Scholar]
- Hoshino A, Fujioka K, Oku T, Suga M, Sasaki YF, Ohta T, Yasuhara M, Suzuki K, Yamamoto K. Physicochemical Properties and Cellular Toxicity of Nanocrystal Quantum Dots Depend on Their Surface Modification. Nano Lett. 2004;4(11):2163–2169. [Google Scholar]
- Hou Y, Gao S. Monodisperse nickel nanoparticles prepared from a monosurfactant system and their magnetic properties. J Mat Chem. 2003;13(7):1510–1512. [Google Scholar]
- Hwang HM, Ray PC, Yu H, He X. Toxicology of designer/engineered metallic nanoparticles. In: Luque R, Varma R, editors. Sustainable Preparation of Metal Nanoparticles: Methods and Applications. Cambridge, United Kingdom: Royal Society of Chemistry; 2012. pp. 190–212. [Google Scholar]
- Kafoury RM, Hernandez JM, Lasky JA, Toscano WA, Jr, Friedman M. Activation of transcription factors-κB (NF-κB) and IL-6 (NF-IL-6) by lipid ozonation products is crucial to interleukin-8 gene expression in human airway epithelial cells. Environ Toxicol. 2007;22(7):159–168. doi: 10.1002/tox.20246. [DOI] [PubMed] [Google Scholar]
- Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Lipid Ozonation Products (LOP) Activate Phospholipases A2, C, and D. Toxicol Appl Pharmacol. 1998;150(2):338–349. doi: 10.1006/taap.1998.8418. [DOI] [PubMed] [Google Scholar]
- Kafoury RM, Pryor WA, Squadrito GL, Zou X, Salgo MG, Friedman M. Induction of Pro-inflammatory mediators in Human Airway Epithelial Cells by Lipid Ozonation Products (LOP) Amer J Respir Crit Care Med. 1999;160(6):1934–1942. doi: 10.1164/ajrccm.160.6.9902025. [DOI] [PubMed] [Google Scholar]
- Krautscheid H, Fenske D, Baum G, Semmelmann M. A new copper selenide cluster with triphenylphosphine as a ligand: [Cu146Se73(PPh3)30] Angew Chem. 1993;105(9):1364–7. (See also Angew Chem, Int Ed Engl. 1993;32(9):1303–5.
- Levard C, Reinsch BC, Michel FM, Oumahi C, Lowry GV, Brown GE. Sulfidation Processes of PVP-Coated Silver Nanoparticles in Aqueous Solution: Impact on Dissolution Rate. Environ Sci Technol. 2011;45(12):5260–5266. doi: 10.1021/es2007758. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Niu J, Chen Y. Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ Sci Technol. 2013;47(18):10293–10301. doi: 10.1021/es400945v. [DOI] [PubMed] [Google Scholar]
- Liu C, Rondinone AJ, Zhang ZJ. Synthesis of magnetic spinel ferrite CoFe2O4 nanoparticles from ferric salt and characterization of the size-dependent superparamagnetic properties. Pure Appl Chem. 2000;72(1–2):37–45. [Google Scholar]
- Lu W, Senapati D, Wang S, Tovmachenko O, Singh AK, Yu H, Ray PC. Effect of surface coating on the toxicity of silver nanomaterials on human skin keratinocytes. Chem Phys Lett. 2010;487(1–3):92–96. doi: 10.1016/j.cplett.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M, Earle MJ, Gilea MA, Gilmore BF, Gormana SP, Seddonb KR. Cytotoxicity of 1-alkylquinolinium bromide ionic liquids in murine fibroblast NIH 3T3 cells. Green Chem. 2011;13:2794–2800. [Google Scholar]
- McShan D, Yu H. DNA damage in human skin keratinocytes caused by multiwalled carbon nanotubes with carboxylate functionalization. Toxicol Ind Health. 2014;30(6):489–498. doi: 10.1177/0748233712459914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShan D, Ray PC, Yu H. Molecular Toxicity Mechanism of Nanosilver. J Food Drug Anal. 2014;22:116–127. doi: 10.1016/j.jfda.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrow H, Bucher S, Hormes J, Brinkmann R, Boennemann H. Model for chainlength-dependent core-surfactant interaction in N(Alkyl)4Cl-stabilized colloidal metal particles obtained from x-ray absorption spectroscopy. J Phys Chem B. 2003;107(16):3684–3689. [Google Scholar]
- Murray CB, Kagan CR, Bawendi MG. Self-organization of CdSe nanocrystallites into three-dimensional quantum dot superlattices. Science. 1995;270(5240):1335–1338. [Google Scholar]
- Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc. 1993;115(19):8706–8715. [Google Scholar]
- Murray CB, Sun S, Doyle H, Betley T. Monodisperse 3d transition-metal (Co, Ni, Fe) nanoparticles and their assembly into nanoparticle superlattices. MRS Bull. 2001;26(12):985–991. [Google Scholar]
- Palla BJ, Shah DO, Garcia-Casillas P, Matutes-Aquino J. Preparation of nanoparticles of barium ferrite from precipitation in microemulsions. J Nanopart Res. 1999;1(2):215–221. [Google Scholar]
- Peng ZA, Peng X. Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J Am Chem Soc. 2001;123(1):183–184. doi: 10.1021/ja003633m. [DOI] [PubMed] [Google Scholar]
- Pernodet N, Fang X, Sun Y, Bakhtina A, Ramakrishnan A, Sokolov J, Ulman A, Rafailovich M. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small. 2006;2(6):766–773. doi: 10.1002/smll.200500492. [DOI] [PubMed] [Google Scholar]
- Pillai V, Kumar P, Multani MS, Shah DO. Structure and magnetic properties of nanoparticles of barium ferrite synthesized using microemulsion processing. Coll Surf A: Physicochem Eng Aspects. 1993;80(1):69–75. [Google Scholar]
- Porta F, Bifulco C, Fermo P, Bianchi CL, Fadoni M, Prati L. Synthesis of spherical nanoparticles of Cu2L2O5 (L = Ho, Er) from W/O microemulsions. Coll Surf A: Physicochem Eng Aspects. 1999;160(3):281–290. [Google Scholar]
- Ray PC. Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Chem Rev. 2010;110(9):5332–5365. doi: 10.1021/cr900335q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PC, Singh AK, Senapati D, Fan Z, Yu H. Toxicity and Environmental Risks of Nanomaterials: An Update. In: Bagchi D, Bagchi M, Moriyama H, Shahidi F, editors. Bio-Nanotechnology: A Revolution in Food, Biomedical and Health Sciences. Blackwell Publishing Ltd; 2013. pp. 733–748. [Google Scholar]
- Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C. 2009;27(1):1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenberger J, Scher EC, Alivisatos AP. A New Nonhydrolytic Single-Precursor Approach to Surfactant-Capped Nanocrystals of Transition Metal Oxides. J Am Chem Soc. 1999;121(49):11595–11596. [Google Scholar]
- Schreder B, Schmidt T, Ptatschek V, Winkler U, Materny A, Umbach E, Lerch M, Mueller G, Kiefer W, Spanhel L. CdTe/CdS clusters with “core-shell” structure in colloids and films. The path of formation and thermal breakup. J Phys Chem B. 2000;104(8):1677–1685. [Google Scholar]
- Seo WS, Jo HH, Lee K, Park JT. Preparation and optical properties of highly crystalline, colloidal, and size-controlled indium oxide nanoparticles. Adv Mat. 2003;15(10):795–797. [Google Scholar]
- Sun S, Murray CB, Doyle H. Controlled assembly of monodisperse ε-Cobalt-based nanocrystals. Mat Res Soc Sym Proc. 1999;577:385–398. Advanced Hard and Soft Magnetic Materials. [Google Scholar]
- Suresh AK, Pelletier DA, Wang W, Moon JW, Gu B, Mortensen NP, Allison DP, Joy DC, Phelps TJ, Doktycz MJ. Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ Sci Technol. 2010;44(13):5210–5215. doi: 10.1021/es903684r. [DOI] [PubMed] [Google Scholar]
- Talapin DV, Rogach AL, Kornowski A, Haase M, Weller H. Highly Luminescent Monodisperse CdSe and CdSe/ZnS Nanocrystals Synthesized in a Hexadecylamine-Trioctylphosphine Oxide-Trioctylphosphine Mixture. Nano Lett. 2001;1(4):207–211. doi: 10.1021/nl0155126. [DOI] [PubMed] [Google Scholar]
- Vestal CR, Zhang ZJ. Synthesis of CoCrFeO4 Nanoparticles Using Microemulsion Methods and Size-Dependent Studies of Their Magnetic Properties. Chem Mat. 2002;14(9):3817–3822. [Google Scholar]
- Viau G, Brayner R, Poul L, Chakroune N, Lacaze E, Fievet-Vincent F, Fievet F. Ruthenium Nanoparticles: Size, Shape, and Self-Assemblies. Chem Mat. 2003;15(2):486–494. [Google Scholar]
- Vogel W, Britz P, Boennemann H, Rothe J, Hormes J. Structure and Chemical Composition of Surfactant-Stabilized PtRu Alloy Colloids. J Phys Chem B. 1997;101(51):11029–11036. [Google Scholar]
- Wang S, Lu W, Tovmachenko O, Rai US, Yu H, Ray PC. Challenge in understanding size- and shape-dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem Phys Lett. 2008;463(1–3):145–149. doi: 10.1016/j.cplett.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-d, Ma C-l, Sun X-d, Li H-d. Preparation of nanocrystalline metal oxide powders with the surfactant-mediated method. Inorg Chem Comm. 2002;5(10):751–755. [Google Scholar]
- Wong EM, Hoertz PG, Liang CJ, Shi B-M, Meyer GJ, Searson PC. Influence of Organic Capping Ligands on the Growth Kinetics of ZnO Nanoparticles. Langmuir. 2001;17(26):8362–8367. [Google Scholar]
- Wu Z, Zhang J, Benfield RE, Ding Y, Grandjean D, Zhang Z, Xin J. Structure and Chemical Transformation in Cerium Oxide Nanoparticles Coated by Surfactant Cetyltrimethylammonium Bromide (CTAB): An X-ray Absorption Spectroscopic Study. J Phys Chem B. 2002;106(18):4569–4577. [Google Scholar]
- Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 2005;158(2):122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Yang X, Gondikas AP, Marinakos SM, Auffan M, Liu J, Hsu-Kim H, Meyer JN. Mechanism of Silver Nanoparticle Toxicity Is Dependent on Dissolved Silver and Surface Coating in Caenorhabditis elegans. Environ Sci Technol. 2012;46:1119–1127. doi: 10.1021/es202417t. [DOI] [PubMed] [Google Scholar]
- Yin H, Too HP, Chow GM. The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials. 2005;26(29):5818–5826. doi: 10.1016/j.biomaterials.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Ying E, Hwang H-M. In vitro evaluation of the cytotoxicity of iron oxide nanoparticles with different coatings and different sizes in A3 human T lymphocytes. Sci Tot Environ. 2010;408(20):4475–4481. doi: 10.1016/j.scitotenv.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Zhang W, Li Y, Niu J, Chen Y. Photogeneration of reactive oxygen species on uncoated silver, gold, nickel, and silicon nanoparticles and their antibacterial effects. Langmuir. 2013;29(15):4647–4651. doi: 10.1021/la400500t. [DOI] [PubMed] [Google Scholar]